Translate this page into:
Emergency preparedness for public health threats, surveillance, modelling & forecasting
For correspondence: Dr Pankaj Dhaka, Centre for One Health, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana 141 004, Punjab, India e-mail: pankaj.dhaka2@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the interconnected world, safeguarding global health security is vital for maintaining public health and economic upliftment of any nation. Emergency preparedness is considered as the key to control the emerging public health challenges at both national as well as international levels. Further, the predictive information systems based on routine surveillance, disease modelling and forecasting play a pivotal role in both policy building and community participation to detect, prevent and respond to potential health threats. Therefore, reliable and timely forecasts of these untoward events could mobilize swift and effective public health responses and mitigation efforts. The present review focuses on the various aspects of emergency preparedness with special emphasis on public health surveillance, epidemiological modelling and capacity building approaches. Global coordination and capacity building, funding and commitment at the national and international levels, under the One Health framework, are crucial in combating global public health threats in a holistic manner.
Keywords
Capacity building
epidemiological modelling
health threats
One Health
preparedness
public health
surveillance
One health approach is integral in enhancing the preparedness for ongoing and upcoming potential public health threats such as emergence of infectious diseases, antimicrobial resistance, environmental pollution, climate change, non-communicable diseases, natural disasters, and bioterrorism12. A proactive, coordinated, interdisciplinary and cross-sectoral approach across human, animal and environmental sectors remain the core pillar of One Health framework to mitigate the public health challenges3. The need for early response to emerging zoonoses before these spill over into human population has been underscored by the gravity of life losses caused by the ongoing COVID-19 pandemic, which has been classified as an ‘emerging disease of probable animal origin’45. A better understanding and early prediction of how an animal pathogen might have crossed species barriers to infect humans and then to an epidemic or pandemic potential can productively prevent many emerging diseases6. Therefore, the holistic One Health programmes are important for the elucidation of various zoonotic pathogen transmission pathways and for conceptualizing policies to prevent the outbreaks at the source of origin3.
In the globalized era, with rapid population and trade mobility, any infectious disease can spread across the world within a span of 36 hours7. The epidemic intelligence-based analysis of the drivers of the emerging infectious diseases in Europe during 2008–2013, categorized ‘travel and tourism’ as the most prominent and frequent disease driver8. With the increasing number of air travel of around 3.6 billion people/year9, the national and international boundaries have become more porous to facilitate the introduction of infectious agents into new regions within a time frame even shorter than the incubation period of most pathogens. The severe acute respiratory syndrome (SARS) pandemic with its epidemic origin in Guangdong, China had flourished into a pandemic affecting“5 countries within 24 hours and to more than 30 countries on 6 continents within 6 months” with 8096 cases and 774 deaths10. Other recent emergence and spread of infectious diseases of global health significance facilitated by air travel include the initiation of the chikungunya epidemic in Europe in 2007 from a single infectious traveler from India11, the pandemic influenza in Mexico12, the spread of New Delhi metallo-beta-lactamase-1 (NDM-1) gene from India to Sweden and to multiple other countries13, the Middle East respiratory syndrome (MERS) epidemic in South Korea, which is the largest epidemic outside Saudi Arabia, from a single infectious traveler returning from Saudi Arabia14, the 2014-2016 West African Ebola virus outbreak costing 11,325 lives, with imported cases in seven countries15, and the 2015-2016 Zika outbreak spread from Brazil to 87 countries and territories16 and more recently the COVID-19 pandemic17. A study on H1N1 pandemic influenza strain highlighted the fact that despite the presence of high-efficiency particulate air (HEPA) filters in aircrafts, the attack rate in a 9-hour flight was estimated as high as 4.3 per cent18. Therefore, in the interconnected world, public health preparedness is the key to avoid devastating losses from such emerging threats1.
The pillars of emergency preparedness for public health threats rely on the integrity of surveillance and forecasting models which aids in mobilizing resources and timely responses19. Public health surveillance is crucial in recognizing new cases of any emerging infections as well as in estimating the present health status of the populations. The epidemiological models support the preparedness and decision making of stakeholders by simulating the probable scenario such as transmission pathways, disease dynamics, along with the evaluation of various alternative intervention strategies20. The predictive information systems based on routine surveillance, modelling and forecasting affect the organizational decisions and public awareness of health-related events. The temporal and spatial risk for many infectious diseases, especially in case of vector-borne diseases, predicted through advanced surveillance and disease models incorporating the environmental data has enhanced the epidemic prevention and control capabilities21.
The emergency preparedness measures should work closely in frame of an integrated global network with national and international relevant stakeholders20. The successes of such global efforts were illustrated to control SARS, the first pandemic of 21st century which depended on a combination of open collaboration and the rapid and accurate communication of surveillance data within and among the countries10. The role of effective risk communication, involvement and coordination among the individuals, healthcare providers, policy makers, community, international organizations and stakeholders is vital for prompt response to emergencies at all stages of the risk prioritization, preparedness and planning22. The operationalization of One Health framework is essential to avoid fragmented planning and implementation of swift and effective response by mobilizing sufficient resources in appropriate time23.
Public health preparedness: An overview
The core realm of global health security is public health preparedness based on the pillars of prevention, early detection and response24. The emergence of COVID-19 pandemic has evoked worldwide concerns across the public health administrators to strengthen the preparedness capabilities25. These measures can help in the improvement of hazard-specific capacity of the countries through the effective priority setting and mobilization of key resources including information, funds, equipment, drugs and response teams, based on their availability and perceived effectiveness2226. The essential components of public health preparedness comprises of robust surveillance system, risk assessment and management, capacity building and maintenance, intersectoral collaboration and international coordination27.
The emergency preparedness to address foreseeable public health problems should be enriched with the information from epidemiological models with valid assumptions, quantitative predictions, and policy needs28. Modelling and simulations are key resources to tackle the unprecedented emergencies effectively by assisting in decision making upon time-pressured situations to interlink theory, policy and practice28. These modelling and simulation exercises need to be combined with participatory surveillance to establish early warning systems for increasing the resilience to combat public health emergencies29. The analytical capability of forecasting has been demonstrated in recent outbreaks of influenza, dengue, Zika, and Ebola in assisting management at policy level for the real-time outbreak response30313233343536. Furthermore, the scaling up of organizational public health workforce is essential for instigating a rapid effective response in case of an emergency. The capacity building of the workforce for adequate and rapid intervention and response should be regularly strengthened by regular training, use of modern epidemiological and molecular tools, technical assistance, periodic assessment and feedback, peer networking, and relevant incentives37. The outline of various components of public health preparedness has been highlighted in Fig. 1.
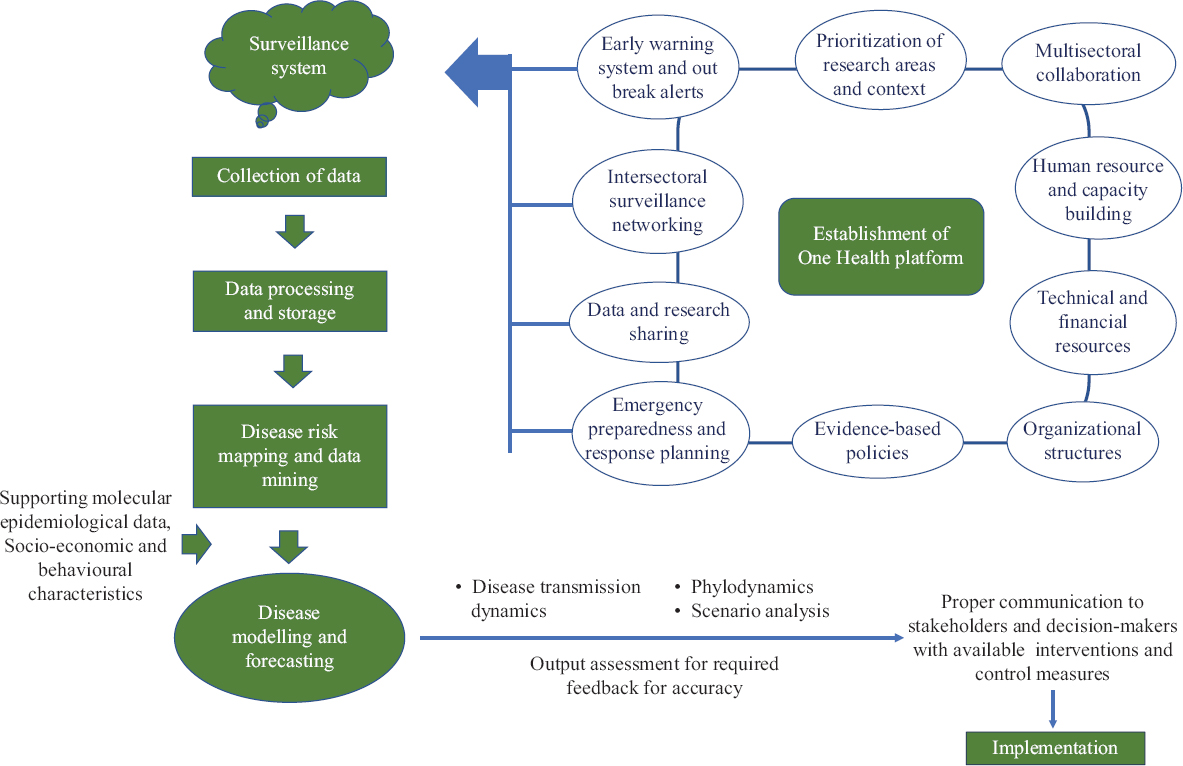
- Various components of public health preparedness.
The ongoing COVID-19 pandemic has demonstrated that the bridging of not only professional silos but also the use of multi-tech approaches is much essential to generate synergistic effects in combatting global pandemic of such scale3839. To cite, various countries have used modern technologies in the form of data science40, computational biology41, medical image processing4243, disease tracking44, prediction models4546, and machine learning and artificial intelligence47 to aid the fight against COVID-19. For examples, the use of software enabled smartphones48, wrist bands49 and facial recognition cameras50 helped in rapid identification of cases, proper source tracking, outbreak forecasting and monitoring of the compliance of quarantine rules. In hospitals, robots for delivery of food and medicines to patients51 and drones for patrolling and broadcasting awareness messages and site disinfections, were employed52. Taiwan, which was applauded globally for its COVID-19 containment efforts, has successfully coupled the information of their national medical insurance database with the immigration and customs database for rapid isolation and tracking of suspected patients53. The use of artificial intelligence in rapid diagnosis of the cases (e.g., use of computational tomography scans43 and radiology images)42, prediction of the possible disease outcomes among patients by virus-host interaction41, new drug molecule discovery54 and development of various suitable vaccine candidates by possible protein structure predictions has been widely employed555657.
Public health surveillance
The public health surveillance is known as the radar of public health, with the two-core objectives of assessing existing disease burden and pattern to guide the control programmes effectively, and early warning and detection of novel pathogens for the rapid instigation of responses22. These systems should have adequate sensitivity and feasibility to be vigilant to detect the alerts from various sources, without compromising the specificity of surveillance system58. The core steps in the design of any surveillance system are depicted in Figure 2.
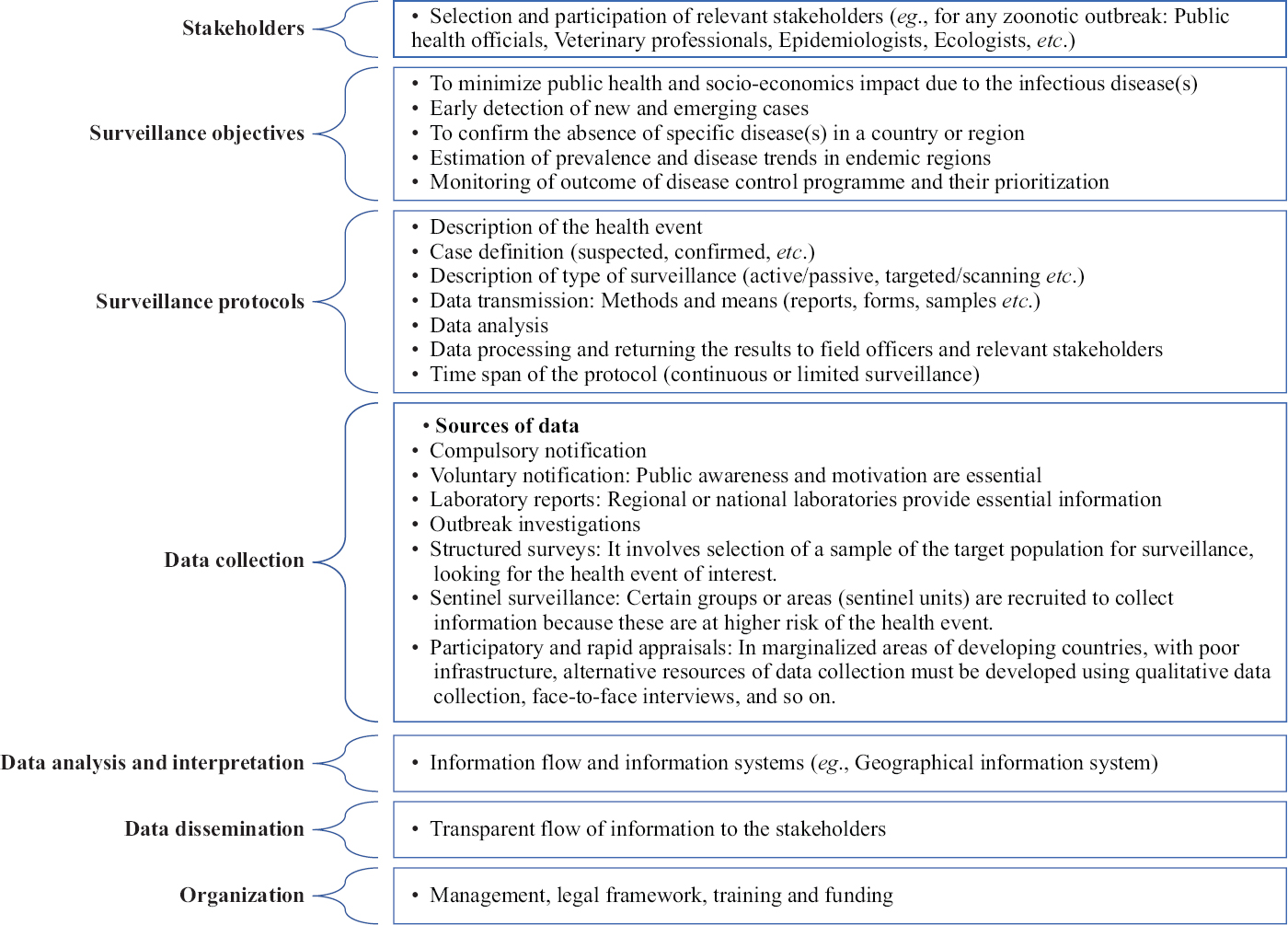
- Core steps in the design of surveillance system.
The complex scenario in developing countries with numerous drivers of infectious diseases at animal and human interface urges the need to strengthen the existing surveillance systems59. The various surveillance systems established for diseases of regional importance need upgradation to tackle numerous emerging infectious diseases59. For example, the Food and Agriculture Organization's (FAO) Emergency Prevention System for Transboundary Animal and Plant Pests and Disease (EMPRES) was initially built on the foundation of community surveillance efforts for Rinderpest eradication60.
Most of the laboratory-based surveillance protocols rely on the conventional culture techniques for identification and isolation of pathogen from the collected samples. These isolates can be further characterized by array of available molecular typing tools, viz., conventional and real-time polymerase chain reaction (PCR); pulsed-field gel electrophoresis (PFGE); amplified fragment length polymorphism (AFLP); random amplification of polymorphic DNA (RAPD); repetitive-element PCR (rep-PCR), variable-number tandem repeat (VNTR) typing; single locus sequence typing (SLST); multi-locus sequence typing (MLST), DNA microarray etc.61. Moreover, the advent of whole-genome sequencing (WGS) as tool with much higher resolving power than traditional molecular methods, greatly improved the speed and accuracy of epidemiologic investigations62. However, implementation of these novel molecular tools to assist conventional laboratory surveillance require proper validation of protocols and trained manpower for their standardized application. The use of molecular tools for the investigation of malaria outbreak included the demonstration of zoonotic transmission of Plasmodium simium in people of the Atlantic Forest in Rio de Janeiro by sequencing of the parasite mitochondrial genome63. The importance of molecular surveillance to prevent the foodborne outbreaks has been highlighted by the researchers, where the use of forensic microbiology based on the WGS of Listeria monocytogenes isolates successfully traced the source of the invasive listeriosis outbreak occurred during 2012-2016 in southern Germany64.
The early detection of the outbreaks allows the public health professionals a window period for coordinating efforts to contain the spread of outbreak and prevent it locally. The early warning and response systems rely on the epidemic intelligence gathered from sentinel and event-based surveillance data22. In sentinel surveillance programmes, the monitoring of at-risk population can generate the outbreak alerts, for example, entry screening of passengers at airport for H1N165, receiving data on influenza like illness from selected healthcare centres66, or designated sentinel sites to monitor the HIV outbreaks among high-risk groups in epidemic areas (e.g., drug users, sex workers, long-distance truck drivers and clinic attendees of sexually transmitted diseases)67. The population based epidemiological tools such as wastewater-based surveillance approaches have been used as an early warning signal in the tracking of infectious agents at the community level, such as, adenoviruses68, hepatitis A69, rotavirus70, poliovirus71 and also for the presence of SARS-CoV-27273.
In addition, the application of zoonotic surveillance for predicting human risk has been highlighted during animal-sentinel approaches which drew the association between crow mortality and mosquito abundance and emergence of West Nile virus among human population74. The identification of potential reservoir hosts and their longitudinal surveillance for the detection of pathogen shedding and related ecological interactions are essential for early prediction of spillover of zoonotic pathogens7576.
The effective implementation of public health surveillance requires the breakage of the sectoral silos by adopting a multisectoral and multi-disciplinary approach. The Global Early Warning System (GLEWS) and World Animal Health Information System (WAHIS) are the two important ongoing supranational global surveillance systems where the multi-institutional coordination and collaboration across disciplines remain a cornerstone component to predict, prevent and control the emerging health threats. GLEWS has been devised as an alert mechanism for major animal diseases including zoonoses at human-animal-ecosystems interface. It is a joint collaborative effort of FAO, World Organization for Animal Health (OIE) and WHO to carry out the disease tracking, analysis and joint risk assessment to generate early warning alerts, and essential response to outbreaks77. The OIE-WAHIS is a web-based system that collects and processes data to monitor the status of OIE listed diseases and to generate the alert messages by its early warning system on animal diseases in real-time to update the member countries78. The risk assessment and modelling are carried out as per the data availability on reported outbreaks, vaccination coverage, livestock movement, land use, wildlife interactions, climatic conditions and surveillance activities carried out by the member countries7778. Thus, the proper standardization of the data and transparent reporting are must for proper dissemination of information to the member countries.
The upgradation of the traditional laboratory based and syndromic surveillance to build hybrid systems with their integration to digital data can improve the speed, sensitivity and specificity of existing surveillance indicators79. The use of participatory surveillance approaches for modelling and forecasting of epidemics has strengthened the real-time response in emergency conditions such as pandemic influenza29. The heterogeneity of data sets in health sector poses a technical challenge for their integration at various spatial scales. However, the use of novel analytical and modelling tools (e.g., multilevel Bayesian statistical approaches) aids in alleviating this challenging issue80. The reliability and validation of surveillance systems highlights the importance of the constant evaluation and subsequent improvement of the methodology.
Disease modelling and forecasting
The models are considered as simplified representation of complex phenomenon which remain an important decision support tool and an aid to communication81. The various uses of epidemiological models include formulation of hypothesis, retrospective analysis from past epidemics, rapid characterization of infectious disease outbreaks, transmission modalities associated with disease outbreaks, contingency planning and facilitating emergency response(s), disease forecasting, resource planning and implementation of public health policy, economic consideration for available intervention tools, and training of public health professionals21828384.
The ongoing COVID-19 pandemic highlighted the applied aspects of epidemiological modelling in terms of disease dynamics85, projected basic reproduction number (R0)86, disease control interventions like social distancing, regional or national lockdown, healthcare capacity estimations87, pharmaceutical distribution and immunisation campaigns88 and other requirements. In such emergency scenario, the experimental studies would have many inherent limitations such as, time consuming, unethical, impractical, or sometimes impossible.
It should be remembered that biological systems are inherently more difficult to model due to their complexity and variability, thereby the input data are more difficult to collect and analyse89. To build a model of disease outbreak, the knowledge of epidemiological parameters of disease is needed so that the relevant components of the model can be put together accurately with correct interactions. Therefore, it is important to match the accuracy of disease modelling with the dynamics of real-world disease transmission. In the past years, dramatic increase in understanding of multifactorial disease dynamics paved to the development of many relevant models with significant implications (e.g., Ebola epidemic-Liberia and Sierra Leone, 2014-201590, COVID-19 pandemics)91. For the development of model for global Zika virus spread, the movement of high-risk population for Zika virus, the ecological niche of the mosquito vectors (i.e., Aedes aegypti and Ae. albopictus) and the data of the environmental temperature profile were used to locate the risk zones92. The risk of the COVID-19 spread outside China was statistically modelled using the aviation data integrated with the numbers of confirmed cases at each potential destination93. However, comprehensive predictive modelling of infectious diseases remains a challenging due to inadequate access to the data on various factors that affect the disease dynamics. Some of the important inputs for robust predictive modelling of infectious diseases have been presented in the Table8182848994.
| Characteristics | Associated parameters |
|---|---|
| Agent | Inherent characteristics: virulence, infectivity and pathogenicity |
| Transmission dynamics: within cell and cell-to-cell transmission; within tissue and tissue-to-tissue transmission; host and multi-host level and population level dynamics | |
| Molecular characterization: genetic make-up, genotypic resistance, genetic relatedness, etc. | |
| Host | Age, sex, immunity status, underlying disease conditions, disease carriers, demographic characteristics, population susceptibility and immunity, geographic networks and host movement |
| Environmental | Environmental hygiene, environmental reservoirs, transmission vehicles, temperature and other climate indicators |
| Socio-economic and behavioural | Personal hygiene and sanitation, cultural/religious practices, prevalence-elastic behaviour |
| Disease related parameters | Latent period, incubation period, infectious period, non-symptomatic cases, chronic cases, possible co-infection dynamics and synergism, deliberate epidemics as in case of bioterrorism |
| Disease surveillance data (if prior outbreaks occurred from same/similar agent) | Transmission dynamics and pathways, sensitivity and specificity of available diagnostic (s), data collection methodology, consideration of previous under reporting, interactions of multiple risk factors, followed medical countermeasures, availability of regional, national and global health resources and infrastructure, availability of novel therapies and interventions, and possible synergistic effects of the interventions |
Development and types of epidemiological models
The important aim of epidemiological models are to provide a systematic, data-driven, transparent, reproducible output, with the ability to describe the uncertainty and key data needed, and comprehensible decision-making framework. The development of epidemiological models remain a complex dynamical system which need coordination of several systems as presented in Fig. 394.
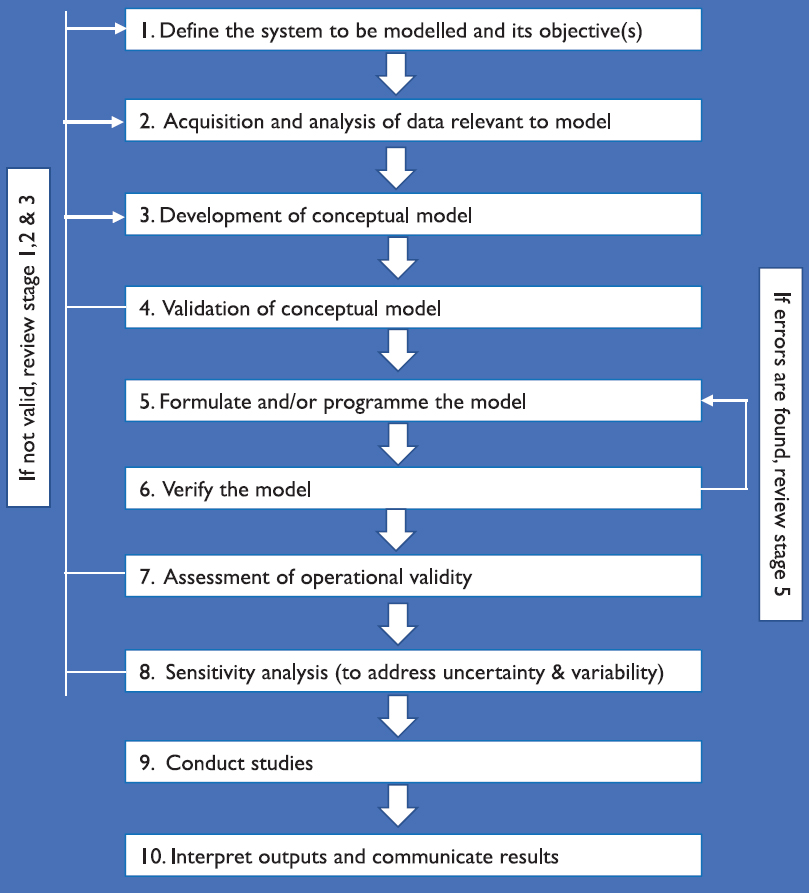
- Different stages of epidemiological model development.
The models can be classified into various types; however, the two well-known classifications of epidemiological models are:
-
Deterministic and stochastic models: Deterministic models use a pre-determined relationship between the model structure and inputs and the associated outputs, whereas, the stochastic models include the ‘effect of chance’, thereby, can produce varying outputs according to the calculation of individual probabilities8194.
-
Compartmental (or state-based) and individual-based (agent-based) models: On the basis of approach to represent the population, compartmental (or state-based) models group individuals into states on the basis of characteristics relevant to the infectious disease processes. e.g., the popular SIR (susceptible–infected–recovered) model95 and its variants. These are quick to form and works best when risk factors of infection are uniform in the population. Whereas the individual-based (agent-based) models explicitly represent the differentiation in biology or behaviour of individuals. Being stochastic, these offer more value when individual heterogeneity in transmission and structure of intervention is important89.
The British statistician George E. P. Box stated that “All models are wrong, but some models are useful”96. The continuous refinement of models to optimally respond to natural outbreaks is important. The realism in the model can be improved by incorporating the treatment of space (e.g., spatial distribution, population contact structure and contact rates) and the effect of available health related infrastructure. It should be remembered that the impact of behavioural factors in a population could have effect on disease dynamics, thus can produce different results from homogeneously mixing populations97. Other major inherent characteristic of models is that the accuracy of the models can be improved only by proper assessment of the real-world data after the outbreak98. For the infectious diseases, where the quality data is available, but the epidemiological knowledge is lacking, analytical modelling, such as regression of various types can be applied to assess the risk factors. Later, with appropriate epidemiological knowledge, these risk factors can be included in simulation models.
Many times, due to incomplete assumptions and inaccuracies in data assessments, the practicality of disease models have been questioned due to overestimation or underestimation of progression of disease outbreak99. In a recent systematic review on the use of prediction models for diagnosis and prognosis of COVID-19, the authors concluded that the prediction models are getting way to the medical literature to support the decision-making process. However, the majority of the models are poorly reported and pose high risk of bias in delivering the information. In addition, the promising models need to be validated appropriately in multiple cohorts through proper data sharing with collaborative efforts to assess their stability and heterogeneity across the various populations settings46.
One of the other limitations of the epidemiological models include multiple interpretation by different stakeholders resulting in the loss of core essence of the conveyed information from the initial model. Sometimes, especially in developing countries, the intervention measures derived from models lacks ground reality in terms of implementation capacity, quarantine limits, availability of logistics, timing, compliance, or extent of completion. Moreover, the lack of required technical skill and the knowledge-practice gap restrict their use by the stakeholders in these regions100. It must be noted that regular examination and frequent model validation is required21, especially in consideration of currently available diagnostics, therapeutics, and other interventions. The generation of participatory approaches in the preparation of policy-oriented models aid in increased incorporation of local knowledge to effectively address the associated environmental and socio-economic implications of infectious diseases101.
Capacity building
The early detection of the pathogen in the 2018 Nipah outbreak in Kerala, India, within a quick time span of 12 hours, due to the presence of trained manpower and the use of advanced genomic tools such as next generation sequencing (NGS), aided in the prompt response and deployment of the multidisciplinary team for the containment of the outbreak102. The detection of such novel pathogens requires well-equipped laboratory networks integrated with robust surveillance system, community participation and knowledge of the socio-economic and environmental factors103.
The standardization of laboratory protocols and their accreditation through periodic assessment assures the accuracy and reliability of the results104. The reference diagnostic frameworks need to be routinely upgraded to equip with latest diagnostic facilities at national, regional, or global levels. The success of the ‘Diagnostic and Laboratory Systems Program (DLSP)’ in Kenya in early detection of infectious disease outbreaks has exemplified the necessity of capacity building of the diagnostic framework and trained manpower105.
The measures for public health preparedness also focus on preparing the public health taskforce with well-defined roles and responsibilities by developing competent training resources, enhancing communications, establishing and sustaining response systems, and providing evaluation parameters for effectiveness and efficiency106. The global public health agencies such as the Centers for Disease Control and Prevention (CDC) have initiated Preparedness and Emergency Response Research Centers (PERRCs) as well as Preparedness and Emergency Response Learning Centers (PERLCs) across the United States to aid in developing such a public health workforce106. The deployment of such trained taskforce in the frontline of emergencies enhances capacity for a timely response to turn aside the global threat. The field epidemiology training programmes exists in many countries to train resource persons and thereby efficiently improving core capacities in the human and animal health sectors107. The tripartite collaboration between FAO, OIE and WHO exhibits a long-lasting strong advocacy for effective multisectoral, multidisciplinary, and transnational collaboration at various levels. The recently published tripartite zoonoses guide provides operational guidance and tool to implement One Health approach at human-animal-environment interface to address emerging zoonoses108.
Conclusion
The advancement in medical education and public health infrastructure coupled with technological development helped in reducing the morbidity and mortality due to infectious diseases in 20th century. These changes were appreciated in terms of increase in life expectancy and shifting of major mortality cause from infectious to chronic degenerative diseases109. However, the recent emergence of public health threats in the globalized world of the 21st century increases the vulnerability of the nations across the world, further demanding the expansion of the present capacity for emergency preparedness and prevention by establishing better early warning systems. A robust surveillance system with the capacity of rapid reporting of newly diagnosed threats; publicising best practices to public health workers, epidemiological disease modelling enabled interventions, simulating transmission dynamics and enhanced forecasting is crucial to mitigate the upcoming emerging public health threats. The multisectoral cooperation and coordination across all the stakeholders under the umbrella of One Health is essential to mount rapid and swift response to the public health challenges.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- One health perspectives on emerging public health threats. J Prev Med Public Health. 2017;50:411.
- [Google Scholar]
- Implementing One Health approaches to confront emerging and re-emerging zoonotic disease threats: lessons from PREDICT. One Health Outlook. 2020;2:1-7.
- [Google Scholar]
- One world, one health: The novel coronavirus COVID-19 epidemic. Med Clin (English ed). 2020;154:175.
- [Google Scholar]
- 2004. Globalization and infectious diseases: a review of the linkages. Available from: https://apps.who.int/iris/handle/10665/68726
- Determinants and drivers of infectious disease threat events in Europe. Emerg Infect Dis. 2016;22:581.
- [Google Scholar]
- 2020. Centers for Disease Control and Prevention. Yellow Book. Chapter 8. Travel by air, land & sea. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-by-airland-sea/air-travel
- Learning from SARS: preparing for the next disease outbreak: workshop summary. Washington, DC: National Academies Press; 2004.
- An outbreak of chikungunya fever in the province of Ravenna, Italy. Euro Surveill. 2007;12:3260.
- [Google Scholar]
- Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361:212-4.
- [Google Scholar]
- Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17:78-85.
- [Google Scholar]
- 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033.
- [Google Scholar]
- Centers for Disease Control and Prevention. 2014-2016 Ebola Outbreak in West Africa. Available from: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html
- Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020;543:34-42.
- [Google Scholar]
- Estimating COVID-19 outbreak risk through air travel. J Travel Med. 2020;20:taaa093.
- [Google Scholar]
- International flight related transmission of pandemic influenza A (H1N1) pdm09: an historical cohort study of the first identified cases in the United Kingdom. Influenza Other Respir Viruses. 2014;8:66-73.
- [Google Scholar]
- 2007. World Health Organization. Everybody's business--strengthening health systems to improve health outcomes: WHO's framework for action. Available from: https://apps.who.int/iris/bitstream/handle/10665/43918/9789241596077_eng.pdf
- Preparedness for Public Health: A Long Story, Short. J Public Health Manag Pract. 2019;25:19-21.
- [Google Scholar]
- Forecasting disease risk for increased epidemic preparedness in public health. Adv Parasitol. 2000;47:309-30.
- [Google Scholar]
- World Health Organization. Early detection, assessment and response to acute public health events: implementation of early warning and response with a focus on eventbased surveillance: interim version (No. WHO/HSE/GCR/ LYO/2014.4). Geneva: WHO; 2014.
- Value-based models for sustaining emergency preparedness capacity and Capability in the United States. Washington, DC: Institute of Medicine; 2014.
- Global Fund contributions to health security in ten countries, 2014–20: mapping synergies between vertical disease programmes and capacities for preventing, detecting, and responding to public health emergencies. Lancet Glob Health. 2021;9:e181-8.
- [Google Scholar]
- Epidemic models of contact tracing: systematic review of transmission studies of severe acute respiratory syndrome and Middle East respiratory syndrome. Comput Struct Biotechnol. 2019;17:186-94.
- [Google Scholar]
- Personal protective equipment supply chain: lessons learned from recent public health emergency responses. Health Secur. 2017;15:244-52.
- [Google Scholar]
- Towards defining core principles of public health emergency preparedness: scoping review and Delphi consultation among European Union country experts. BMC Public Health. 2020;20:1482.
- [Google Scholar]
- Managing public health crises: the role of models in pandemic preparedness. Influenza Other Respir Viruses. 2009;3:75-9.
- [Google Scholar]
- Combining participatory influenza surveillance with modelling and forecasting: Three alternative approaches. JMIR Public Health Surveill. 2017;3:e83.
- [Google Scholar]
- Improving the evidence base for decision making during a pandemic: the example of 2009 influenza A/H1N1. Biosecur Bioterror. 2011;9:89-115.
- [Google Scholar]
- Modelling infectious disease dynamics in the complex landscape of Global Health. Science. 2015;347:aaa4339.
- [Google Scholar]
- Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335-6.
- [Google Scholar]
- Model-based projections of Zika virus infections in childbearing women in the Americas. Nat Microbiol. 2016;1:1-7.
- [Google Scholar]
- The RAPIDD Ebola forecasting challenge: Model description and synthetic data generation. Epidemics. 2018;22:3-12.
- [Google Scholar]
- Technology to advance infectious disease forecasting for outbreak management. Nat Commun. 2019;10:1-4.
- [Google Scholar]
- Building capacity for evidence-based public health: reconciling the pulls of practice and the push of research. Annu Rev Public Health. 2018;39:27-53.
- [Google Scholar]
- Mapping the landscape of artificial intelligence applications against COVID-19. J Artif Intell Res. 2020;69:807-45.
- [Google Scholar]
- A review of modern technologies for tackling COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:569-73.
- [Google Scholar]
- Leveraging data science to combat COVID-19: A comprehensive review. IEEE Trans Artif Intell. 2020;1:85-103.
- [Google Scholar]
- COVID-19 Disease Map, building a computational repository of SARS-CoV-2 virus-host interaction mechanisms. Sci Data. 2020;7:1-4.
- [Google Scholar]
- Automatic detection of coronavirus disease (COVID-19) using x-ray images and deep convolutional neural networks. arXiv 2003 10849. 2020
- [Google Scholar]
- A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19) Eur Radiol 2021 doi: 10.1007/s00330-021-07715-1
- [Google Scholar]
- Abnormal respiratory patterns classifier may contribute to large-scale screening of people infected with COVID-19 in an accurate and unobtrusive manner. arXiv:2002.05534 2020
- [Google Scholar]
- Effect of weather on COVID-19 spread in the US: A prediction model for India in 2020. Sci Total Environ. 2020;728:138860.
- [Google Scholar]
- Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ. 2020;369:m1328.
- [Google Scholar]
- Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:337-9.
- [Google Scholar]
- Using Smartphones and Wearable Devices to Monitor Behavioral Changes During COVID-19. J Med Internet Res. 2020;22:e19992.
- [Google Scholar]
- Wearable sensors for COVID-19: a call to action to harness our digital infrastructure for remote patient monitoring and virtual assessments. Front Digit Health. 2020;2:8.
- [Google Scholar]
- Penang Institute. Smart city technologies take on COVID-19. Available from: https://penanginstitute.org/publications/issues/smart-city-technologies-take-on-covid-19/
- Mitigating loneliness with companion robots in the COVID-19 pandemic and beyond: an integrative framework and research agenda. J Serv Manag. 2020;6:1149-62.
- [Google Scholar]
- The uses of drones in case of massive epidemics contagious diseases relief humanitarian aid: Wuhan-COVID-19 crisis. SSRN 2020 doi:10.2139/ssrn.3546547
- [Google Scholar]
- Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341-2.
- [Google Scholar]
- Artificial intelligence in COVID-19 drug repurposing. Lancet Digit Health. 2020;2:E667-76.
- [Google Scholar]
- Combining immunoprofiling with machine learning to assess the effects of adjuvant formulation on human vaccine-induced immunity. Hum Vaccines Immunother. 2020;16:400-11.
- [Google Scholar]
- COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front Immunol. 2020;11:1581.
- [Google Scholar]
- A materials-science perspective on tackling COVID-19. Nat Rev Mater. 2020;5:847-60.
- [Google Scholar]
- The challenge to know and control: Disease outbreak surveillance and alerts in China and India. Glob Public Health. 2012;7:695-716.
- [Google Scholar]
- Driving improvements in emerging disease surveillance through locally relevant capacity strengthening. Science. 2017;357:146-8.
- [Google Scholar]
- Rinderpest eradication: appropriate technology and social innovations. Science. 2012;337:1309-12.
- [Google Scholar]
- ESCMID Study Group of Epidemiological Markers (ESGEM). Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Euro Surveill. 2013;18:20380.
- [Google Scholar]
- Integrating advanced molecular technologies into public health. J Clin Microbiol. 2017;55:703-14.
- [Google Scholar]
- Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health. 2017;5:e1038-46.
- [Google Scholar]
- Molecular tracing to find source of protracted invasive listeriosis outbreak, Southern Germany, 2012–2016. Emerg Infect Dis. 2017;23:1680.
- [Google Scholar]
- Entry screening to delay local transmission of 2009 pandemic influenza A (H1N1) BMC Infect Dis. 2010;10:1-4.
- [Google Scholar]
- The duration and magnitude of influenza epidemics: a study of surveillance data from sentinel general practices in England, Wales and the Netherlands. Eur J Epidemiol. 1999;15:467-73.
- [Google Scholar]
- Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol. 2006;72:7894-6.
- [Google Scholar]
- Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80:6771-81.
- [Google Scholar]
- Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011;45:1287-97.
- [Google Scholar]
- Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J Infect Dis. 2014;210:S294-303.
- [Google Scholar]
- First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764.
- [Google Scholar]
- Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci Total Environ. 2020;730:138875.
- [Google Scholar]
- West Nile virus surveillance in Connecticut in 2000: an intense epizootic without high risk for severe human disease. Emerg Infect Dis. 2001;7:636-42.
- [Google Scholar]
- Prioritizing surveillance of Nipah virus in India. PLOS Negl Trop Dis. 2019;13:e0007393.
- [Google Scholar]
- Nipah virus dynamics in bats and implications for spillover to humans. Proc Natl Acad Sci USA. 2020;117:29190-201.
- [Google Scholar]
- Global Early Warning System. Available from: http://www.glews.net/
- World Organisation for Animal Health: The new OIEWorld Animal Health Information System (OIE-WAHIS). Available from: https://www.oie.int/animal-health-inthe-world/the-world-animal-health-information-system/the-world-animal-health-information-system/
- Global infectious disease surveillance and detection: assessing the challenges-finding solutions. Workshop summary. In: Global infectious disease surveillance and detection: assessing the challenges. Workshop summary. Washington DC: The National Academies Press; 2007.
- [Google Scholar]
- Handling big data: research challenges and future directions. J Supercomput. 2016;72:1494-516.
- [Google Scholar]
- Model-driven decision support systems: Concepts and research directions. Decis Support Syst. 2007;43:1044-61.
- [Google Scholar]
- Mathematical models to characterize early epidemic growth: A review. Phys Life Rev. 2016;18:66-97.
- [Google Scholar]
- Common swine models of cardiovascular disease for research and training. Lab Anim (NY). 2016;45:67-74.
- [Google Scholar]
- Using “outbreak science” to strengthen the use of models during epidemics. Nat Commun. 2019;10:1-3.
- [Google Scholar]
- Investigating the dynamics of COVID-19 pandemic in India under lockdown. Chaos Soliton Fractals. 2020;138:109988.
- [Google Scholar]
- The reproduction number of COVID-19 and its correlation with public health interventions. Comput Mech. 2020;66:1035-50.
- [Google Scholar]
- Analysis of the mitigation strategies for COVID-19: from mathematical modelling perspective. Chaos Soliton Fractals. 2020;138:109968.
- [Google Scholar]
- A global survey of potential acceptance of a COVID-19 vaccine. Nat med. 2020;27:225-28.
- [Google Scholar]
- Modelling and simulation of biological systems with stochasticity. In Silico Biol. 2004;4:293-309.
- [Google Scholar]
- Estimating the future number of cases in the Ebola epidemic--Liberia and Sierra Leone, 2014–2015. MMWR Suppl. 2014;63:1-14.
- [Google Scholar]
- Phase-adjusted estimation of the number of coronavirus disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:1-8.
- [Google Scholar]
- Anticipating the international spread of Zika virus from Brazil. The Lancet. 2016;387:335-6.
- [Google Scholar]
- The predictive capacity of air travel patterns during the global spread of the COVID-19 pandemic: risk, uncertainty and randomness. Int J Environ Res Public Health. 2020;17:3356.
- [Google Scholar]
- A contribution to the mathematical theory of epidemics. Proc R Soc Lond A. 1927;115:700-21.
- [Google Scholar]
- Modelling the influence of human behaviour on the spread of infectious diseases: a review. J R Soc Interface. 2010;7:1247-56.
- [Google Scholar]
- Modelling the global spread of diseases: A review of current practice and capability. Epidemics. 2018;25:1-8.
- [Google Scholar]
- Perspectives on model forecasts of the 2014–2015 Ebola epidemic in West Africa: lessons and the way forward. BMC Med. 2017;15:42.
- [Google Scholar]
- Bridging the gap between evidence and policy for infectious diseases: How models can aid public health decision-making. Int J Infect Dis. 2016;42:17-23.
- [Google Scholar]
- Forecasting and control of emerging infectious forest disease through participatory modelling. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180283.
- [Google Scholar]
- Towards global health security: Response to the May 2018 Nipah virus outbreak linked to Pteropus bats in Kerala, India. BMJ Glob Health. 2018;3:e001086.
- [Google Scholar]
- Detection of emerging zoonotic pathogens: an integrated one health approach. Annu Rev Anim Biosci. 2018;6:121-39.
- [Google Scholar]
- The neglected dimension of global security—a framework for countering infectious-disease crises. N Engl J Med. 2016;374:1281-7.
- [Google Scholar]
- Building laboratory capacity to detect and characterize pathogens of public and global health security concern in Kenya. BMC Public Health. 2019;19(Suppl 3):477.
- [Google Scholar]
- Preparedness and emergency response learning centers: supporting the workforce for national health security. J Public Health Manag Pract. 2014;20:S7-16.
- [Google Scholar]
- An evaluation of the global network of field epidemiology and laboratory training programmes: a resource for improving public health capacity and increasing the number of public health professionals worldwide. Hum Resour Health. 2013;11:1-7.
- [Google Scholar]
- Food and Agriculture Organization of the United Nations, World Organisation for Animal Health, World Health Organization. Taking a multisectoral, One Health approach: A tripartite guide to addressing zoonotic diseases in countries. https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/EN_TripartiteZoonosesGuide_webversion.pdf






