Translate this page into:
Efficacy of moxifloxacin & econazole against multidrug resistant (MDR) Mycobacterium tuberculosis in murine model
Reprint requests: Dr U.D. Gupta, National JALMA Institute for Leprosy & Other Mycobacterial Diseases (ICMR), Tajganj, Agra 282 004, Uttar Pradesh, India e-mail: gupta.umesh95@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Studies have shown the bactericidal potential of econazole and clotrimazole against Mycobacterium tuberculosis under in vitro and ex vivo conditions along with their synergism with conventional antituberculosis drugs. These molecules were also found to be effective against different multidrug resistant (MDR) M. tuberculosis isolates in vitro. Hence the present study was designed to evaluate the in vivo antimycobacterial potential of moxifloxacin and econazole alone and in combination against multidrug resistant tuberculosis (MDR-TB) in a mice model.
Methods:
Mice were infected with 2.5×107 bacilli of MDR strain of M. tuberculosis by aerosol route of infection. After four weeks of infection, chemotherapy was started orally by moxifloxacin 8.0 mg/kg body wt and econazole 3.3 mg/kg alone and in combination, as well as with four first line anti-tuberculosis drugs as a positive control. The animals were sacrificed and the lungs and spleen were excised under aspetic conditions. The tissues were homogenized with sterile normal saline, an aliquot of the homogenate was plated on Middlebrook 7H11 agar supplemented with oleate albumin dextrose catalase (OADC) and incubated at 37°C for four weeks. The number of visible and individual colonies were counted.
Results:
The first line anti-tuberculosis drugs (RIF+INH+EMB+PZA) after eight weeks of therapy had no impact as the bacillary load in lungs and spleens remained unchanged. However, econazole, moxifloxacin alone as well as in combination significantly reduced the bacillary load in lungs as well as in spleens of MDR-TB bacilli infected mice.
Interpretation & conclusions:
Co-administration of the two drugs (econazole and moxifloxacin) to MDR-TB strain JAL-7782 infected mice exhibited additive effect, the efficacy of the drugs in combination being higher as compared with ECZ or MOX alone. These results were substantiated by histopathological studies. This study suggests the utility of econazole for the treatment of MDR tuberculosis and warrants further work in this direction.
Keywords
Anti-tuberculosis drugs
azoles
fluoroquinolones
multidrug resistance
tuberculosis
Fluoroquinolones (FQs) have been found to exhibit potent anti-mycobacterial activity under in vitro123 and in vivo conditions456 and hence recommended by the WHO as the second and third-line chemotherapy for multidrug resistant tuberculosis (MDR-TB)7. Inclusion of fluoroquinolone drugs as first-line chemotherapy is significant since these do not exhibit any cross-reactivity with the conventional anti-tuberculosis drugs (ATDs)8. Substitution of frontline ATDs with moxifloxacin (MOX) has shown a reduction in the treatment duration against murine TB9. Moxifloxacin is a synthetic broad spectrum 8-methoxy fluoroquinolone antibacterial agent. The bactericidal action of moxifloxacin results from inhibition of topoisomerase II (DNA gyrase) and topoisomerase IV required for DNA replication, transcription, repair and recombination. Moxifloxacin has also been shown to be as effective as ethambutol and isoniazid in the treatment of pulmonary TB10.
Inhibition of biosynthesis of glycopeptidolipids, which maintain the integrity of the mycobacterial cell envelope, has been the mainstay of drug development against mycobacteria. Apart from the known ATDs possessing such action, azoles have been shown to inhibit the growth of Mycobacterium smegmatis and M. tuberculosis H37 Ra under in vitro conditions11. Econazole (ECZ) was found to bind to various cytochrome P450 (CYP450) enzymes in M. tuberculosis, inhibit endogenous respiration, interact with membrane phospholipids, inhibit purine uptake and decrease phospholipid biosynthesis. Two clinically approved antifungal azole drugs, clotrimazole (CTZ) and econazole inhibited the growth of M. tuberculosis H37 Rv under in vitro and ex vivo conditions12. The low minimum inhibitory concentration (MIC90 0.120 µg/ml) and low minimum bactericidal concentration (MBC 0.125 µg/ml) values in comparison to rifampicin (RIF, MIC 0.2l µg/ml) demonstrated their anti-mycobacterial activity. Additionally, the azole drugs exhibited a synergistic activity with rifampicin and isoniazid (INH) on the basis of reduction in colony forming units (cfu)12. The two azole drugs also showed strong antimycobacterial activity against latent/persistent M. tuberculosis under in vitro conditions13, against murine tuberculosis14, as well as against MDR-TB strains15, with possible indications of replacement of RIF and INH with ECZ. Chemotherapy of murine TB with ECZ, MOX and RIF resulted in total bacterial clearance from lungs and spleen within eight weeks compared to the conventional 4-ATDs treatment16 and hence can be considered among the most potent regimens. This study was undertaken to evaluate the chemotherapeutic potential of MOX and ECZ alone and in combination against MDR-TB strains in a murine model.
Material & Methods
Moxifloxacin and econazole were acquired as gift samples from Jai Radhey Sales, Gujarat, and Gufic Biosciences Ltd, Gujarat, India, respectively. The cultures of M. tuberculosis H37Rv (susceptible strain) and MDR-TB strain JAL 7782 were originally obtained from Mycobacterial Repository of National JALMA Institute for Leprosy & Other Mycobacterial Diseases (NJIL & OMD), Agra and were maintained on Lowenstein-Jensen (L-J) medium.
A total of 62 BALB/c mice (28 mice for M. tuberculosis H37Rv strain and 34 mice for JAL 7782) of either sex weighing 18-20 g and 7-8 wk old were procured from the animal house, National Institute of Nutrition, Hyderabad, India. The mice were housed in Biosafety Level-3 (BSL-3) laboratory for Animal Experiments and provided with pellet diet and water ad libitum. The study was approved by the NJIL & OMD's Animal Ethics Committee as well as the Institute's Biosafety Committee (IBSC).
Experimental infection in mice: The mice were infected with 2.5x107 bacilli of M. tuberculosis H37Rv (susceptible strain) or MDR-TB strain JAL 7782 by aerosol route using Aerosol Inhalation Chamber, Glascol, USA17. After one day and four weeks of infection, four animals were sacrificed and their, lungs and spleen were excised under aseptic conditions17. The tissues were homogenized with sterile normal saline. An aliquot of the homogenate was plated on Middlebrook 7H11 agar supplemented with oleate albumin dextrose catalase (OADC) (Backton & Dickinson, USA) and incubated at 37°C for four weeks. The number of visible and individual colonies were counted.
Chemotherapeutic studies: The mice were segregated into the following groups: With M. tuberculosis H37Rv (susceptible strain): 28 mice (4 mice to determine initial bacterial load; 24 mice were divided into 4 groups of 6 mice each); group 1, untreated; group 2, ECZ [3.3 mg/kg body weight, twice daily (112 doses)]; group 3, MOX (8 mg/kg body weight), once daily (56 doses); and group 4, ECZ+MOX [3.3 mg/kg twice daily (112 doses) + 8 mg/kg once daily (56 doses)].
With MDR –TB strain JAL-7782: 64 mice (4 mice to determine initial bacterial load; 60 mice were divided in 10 groups of 6 mice each ---5 groups for 4 wk chemotherapy and 5 groups for 8 wk chemotherapy): group 1: untreated; group 2: RIF treated [10 mg /kg body weight), once daily (56 doses)]; INH treated [5mg/kg body weight, once daily (56 doses)]; PZA (pyrazinamide) treated [25 mg/kg body weight, once daily (56 doses)]; EMB (ethambutol) treated [15mg/kg body weight, once daily (56 doses)]; group 3: ECZ treated [3.3 mg/kg body weight, twice daily (112 doses)]; group 4: MOX treated [8mg/kg body weight, once daily (56 doses)]; and group 5: ECZ and MOX treated [3.3 mg/kg ECZ twice daily and 8.0 mg/kg MOX – once daily (56 doses)].
The drug doses used throughout the study were as described in our publication16.
Administration of drugs: The drug stocks (ECZ and MOX and first-line ATDs) were prepared fresh daily; ECZ was dissolved in 1:4 ratios of methanol and distilled water (1 mg of ECZ was dissolved in 150 μl of methanol and 600 μl of distilled water - stock for 15 mice) and, RIF was dissolved in 1×PBS, whereas other drugs (MOX, INH, PZA and EMB); and were dissolved in sterile distilled water as mg/kg body weight. A dose of 50 µl of each drug was given orally to each mouse in the corresponding groups. After completion of the dosing period (8 wk for M. tuberculosis H37 Rv as well as for JAL 7782 four mice from each group were sacrificed and lungs and spleen homogenates were enumerated for basal colony forming units (cfu).
Histopathology: Two mice from each group were sacrificed, the lungs excised and subjected to routine histological processing. The sections were stained with haematoxylin and eosin followed by microscopic examination. The severity of infection was graded on a scale of 0 to IV in ascending order.
Statistical analysis: Student's t test was applied to assess the differences in cfu among the different groups.
Results
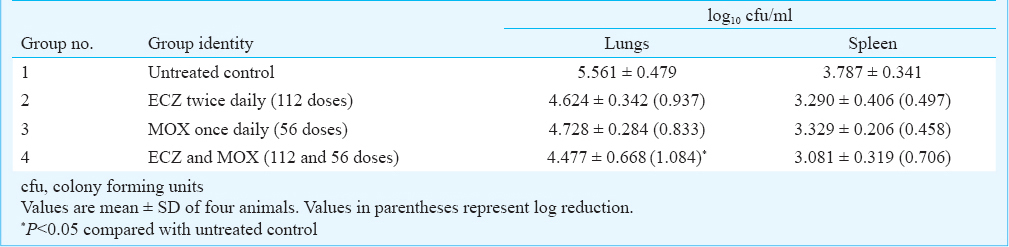
Basal colony forming units (cfu) in lungs and spleen: On aerosol infection with 2.5x107 bacilli of M.tuberculosis H37Rv (susceptible strain of TB), the lungs showed the presence of 5.561 ± 0.479 log10 cfu while the spleen showed the presence of 3.79 ± 0.341 log10 cfu. On aerosol infection with 2.5x107 bacilli of MDR-TB strain JAL-7782, the lungs showed the presence of log10 7.088 cfu and the spleen showed log10 4.18 cfu. The cfu in the lungs and spleen in the two groups of infected animals served as the baseline infection load, prior to oral drug administration.
Chemotherapeutic efficacy of ECZ and MOX in M. tuberculosis H37Rv infected mice: The drugs ECZ (112 doses) and MOX (56 doses) were administered individually to mice infected with M. tuberculosis H37Rv (Table I). Administration of ECZ and MOX in two independent groups showed a reduction in cfu (from baseline infection load) in lungs and spleen. The reduction in cfu with both drugs was comparable as well as significant compared to untreated control. However, co-administration of ECZ (112 doses)+ MOX (56 doses) showed a significant (P<0.05) reduction in cfu (1.1 log10 cfu and 0.71 log10 reduction) in lungs and spleen, respectively while the effect observed with the individual drugs were comparatively lower [significantly only for lungs (P<0.05)].
Chemotherapeutic efficacy of ECZ and MOX in MDR-TB strain JAL-7782 infected mice on oral anti-tuberculosis drugs: Mice were infected by aerosol route with MDR-TB strain JAL 7782 and treated orally for four and eight weeks with first line ATDs, MOX and ECZ. Chemotherapy beyond four weeks was based on the presumption that 4-wk chemotherapy would not be sufficient for reducing the bacterial load of MDR strains. Hence, additional groups of mice infected with MDR-TB strain JAL-7782 were treated with the drugs for eight weeks.
Chemotherapy with first-line ATDs for four weeks showed no reduction in cfu in lungs and spleens of mice (Table II). However, further chemotherapy up to eight weeks, showed only a slight reduction in cfu in both lungs and spleen of infected mice. Chemotherapy with ECZ and MOX for 4-wk showed 1.38 and 1.34 log10 reduction, respectively in cfu in lungs. The reduction in cfu in the spleen was negligible with both MOX and ECZ individually. However, administration of a combination of MOX with ECZ for four weeks showed significant 1.73 log10 reduction in cfu in lungs (P<0.01) and 0.62 log10 in spleen (P<0.05).
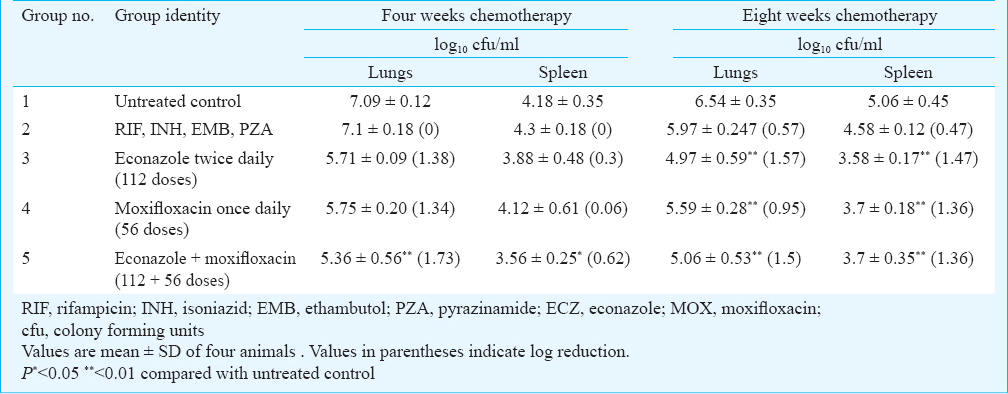
The mice were treated for 8-wk with ECZ and MOX individually as well as in combination, ECZ showed a significant (P<0.01) reduction of 1.57 log10 and 1.47 log10 reduction in lungs and spleen, respectively compared with untreated control, while with MOX, there was a 0.95 log10 and 1.36 log10 reduction in cfu of lungs and spleen, respectively. The combination of ECZ and MOX for 8-wk reduced the bacterial load in lungs and spleen by 1.5 log10 and 1.36log10, respectively which were significantly different from untreated control (P<0.01).
Histopathological observations of infected lungs: Histopatholological observations of lungs from mice infected with MDR-TB strain JAL-7782 and untreated or treated with ECZ and MOX were graded on a scale of 0 to IV, on the basis of negligible to severe consolidation, respectively. The lungs from an infected but untreated mouse showed a complete lobar pneumonia consolidation by tuberculous granulation tissue (grade IV), whereas the lungs from MOX treated mice showed a fairly large area of consolidation by fat tuberculous pneumonia, both in the central and peripheral zone (grade II). The lungs of mice treated with ECZ were nearly normal with a tiny focus of lymphocytes (grade 0). In contrast, lungs of ECZ and MOX treated mice showed focal areas of consolidation due to tuberculous inflammation (grade III) (Figure).
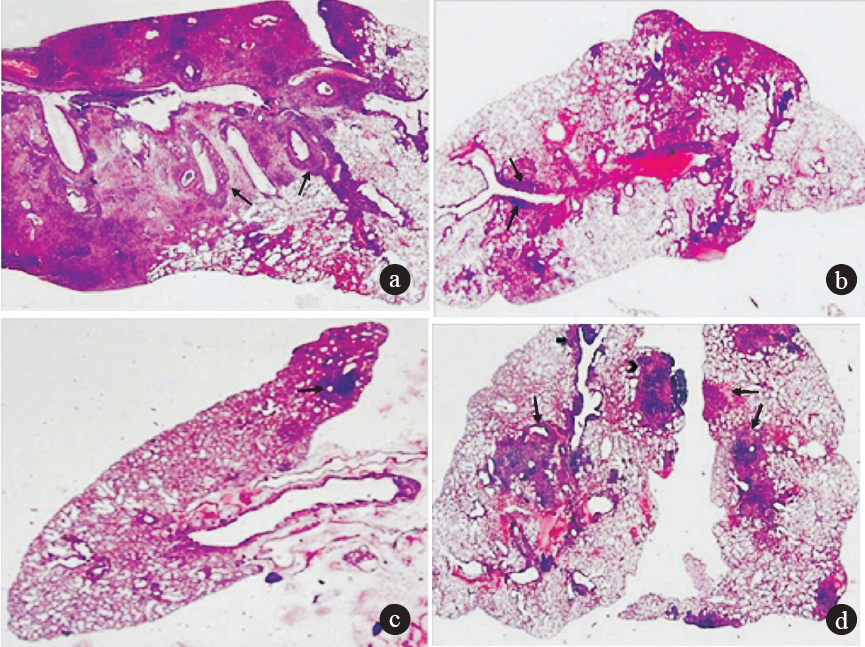
- Light photomicrographs of lung sections from mice infected with MDR-TB strain JAL-7782 with haematoxylin-eosin stain. (a) Positive control: An almost complete lobar pneumonia consolidation by tuberculous granulation tissue including pale staining histiocytes and dark clusters of lymphocytes and peribronchial lymphocytic cuffing (arrow) (Grade 0; maximum consolidation) (b) MOX treated: Lung showing fairly large area of consolidation by fat tuberculous pneumonia, both in the central and peripheral zone. Small focus of lymphocytic cuffing around bronchi (arrows). (Grade II moderate consolidation) (c) ECZ treated: Nearly normal lung with a tiny focus of lymphocytes and pale histiocytes (arrow). The central zone shows collapse and some interstitial inflammation. Grade 0: minimum consolidation. (d) Both lungs show focal areas of consolidation by tuberculous inflammation (arrows). There is a large peripheral sub pleural focus, an irregular zone at the hilum and a peribronchial cuffing by lymphoid cells (arrow). (grade III; focal areas of consolidation) (magnification 20x).
Discussion
The chemotherapeutic regimen for tuberculosis patients involves use of first-line drugs (rifampicin, isoniazid, pyrazinamide and ethambutol) for a period of 6-8 months. Administration of improper treatment in drug-susceptible TB patients as well as patient non-compliance, results in the emergence of drug-resistant and multidrug-resistant TB strains which have posed new challenges in controlling the disease. According to the World Health Organization, drug-resistant M. tuberculosis isolates are found in at least 72 countries at a rate ranging from 3 to 41 per cent7. The current regimens for MDR-TB are complicated due to long duration, high toxicity, poor tolerance and significant cost, resulting in poor outcomes1819. Hence, there is a pressing need to investigate new drugs with potent antimycobacterial activity.
Earlier investigations revealed the antimycobacterial potential of econazole against MDR-TB strains with an MIC90 of 0.120-0.125 µg/ml and MBC>99.99 of 0.120-0.15 µg/ml which were similar to those reported earlier against M. tuberculosis H37 Rv and other virulent strains121314. The presence of multiple targets for econazole in M. tuberculosis was validated by in vitro binding studies of ECZ to various CYP450 enzymes20. The efficacy of MOX and ECZ against TB was initially tested in mice infected with susceptible strains of M. tuberculosis H37Rv. Administration of ECZ and MOX individually showed a comparable reduction in cfu in lungs and spleen. The co-administration of ECZ with MOX showed a significant reduction in cfu in lungs as compared with individual drugs, demonstrating the efficacy of drugs in combination. The additive effect of ECZ and MOX can be explained on the basis that the two drugs have different targets; econazole is known to inhibit the biosynthesis of glycopeptidolipids which are responsible for maintaining the integrity of the mycobacterial cell envelope21. Treatment with ECZ might, therefore, inhibit efficient cell wall formation thereby augmenting the penetration of other drugs such as MOX.
Chemotherapy with first line ATDs for four and eight weeks in mice infected with MDR-TB strain JAL-7782 showed no reduction in cfu in lungs and spleens, indicating the inefficacy of first-line ATDs as chemotherapeutic agents for treating MDR-TB infections in a murine model. In contrast, MDR-TB infected mice treated for 4-wk with ECZ and MOX individually, showed about 1.3 log10 cfu reduction in lungs while no effect was seen in the spleen. Since the mice were infected through the aerosol route and treatment with the drugs orally was for a short period (4 wk), short-course chemotherapy was considered insufficient for the bacterial clearance of MDR-TB strains, especially from the spleen. The synergistic effect of a combination of ECZ and MOX supports their role for pulmonary lung MDR-TB therapy.
The tuberculous granuloma in lungs of humans and mice have a large complement of T lymphocytes, some B lymphocytes, dendritic cells, neutrophils, fibroblasts and extracellular components resulting in consolidation in variable areas of lungs22. The lungs from mice infected with JAL-7782 showed a typical consolidated lung with degenerative changes due to the declining conditions of animals suffering from systemic TB. There was complete lobar pneumonia consolidation by tuberculous granulation tissue comprising histiocytes and clusters of lymphocytes. The lungs from MDR-TB infected mice treated with MOX showed a fairly large area of consolidation by fat tuberculous pneumonia, both in the central and peripheral zone of lungs. The lungs from ECZ-treated mice were nearly normal with a minuscule focus of lymphocytes and histiocytes. In contrast, with co-administration of ECZ and MOX, the lungs showed focal areas of consolidation caused by tuberculous inflammation.
It seems that in comparison to MOX, ECZ has a greater potential for clearance of MDR-TB from the lungs. It has a characteristic feature of binding to multiple targets on the Mycobacterium and ability to inhibit the synthesis of glycopeptidolipids necessary to maintain the cell wall integrity. Based on the above findings it can be concluded that econazole and moxifloxacin in combination show greater efficacy as compared with the individual drugs. Based on histopathological results ECZ appears to be a better drug for treatment of MDR-TB. However, further studies are required to confirm these findings.
Acknowledgment
Authors acknowledge Dr B.N. Datta for carrying out the histopathology studies. This work was supported by a grant from Indian Council for Medical Research (ICMR), New Delhi.
References
- In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20:464-7.
- [Google Scholar]
- Moxifloxacin, ofloxacin, sparfloxacin and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007;51:576-82.
- [Google Scholar]
- Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:653-7.
- [Google Scholar]
- Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2003;47:3117-22.
- [Google Scholar]
- Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob Agents Chemother. 2002;46:1875-9.
- [Google Scholar]
- World Health Organization (WHO). Stop TB Department. Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency Update 2008 [WHO/HTM/TB/2008.402] Geneva: WHO; 2008.
- [Google Scholar]
- Selection of a moxifloxacin dose that suppresses drug resistnace in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modelling. J Infect Dis. 2004;190:1642-51.
- [Google Scholar]
- Moxifloxacin containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170:1131-4.
- [Google Scholar]
- Moxifloxacin as an alternative or additive therapy for treatment of pulmonary tuberculosis. Ann Pharmacother. 2011;45:1439-44.
- [Google Scholar]
- Azole-antifungal binding to a novel cytochrome P450 from Mycobacterium tuberculosis. Biochem Pharmacol. 2001;61:1463-70.
- [Google Scholar]
- In vitro and ex vivo antimycobacterial potential of azole drugs against M. tuberculosis H37RV. FEMS Microbiol Lett. 2005;251:19-22.
- [Google Scholar]
- The potential of azole antifungals against latent/persistent tuberculosis. FEMS Microbial Lett. 2006;258:200-3.
- [Google Scholar]
- Azole antifungals as novel chemotherapeutic agents against murine tuberculosis. FEMS Microbiol Lett. 2006;261:181-6.
- [Google Scholar]
- Antimycobacterial activity of econazole against multidrug-resistant strains of Mycobacterium tuberculosis. Int J Antimicrob Agents. 2006;28:543-4.
- [Google Scholar]
- Novel chemotherapy for tuberculosis: chemotherapeutic potential of econazole and moxifloxacin- loaded PLG nanoparticles. Int J Antimicrob Agents. 2008;31:142-6.
- [Google Scholar]
- Inhaled microparticles containing clofazimine is efficacious in the treatment of experimental tuberculosis in mice. Antimicrob Agents Chemother. 2013;57:1050-2.
- [Google Scholar]
- Influence of multidrug resistance on tuberculosis treatment outcomes with standardized regimens. Am J Respir Crit Care Med. 2008;178:306-12.
- [Google Scholar]
- Predictors of poor treatment outcome in multi and extensively drug-resistant pulmonary TB. Eur Respir J. 2009;33:1085-94.
- [Google Scholar]
- Atomic structure of Mycobacterium tuberculosis CYP1221 to 1.6A reveals novel features of cytochrome P450. J Biol Chem. 2003;278:5141-7.
- [Google Scholar]
- Altered expression profile of mycobacterial surface glycopeptidolipids following treatment with antifungal azole inhibitors econazole and clotrimazole. Microbiology. 2005;151:2087-95.
- [Google Scholar]






