Translate this page into:
Eliminating HIV & AIDS in India: A roadmap to zero new HIV infections, zero discrimination & zero AIDS-related deaths
*For correspondence: cpalmer@burnet.edu.au
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
The HIV and AIDS epidemic in India: Getting to zero
In 2011, the World Health Organization Member States adopted a new global health sector strategy to implement initiatives to reduce HIV prevalence and AIDS-related deaths1. The United Nations Programme on HIV and AIDS (UNAIDS) also targets to end AIDS as a public health threat by 20302. At the end of 2013, India had the third largest number of people living with HIV (PLHIV) in the world and accounted for about four out of ten PLHIV in Asia. There are 2.1 million PLHIV in India, of whom 790,000 are women3. Key populations at very high risk of HIV transmission are men who have sex with men (MSM) and people who inject drugs. These groups are nearly 20-30 folds higher at risk than in the general population (0.3%), closely followed by female sex workers and migrants3.
HIV and AIDS response in India
India has made a great progress in controlling HIV since the beginning of the epidemic. The National AIDS Control Organization (NACO) realized early on that the western model of specialist physician management and advanced laboratory monitoring was not feasible in India. From 2004 onwards, the NACO set up antiretroviral treatment (ART) centres, which provided one of the world's largest free ART, and HIV testing and counselling sites all across the country. The current programme, National AIDS Control Programme IV (NACP-IV) (2012-2017) is aimed at diagnosing and reducing annual new HIV cases by 50 per cent through comprehensive HIV treatment, education, care and support for the general population and to build on targeted interventions for the key affected groups and those at a high risk of HIV transmission4.
The NACO estimated that around 1,300,000 PLHIV needed ART in 20155. Despite this progress, only 43 per cent of adults living with HIV are on ART and only 74 per cent of all PLHIV in India are thought to be aware of their HIV status67. Though this is well short of the global ‘90-90-90’ target by the year 2020 (which is, 90% of PLHIV know their status; 90% of diagnosed individuals receiving treatment and 90% treated individuals have an undetectable viral load), but India has successfully achieved the 6th Millennium Development Goal of halting and reversing the HIV epidemic5. Diagnosis of annual new HIV cases in India have declined by more than 50 per cent during the past decade. The six highest prevalence States (Karnataka, Maharashtra, Manipur, Odisha, Andhra Pradesh and Telangana) all have shown a declining trend, in terms of HIV prevalence3.
Challenges in meeting the demands of a diverse culture
India is a large, socially diverse and complex country, which is a challenge when trying to implement any national medical programme. To widen their reach towards people from different socio-economic backgrounds, targeted interventions supported by the NACO (such as the Project Pehchan, Avahan, Sonagachi Project, Project Kavach, as well as HIV education and awareness through Link Worker Scheme, Red Ribbon Express and The Condom Social Marketing Programme) play innovative roles in financing and providing healthcare services, particularly for the key affected groups and those at high risk of HIV transmission. Community and peer-based approaches to sharing prevention tools and increasing awareness about HIV and AIDS have proven to be effective8.
What is now concerning is that the low-prevalence States of Uttarakhand, Assam, Meghalaya, Haryana and Uttar Pradesh show rising trends in the past four years3. Tripura and Sikkim have recorded a relatively steep climb in HIV prevalence3. Given the regional differences in the rates of prevalence of HIV in India, the challenges and solutions for ‘Getting to Zero’ will vary considerably from State to State.
HIV pre-exposure prophylaxis in India
At the end of 2015, a ‘Pre-Exposure Prophylaxis’ (PrEP) demonstration project was rolled out in Asia's largest red light zone, Sonagachi in Kolkata910. The daily use of tenofovir/emtricitabine (Truvada) combination as oral PrEP has been found to be effective in several clinical trials11. PrEP is yet to be introduced across India, and there is no national PrEP policy or guidance at present. However, this ongoing demonstration project can be used to effectively inform the implementation of PrEP all over India.
The drugmaker, Cipla received clearance to use generic Truvada for PrEP in early 201612. The next important step will depend on the NACO and inclusion of convenient formulations, such as long-acting injectable antiretrovirals, cabotegravir and rilpivirine, or slow-release dapivirine intravaginal rings, gels as well as more ambitious developments such as subdermal implants and patches in the national programme13.
HIV and lesbian gay bisexual transgender criminalization in India
After more than a quarter century of the HIV epidemic, it is the considerable burden of stigma that comes with HIV which has shown to create fear about HIV testing and disclosure and drive PLHIV underground with no access to support, treatment or care14. The revival of the 2014 HIV and AIDS Prevention and Control Bill and the Union Cabinet's approval for provisions that makes discrimination against people living with the virus punishable are positive steps towards the ‘Getting to zero’ efforts.
The magnitude and nature of the HIV and AIDS epidemic require an environment free of stigma and discrimination to reach the zero goals. Some countries, in particular Australia, are effectively working towards achieving this goal. Australia's strength lies in its public education and management of HIV and AIDS as a public health issue and the understanding and response by all levels of society to the epidemic15.
In February 2016, Indian Supreme Court agreed to examine the constitutional validity of Section 377 of the Indian Penal Code that legitimises criminalization of homosexuality16. India's lesbian, gay, bisexual, and transgender community is beginning to gain more recognition and acceptance in the mainstream society. The current need is for interventions that support openness and disclosure and that help protect those with HIV from discrimination and stigma.
Taking advantage of India's advances in technology and drug development to fight HIV & AIDS
India has emerged as a world leader in the production of generic pharmaceuticals17. Indian generic manufacturers dominate the antiretroviral (ARV) market and have played an exceptional role in providing quality-assured ARVs at low prices to people with HIV and AIDS in developing countries16. However, India continues to battle with antiretroviral drug shortages, one that could derail the impact of the various free interventions and scaled-up prevention strategies being undertaken globally, including the NACP in India.
In the HIV landscape, prevention trials on microbicides and vaccines are underway globally. Three phase I HIV vaccine trials have been completed so far by the National AIDS Research Institute in Pune and the National Institute for Research on Tuberculosis in Chennai18192021. Conducting HIV vaccine trials, especially in countries such as India, requires cooperation and coordination from different segments of society to build the capacity and to conduct clinical trials conforming to ethical framework on par with international standards.
India's multidisciplinary approach to fight HIV & AIDS
A standardized system with high emphasis on counselling and a multidisciplinary approach present within the public HIV healthcare system will have a positive impact on adherence levels and virological suppression among patients2223. The general consensus for an effective global approach towards diminishing the burden of HIV worldwide is to ‘test and treat’. In India, almost one quarter of PLHIV are unaware of their HIV status7. HIV-positive individuals continue to be detected late in the course of disease progression, with 85 per cent registering for ART when their CD4 count is already <250 cells/μl, making them vulnerable to AIDS24. Universal testing of the general population every five years, and annual screening among high-risk groups and in high-prevalence districts combined with the expansion of ART services, and earlier ART initiation, will improve outcomes in those with HIV, decrease HIV transmission and improve cost-effectiveness in India. Novel anti-HIV treatment modalities such as potent broadly neutralizing antibodies2526 and advances in understanding new ways to strengthen the immune system by modifying how immune cells use energy27 will be the key to mitigate for scenarios in which viral resistance threatens virologic responses to the current ART regimen.
In India, effective technical support and enhanced monitoring with the involvement of local communities, government and health and research organizations is needed to achieve the reductions required to end the HIV and AIDS epidemics as a public health threat by 2030. At the moment, Australia is one of the few countries that will surpass the ambitious UNAIDS target of ‘90-90-90’ by 2020. Effective Australian Health Promotion Policy that was able to contain the epidemic in Australia included the following efforts28: (i) Health promotion programmes involving the affected communities in discussion and debate about the range and nature of measures it could take to reduce the impact of the epidemic; (ii) Engagement with HIV-positive people for all phases of programme design, from initial concept through the development of content and delivery; and (iii) Campaigns targeting high-risk behaviours rather than high-risk groups.
Over the past decade, India has made a significant progress in tackling its HIV epidemic. For ongoing improvement in HIV response, India needs an effective prevention programme (PrEP), protection against discrimination, reduced stigma, strong leadership and advocates, greater access to routine HIV screening and, most importantly, treatment and optimum patient care (Figure).
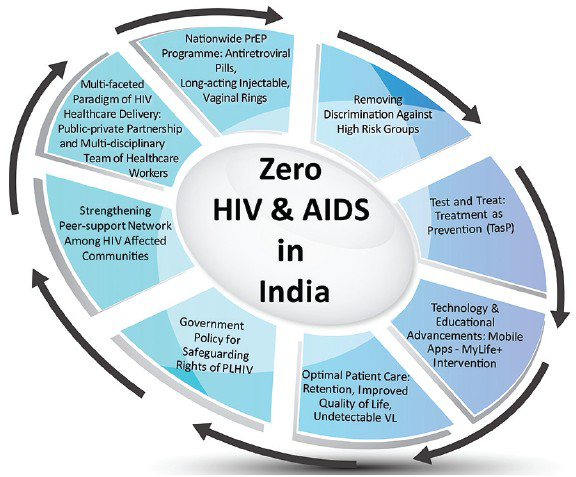
- Getting to zero: A multifaceted approach consisting of optimal healthcare delivery, strengthening peer support, technological advances and biomedical interventions. PLHIV, people living with HIV; PrEP, pre-exposure prophylaxis; VL, viral load.
Acknowledgment
Authors acknowledge Shri A. Mannan for assistance with graphics.
References
- UNAIDS Report on the Global AIDS Epidemic: 2012. Global Report. Available from: http://www.unaids.org/sites/default/files/media_asset/20121120_UNAIDS_Global_Report_2012_with_annexes_en_1.pdf
- UNAIDS: 2016-2021 Strategy. On the Fast-Track to end AIDS. Available from: http://www.unaids.org/sites/default/files/media_asset/20151027_UNAIDS_PCB37_15_18_EN_rev1.pdf
- National AIDS Control Organisation (NACO). Department of AIDS Control, Ministry of Health & Family Welfare, Government of India. Annual Report 2013-14. Available from: http://164.100.12.21/upload/2014%20mslns/NACO_English%202013-14.pdf
- Department of AIDS Control, Ministry of Health & Family Welfare, Government of India. NACO AIDS Control Programme Phase-IV (2012-2017): Strategy Document. Available from: http://naco.gov.in/sites/default/files/Strategy_Document_NACP%20IV.pdf
- National AIDS Control Organisation (NACO), National Institute of Medical Statistics (NIMS), Government of India. India HIV Estimations 2015. Available from: http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf
- UNAIDS 2016. Prevention Gap Report. Available from: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf
- National AIDS Control Organisation (NACO). Integrated Counselling and Testing Centre. Available from: http://naco.gov.in/integrated-counselling-and-testing-centre
- Impact of community-based interventions on HIV knowledge, attitudes, and transmission. Infect Dis Poverty. 2014;3:26.
- [Google Scholar]
- The Sonagachi Project: A sustainable community intervention program. AIDS Education and Prevention. 2004;16:405-14.
- [Google Scholar]
- 2015. The Economic Times. Anti-AIDS medicine project likely to take off at Sonagachi. Available from: http://economictimes.indiatimes.com/news/politics-and-nation/anti-aids-medicine-project-likely-to-take-off-at-sonagachi/articleshow/45715559.cms
- 2016. The Hindu Business Line. Cipla gets approval to sell its version of Truvada in India. Available from: http://www.thehindubusinessline.com/companies/cipla-gets-approvalto-sell-its-version-of-truvada-in-india/article8637483.ece
- PREP is a key HIV prevention strategy, but how do we chart its success? 2015. Australian Federation of AIDS Organisations (AFAO). 13 Available from: https://www.afao.org.au/library/hiv-australia/volume-13/vol-13-number-2-horizons/how-to-chart-success-of-prep#.WOci7UWGPct
- [Google Scholar]
- Perception of patients with HIV/AIDS from stigma and discrimination. Iran Red Crescent Med J. 2015;17:e23638.
- [Google Scholar]
- 2014. Australian Government: Department of Health. Seventh National HIV Strategy 2014-2017. Available from: https://consultations.health.gov.au/ohpd-health-protection-policy-branch/test-national-strategies-for-blood-borne-viruses-a/supporting _documents/Draft%207th%20National%20HIV%20Strategy%2020142017%20D14818811.PDF
- 2016. Indiatimes. Everything you need to know about section 377 of Indian Penal Code and the story so far. Available from: http://www.indiatimes.com/news/india/lgbtq-everything-yo1uneed-to-know-about-section-377-of-the-indian-constitution-andthe-story-so-far-256826.html
- A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc. 2010;13:35.
- [Google Scholar]
- Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised Trial in HIV-uninfected Indian volunteers. PLoS One. 2013;8:e55831.
- [Google Scholar]
- A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses. 2009;25:1107-16.
- [Google Scholar]
- Safety & immunogenicity of tgAAC09, a recombinant adeno-associated virus type 2 HIV-1 subtype C vaccine in India. Indian J Med Res. 2010;132:168-75.
- [Google Scholar]
- A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses. 2008;24:873-80.
- [Google Scholar]
- Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43:S23-35.
- [Google Scholar]
- Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442.
- [Google Scholar]
- Are persons living with HIV timely accessing ART services in India? J Indian Med Assoc. 2009;107:288-90.
- [Google Scholar]
- A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105-9.
- [Google Scholar]
- HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556-60.
- [Google Scholar]
- Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis. EBioMedicine. 2016;6:31-41.
- [Google Scholar]
- Australian Federation of AIDS Organisation. Available from: https://www.afao.org.au/what-we-do/health-promotion





