Translate this page into:
Enabling One Health solutions through genomics
*For correspondence: trinad.chakraborty@mikrobio.med.uni-giessen.de
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Ecological interactions between different domains of life impact the balance of ecosystems and human health. This point is made with destructive clarity by the current coronavirus disease (COVID-19) outbreak, caused by SARS-CoV-2. The pandemic has its origin as a zoonotic pathogen but its transmission pathway to humans is presently unknown1. As the pandemic progressed, it became clear that many other animals and in particular mammals, were secondary targets of the disease. Also, the presence of virus in sewage and waste water, and probably also in packaged frozen food, represents enormous reservoirs of virus loads and a potential for increased infections through evolution of more transmissable virus variants2. This will invariably affect the efficacies of both detection methods as well as the vaccines currently in use. Thus, to respond to the current challenges the world is facing, an integrated vision that humans, animals, the environment and climate are intricably linked is more important than ever3.
The implementation of genomic sequencing and molecular epidemiology in public health has been largely driven by the detection of viruses during the outbreaks and pandemics4. In 2009, genome sequencing of virus during the outbreak of A(H1N1)pdm 09 suggested that the pandemic had begun almost two months earlier before the first reported case5. Sequencing of outbreak isolates of the Middle-East respiratory syndrome (MERS) virus identified presence of the virus in camels and allowed the documentation of independent cases of transmission from camels to humans6. Wide scale genome sequencing introduced during the 2013-2016 Ebola virus outbreak enabled identification of the sentinel case in the outbreak and phylodynamic insights from large sequencing efforts demonstrated that the outbreak was maintained by human-to-human transmission rather than repeated zoonotic introduction7. In the current pandemic, as SARS-CoV-2 continues to acquire genetic changes, accelerated determination of genome sequences provides the tool vital to monitor and validate the expected sensitivity of various diagnostic assays even within a locality8.
The need to determine origins and transmission routes for an assessment of the emergence and expansion of antimicrobial resistance (AMR) worldwide is an urgent problem. Between 2010 and 2015, the total aggregated consumption of antibiotics in 76 countries increased by 65 per cent from 21.1. to 34.8 billion defined daily doses (DDD), with the greatest increases reported in rapidly emerging economies9. This constitutes a major cause for the emergence of AMR worldwide. The increase in AMR in human health settings is associated with the overuse, misuse as well as an unwarranted use of antimicrobials, either through self-diagnosed medication or over-prescription by medical practioners. This is facilitated through easy and/or illegal access to antimicrobial medication without a prescription10. However, this is dwarfed by the mass usage of antibiotics in livestock farming and aquaculture10. The soaring global demand for meat in low- and middle income countries has led to the growth in meat production levels of 68, 64, and 40 per cent in Africa, Asia and South America, respectively11. The use of antimicrobials in livestock production is enormous comprising 73 per cent of the entire global consumption12. Currently, global consumption of antibiotics is estimated to be 131,000 tons annually. This is predicted to rise by over 60 per cent to around 200,000 tons by 203011. This contributes to the rise in contamination of water bodies and environments by large-scale pharmaceutical production facilities as well as discharge from communities dedicated to healthcare and livestock with antimicrobials. Environmental contamination with pharmaceutical waste provides selection for antimicrobial resistance and promotes transfer of AMR genes among bacterial communities in the environment13.
To deal with the rise in AMR and emerging infectious diseases, a One Health approach needs to be considered. The protection of human life cannot be a sole concern. Our well-being as a species requires environmental sustainability, and various disciplines are required to interact with one another to provide new tools and methods for research and implementation of effective services to inform policy decisions and regulations to the benefit of health in animals and humans, the environment and climate for future generations. Collaboration among multiple disciplines is necessary and this approach is fundamental to understand the concept of One Health14.
A transdisciplinary approach to One Health considers animals, humans and their shared settings together with their linked environments (Fig. 1). To promote the well-being of the animal-human-ecosystem interface, increased efforts to improve understanding of health and disease processes is needed14. To be effective it should be able to predict, detect, prevent and control infectious hazards and other issues affecting health and well being15. These efforts contribute to sustainable development goals and to improve equity in urban and rural populations.
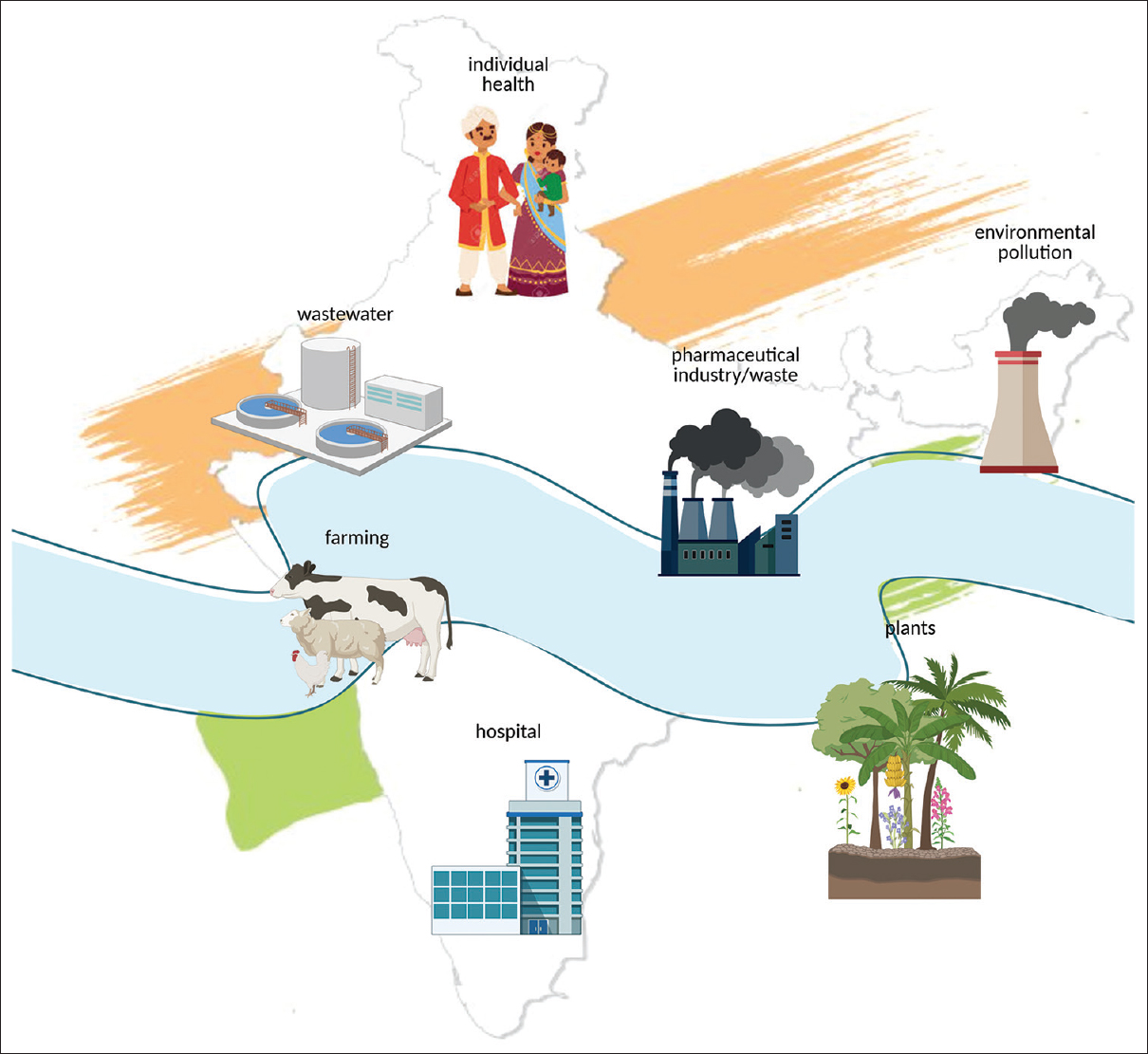
- Ecosystems involved in the transfer and spread of antimicrobial resistance emphasizing a One Health approach (created with tools from: https://biorender.com/).
Global threat posed by antibiotic-resistant bacteria
In the European Union (EU) and the European Economic Area, about 670,000 infections and 33,110 deaths were attributed to antibiotic-resistant bacteria in 201516. By the year 2050, antibiotic-resistant bacteria may, on a global scale, lead to the death of up to ten million people at a cost of 94 trillion Euros1718. However, the increasing prevalence of antibiotic resistance is also a problem in numerous other areas, such as with farm animals, plants, food and the environment19. In 2018, the World Health Organization (WHO) published a priority list for the development of new antibiotics against pathogenic bacteria. Carbapenem-resistant, Gram-negative bacteria (Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, referred to as ESKAPE pathogens) were of highest concern. These multi-resistant bacteria have become increasingly more frequent in recent years20.
The concern is that we are reaching a post-antibiotic era, in which bacterial infections will become virtually impossible to treat with antibiotics. Counteracting this threat, by developing new antibiotics, for example, requires precise knowledge of the bacteria involved. For this purpose, their characteristics must be analysed as accurately as possible and for as many bacteria as feasible. Among the important tools advanced to promote the fight against AMR is the development of balanced and comprehensive epidemiological and ecological surveillance networks at the regional, national and international scales21.
Use of genome sequencing in antibiotic resistance research
The cost of curbing local and regional outbreaks and particularly that of a global pandemic has enormous consequences in social, economic and ecological dimensions22. A more rational approach, far less costly and with probably more societal support, would be the development of surveillance and interception systems based on detailed knowledge of emerging pathogens and their environmental interactions. When linked to strategies for advanced diagnostics, therapeutics and vaccine development, these are anticipatory and can be preemptive in consequence.
The ecological and pathological consequences of animal, human and enivronmental interactions with and among viruses and other microorganisms have remained poorly understood because of their invisible scales23. The scope of characterizing these interactions appeared to be an impossible task a few years ago, but an exponential rise in the output capacities of DNA sequencing platforms coupled together with steep decrease in sequencing costs provided the technical and economic breakthroughs needed to address these challenges24.
Early containment of antibiotic-resistant pathogens to prevent and stop spread of emerging or highly prevalent resistance genes requires different scales of surveillance programmes and epidemiological analysis21. The increase in recently sequenced genomes of antibiotic-resistant bacteria has revealed the overall diversity of antibiotic resistance mechanisms involving innate and adaptive components25. Innate antibiotic resistance is the intrinsic resistance imparted by the chromosomally encoded genes and/or naturally occurring variants that modify efflux pumps, etc., and is disseminated vertically as clones26. On the other hand, adaptive antibiotic resistance is often acquired on segments of highly mobile genetic elements (MGEs) that encode enzymes which can detoxify or modify antibiotics and their cellular targets. The MGEs are often transferred horizontally, very frequently by extrachromosomal entities called plasmids that can in very short evolutionary time scale, lead to very large reservoirs of multi-resistant bacteria comprising many different clones and even species27.
Genome-based surveillance systems
The increased recognition that whole genome-based sequencing (WGS) has an important role in improving public health is being applied to national surveillance of infectious diseases4. In the EU and the United States the widespread adoption of WGS has improved the accuracy and effectiveness of disease surveillance as well as outbreak investigation28. For real impact of this strategy on public health policies such as through enhanced assessment of disease or changes in drug resistance transmission dynamics, it requires the communication of actionable and timely results. This requires fostering of a multidisciplinary approach to interprete information derived. from epidemiological data and pathogen sequence analysis as key competencies to guide public health action29. Trained staff to coordinate exchange of data between public health institutions, together with those overseeing the monitoring of livestock, food chains and environmental laboratory network partners will be needed. Multidisciplinary expertise is needed, between scientists, statisticians, clinicians, veterinarians, public health experts and policymakers, to prioritize and identify high impact diseases or drug resistance emergence, to establish where genome sequence information is truly useful for public health interventions30.
It is clear that the public health emergencies of the current pandemic has fuelled the rise of WGS-based typing methods. This has enabled rapid identification of pathogens and is now being used to understand their reservoirs and origins, the paths and modes of transmission, derive and map genetic diversity, and describe outbreak dynamics31. The genome analysis can estimate aspects of disease dynamics in the absence of epidemiological data and unanalysed samples. For AMR, the in silico prediction of phenotype, innate and acquired antimicrobial resistance mechanisms, colonization and virulence determinants and correlates of epidemiological/ecological fitness associated with transmission, for so-called high-risk clones, can be determined32. Finally, the generation of AMR-based epidemiological landscapes across space and time will enable prediction of emergence and inform on policy measures in One Health.
During surveillance, both high-quality genomic information and the resolution of genetic relatedness is required to address disease-specific objectives. For example, enhanced resolution strategies and precision enable detection and common source tracing during cross-community infection outbreaks. Also, the monitoring of vaccine antigen expression among circulating pathogens and changes in antigen diversity in large populations are highly relevant33.
Challenges in microbial bioinformatics
The decrease in the costs of DNA sequencing has accelerated sequence generation. It has been estimated that the annual acquisition of raw data worldwide would excede one zetta byte to one trillion Gb in 202534. This increases the demand for local data processing as well as the need of scalable, federated and nearby computing infrastructures for large scale computations as primarily manifested in the rise of cloud computing35. By sharing the physical computational backbone between numerous users, synergistic effects such as limiting the overprovisioning for peak loads and shared spare resources has reduced costs and increased literacy and know-how to use large IT infrastructures36.
This huge increase in data opens new scientific questions and constantly requires new algorithms and bioinformatic approaches. This has contributed to the genesis of a large set of bioinformatics software tools and databases37. Robust implementations of analysis workflows need to produce reproducible results on different machines among multiple iterations. A central role of these workflows is to enable scalability and modern analysis workflows must be able to cover broad ranges of data sizes. Smaller numbers of cases need to return rapid results while production of real-time data might scale to very large datasets. This often requires either vertical or horizontal distribution workflows to compute clusters of different types and sizes.
In order to achieve a focused and comprehensive analysis of genome sequence data, we developed the analytical software ASA3P (Automatic Bacterial Isolate Assembly, Annotation and Analyses Pipeline)38. ASA3P has been optimized to process sequence data obtained by applying leading sequencing technologies. Initially, the analysis software subjects the genome sequence data to a quality control procedure and removes faulty data. The remaining data are then used to derive the genetic information of the individual bacteria (genetic fingerprint). The genetic fingerprints of several bacteria can subsequently be directly compared. ASA3P creates high-resolution genetic fingerprints of hundreds of bacteria within hours, a task that would have taken several weeks or even months to complete in the days of manual approaches38. This was accomplished using technical adjustments, allowing the optimal exploitation of the enormous capacities of a cloud-based computing infrastructure.
A proposal for implementing a genome-based One Health initiative in india
At the UN in September 2016, the world leaders highlighted an unprecedented level of attention to control the spread of infections resistant to antimicrobials39. A broad, coordinated approach was mooted to address the primary causes of AMR across human and animal health and agriculture. Since then, the World Health Organization (WHO), Food and Agriculture Organization (FAO), and World Organization of Animal Health (OIE) working in conjunction with other key organizations are developing policy frameworks for global action40. It is anticipated that these global policy frameworks will translate into national action plans in many participating countries. A key tenet is the collection of state-of-the art-information from regional and national databases and interdisciplinary research incorporating multiple sectors, domains and disciplines including health, natural, social and economic sciences. Research into implementation represents a key area of practice as only a good understanding of contextual and real-world factors will create the necessary impact required.
Here, we propose the creation of a Centre for Interdisciplinary Research and Solutions on AMR (CIRS-AMR) within the One Health Initiative for the purposes outlined above (Fig. 2). As AMR poses an urgent threat, the aim is to safeguard the use of antimicrobials to support the achievement of Sustainable Development Goals particularly regarding human and animal health, welfare, equity and economic growth. As outlined in JimO´Neill's report on AMR, investment in AMR containment is expected to give a high economic rate of return and should be of the highest priority among public sector investments18.

- The Million Vaibhav Proposal to promote consciousness and to foster a One Health approach to antimicrobial resistance (created with tools from: https://biorender.com/). This figure was generated exclusively for the purpose of this article.
A proposal of high impact and visibilty would be the implementation of a national, transsectoral proposal examining the genetic context of one million microorganisms in a One Health Context (The Million Vaibhav Project). This would be in keeping with the WHO recommendations on the use of genomic surveillance for AMR40 and be part of a global strategy for infectious disease and foodborne outbreak control, for case definition as well as enabling source attribution. Genomic surveillance would also enhance elimination strategies. It would monitor vaccine effectiveness by assessing the increase, decline or replacement of specific lineages. It would identify failures in control efforts, reveal potential reservoirs, promote outbreak investigation and detect new emerging strains.
Such an initiative would require harnessing and improving sequencing infrastructure, promoting computational infrastructure and bioinformatic tools for automated platforms to process and analyze genome data with readily interpretation formats and actionable information41. As detailed above, combinations involving on-site and cloud-based computing infrastructure could be developed. To support genomic surveillance service statisticians, bioinformaticians and molecular epidemiologists would be needed for data analysis and interpretation. Field epidemiologists and disease specialists would interpret the data generated and public health experts would use these findings for de/refining policy and communication42. This would generate high standards in the collection, storage and distribution of specimens, genome archiving as well as biorepository storage and sharing. The overaching benefits of rapid data sharing, which is to be developed would realize disease control and pandemic prevention43.
Ensuring efficiency and effectiveness of the use of genome-based data in routine surveillance systems is crucial. This includes assessing completeness of data, timely data collection and the use of data for policy decisions. A sustainability framework would also need to be established and would best be integrated into national disease control programmes. Finally, developing genome-based surveillance will need guidance, leadership and coordination that would be mandated within the One Health Initiative of India.
Acknowledgment:
The first author (TC) acknowledges the assistance of Dr Luigi La-Pietra with the Figures.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Unraveling the Zoonotic Origin and Transmission of SARS-CoV-2. Trends Ecol Evol. 2021;36:180-4.
- [Google Scholar]
- Dynamics of severe acute respiratory syndrome coronavirus 2 genome variants in the feces during convalescence. Genet Genomics. 2020;47:610-7.
- [Google Scholar]
- Biodiversity and Health: Linking life, ecosystems and societies. London: Elsevier, ISTE Press; 2017.
- Whole genome sequencing in clinical and public health microbiology. Pathology. 2015;47:199-210.
- [Google Scholar]
- Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605-15.
- [Google Scholar]
- Genomic sequencing and analysis of eight camel-derived Middle East respiratory syndrome coronavirus (MERS-CoV) isolates in Saudi Arabia. Viruses. 2020;12:611.
- [Google Scholar]
- Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature. 2017;544:309-15.
- [Google Scholar]
- Genomic sequencing of SARSCoV-2: a guide to implementation for maximum impact on public health. Geneva: WHO; 2021.
- Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463-70.
- [Google Scholar]
- Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112:5649-54.
- [Google Scholar]
- Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:eaaw1944. doi: 10.1126/science.aaw1944
- [Google Scholar]
- Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms. 2019;7:180.
- [Google Scholar]
- The Lancet. Zoonoses: beyond the human-animalenvironment interface. Lancet. 2020;396:1. doi: 10.1016/S0140-6736(20)31486-0
- [Google Scholar]
- Ecological determinants of health: food and environment on human health. Environ Sci Pollut Res Int. 2017;24:9002-15.
- [Google Scholar]
- Sciworthy. The impact of antibiotic resistant infections in Europe. Available from: https://sciworthy.com/the-impact-ofantibiotic-resistant-infections-in-europe/
- Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56-66.
- [Google Scholar]
- Review on Antimicrobial Resistance. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J Glob Antimicrob Resist. 2020;20:170-7.
- [Google Scholar]
- Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318-27.
- [Google Scholar]
- Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109:309-18.
- [Google Scholar]
- Socio-economic impacts of COVID-19 on household consumption and poverty. Econ Dis Cli Cha. 2020;4:453-79.
- [Google Scholar]
- Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17:333-51.
- [Google Scholar]
- Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015
- [Google Scholar]
- CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517-25.
- [Google Scholar]
- Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:e00088-17.
- [Google Scholar]
- The use of next generation sequencing for improving food safety: Translation into practice. Food Microbiol. 2019;79:96-115.
- [Google Scholar]
- Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. 2018;19:9-20.
- [Google Scholar]
- Whole genome sequencing: Bridging One-Health surveillance of foodborne diseases. Front Public Health. 2019;1:172.
- [Google Scholar]
- Phylodynamic applications in 21st century global infectious disease research. Glob Health Res Policy. 2017;2:13-22.
- [Google Scholar]
- Evolution of antibiotic resistance is linked to any genetic mechanism affecting bacterial duration of carriage. Proc Natl Acad Sci U S A. 2017;114:1075-80.
- [Google Scholar]
- One Health-Its importance in helping to better control antimicrobial resistance. Trop Med Infect Dis. 2019;4:22.
- [Google Scholar]
- eInfochips. Importance of cloud computing for large scale IoT solutions. Available from: https://www.einfochips.com/blog/importance-of-cloud-computing-for-large-scale-iotsolutions/
- Network and server resource management strategies for data centre infrastructures: A survey. Computer Networks. 2016;106:209-25.
- [Google Scholar]
- Tools and data services registry: a community effort to document bioinformatics resources. Nucleic Acids Res. 2016;44:D38-47.
- [Google Scholar]
- An automatic and scalable pipeline for the assembly, annotation and higher-level analysis of closely related bacterial isolates. PLoS Comput Biol. 2020;16:e1007134 ASA3P.
- [Google Scholar]
- Political Declaration of the high-level meeting of the general assembly on antimicrobial resistance : draft resolution. Available from: https://sdg.iisd.org/news/unga-adoptspolitical-declaration-on-antimicrobial-resistance-discusseslinks-with-sdgs/
- Global Antimicrobial Resistance Surveillance System (GLASS). Available from: https://www.who.int/glass/resources/publications/molecular-methodsfor-amr-diagnostics/en/
- Whole genome sequencing for foodborne disease surveillance. Available from: https://www.who.int/publications/i/item/whole-genome-sequencingfor-foodborne-disease-surveillance
- Genomic-informed pathogen surveillance in Africa: opportunities and challenges. Lancet Infect Dis 2021 S1473-3099.30939-7
- [Google Scholar]
- Next generation sequencing and bioinformatics methodologies for infectious disease research and public health: Approaches, applications, and considerations for development of laboratory capacity. J Infect Dis. 2020;221(Suppl 3):S292-307.
- [Google Scholar]





