Translate this page into:
Efficacy of indigenous plant extracts on the malaria vector Anopheles subpictus Grassi (Diptera: Culicidae)
Reprint requests: Dr A. Abdul Rahuman, Unit of Bioactive Natural Products, Post Graduate & Research Department of Zoology, C. Abdul Hakeem College, Melvisharam 632 509, Vellore District, Tamil Nadu, India e-mail: abdulrahuman6@hotmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Mosquito control is facing a threat due to the emergence of resistance to synthetic insecticides. Insecticides of plant origin may serve as suitable alternative biocontrol techniques in the future. The purpose of the present study was to assess the ethyl acetate, acetone and methanol extracts of Andrographis paniculata, Eclipta prostrata and Tagetes erecta leaves tested for oviposition-deterrent, ovicidal and repellent activities against malaria vector, Anopheles subpictus Grassi (Diptera: Culicidae).
Methods:
The dried leaves of the three plants were powdered mechanically and extracted with ethyl acetate, acetone and methanol. One gram of crude extract was first dissolved in 100 ml of acetone (stock solution). From the stock solution, test solution concentrations of 31.21- 499.42 mg/l for oviposition- deterrence assay and repellency and 15.60 - 998.85 mg/l were used in ovicidal assay. The percentage oviposition- deterrence, hatching rate of eggs and protection time were calculated. One-way analysis of variance was used for the multiple concentration tests and for per cent mortality to determine significant treatment differences.
Results:
The percentage of effective oviposition repellency was highest at 499.42 mg/l and the lowest at 31.21 mg/l in ethyl acetate, acetone and methanol extracts of A. paniculata, E. prostrata and T. erecta. The oviposition activity index (OAI) value of ethyl acetate, acetone and methanol extracts of A. paniculata, E. prostrata and T. erecta at 499.42 mg/l were -0.91, -0.93, -0.84, -0.84, -0.87, -0.82, -0.87, -0.89 and -0.87, respectively. Mortality (no egg hatchability) was 100 per cent with ethyl acetate and methanol extracts of A. paniculata, E. prostrata and T. erecta at 998.85 mg/l. The maximum adult repellent activity was observed at 499.42 mg/l in ethyl acetate extracts of A. paniculata, E. prostrata and methanol extracts of T. erecta, and the mean complete protection time ranged from 120 to 150 min with the different extracts tested.
Interpretation & conclusions:
The acetone extract of A. paniculata, methanol extract of E. prostrata and T. erecta showed good oviposition-deterrent, ovicidal and repellent activities respectively. These results suggest that the leaf extracts of A. paniculata, E. prostrata and T. erecta may have the potential to be used as an ideal eco-friendly approach for the control of the An. subpictus.
Keywords
Anopheles subpictus
ovicidal
oviposition-deterrent
plant extracts
repellent activities
In India, malaria is one of the most important causes of direct or indirect infant, child, and adult mortality. About 2 million confirmed malaria cases and 1,000 deaths are reported annually, although 15 million cases and 20,000 deaths are estimated by WHO South East Asia Regional Office. India contributes 77 per cent of the total malaria in Southeast Asia1. Anopheles subpictus is known to transmit malaria and filariasis. A study of multiple host-feeding in field populations and its specific role in transmitting malaria in Sri Lanka, revealed that multiple blood feeding within the same gonotrophic cycle was attributed to a local ‘frequent feeding strategy’ in this primarily zoophagic and endophilic malaria vector2. An. subpictus Grassi is distributed throughout India, Afghanistan, Borneo, China, Malaysia, Philippines, Sri Lanka, Java and Indonesia. It is a dominant species in Haryana and Uttarakhand States3. Though it is a non-vector species, infected specimens with malaria parasite have been reported from India, Indonesia and Java4. An. culicifacies is the main vector of malaria, and An. subpictus is a significant secondary vector in Sri Lanka5. Conventional synthetic pesticides are used for mosquito control. However, unsystematic prolonged application of these pesticides can have adverse effects on the environment, as well as cause residual effects and induce the development of resistance to the pesticide by the vector6.
Andrographis paniculata is well known plant in Asia and animal studies have shown that extracts are biologically active. It has a broad range of pharmacological effects, showed 100 per cent mortality against Dipetalonema reconditum7 and the methanol and ethyl acetate extracts were tested on Callosobruchus chinensis8. Eclipta prostrata is a common plant growing in moist soils throughout India up to a height of 6000 ft. Lethal activity of methanol whole plant extracts of E. prostrata tested against larvae of Lycoriella ingénue and Coboldia fuscipes using residual contact toxicity9 and the ethanol extract was tested for larvicidal activity against Aedes fluviatilis10. Tagetes erecta methanol and dichloromethane extracts showed a significant activity against Sitophilus oryzae11. Elango et al12 have reported that the acetone, chloroform, ethyl acetate, hexane, and methanol extracts of leaves of A. paniculata, E. prostrata and T. erecta were tested against fourth instar larvae of An. subpictus and Cx. tritaeniorhynchus. The chemicals derived from plants have been projected as weapons in future mosquito control programme as these are shown to function as general toxicant, growth and reproductive inhibitors, repellents and oviposition-deterrent13. The methanol, benzene and acetone extracts of leaves of Cassia fistula were studied for the larvicidal, ovicidal and repellent activity against Ae. aegypti14. The ethyl acetate extracts of Hyptis suaveolens, Rhododendon tomentosum, H. Harmaja and Myrica gale significantly reduced biting activity of Ae. aegypti15. The present study was carried out to assess the efficacy of leaf extracts of A. paniculata, E. prostrata and T. erecta oviposition, ovicidal and repellent activity against malaria vector, An. subpictus.
Material & Methods
Collection and preparation of plant extracts: The leaf of A. paniculata (Burm.f.) Wall. ex Nees. (Acanthaceae), E. prostrata L. (Asteraceae) and T. erecta L. (Compositae) were collected from the Tamil Nadu Medical Plant Farms and Herbal Medicine Corporation Limited, medicinal plant farm, Arumbakkam, Chennai, Tamil Nadu, and the taxonomic identification was made by Dr C. Hema, Department of Botany, Arignar Anna Government Arts College for Women, Walajapet, Vellore, India. The voucher specimen was numbered and kept in our research laboratory, Unit of Bioactive Natural Products, Post Graduate & Research Department of Zoology, C. Abdul Hakeem c0 ollege, Melvisharam, Vellore district, Tamil Nadu, India, for further reference. The study protocol was approved by the ethics committee.
Preparation of plant extracts: The leaves were dried for 7-14 days in the shade at the environmental temperatures (27-37° C day time). The dried leaves (800 g) were powdered mechanically using commercial electrical stainless steel blender and extracted with ethyl acetate (2,200 ml, Qualigens, India), acetone (1,200 ml, Qualigens) and methanol (2,500 ml, Qualigens) in a Soxhlet apparatus (boiling point range 60-80°C) for 8 h. The extract was concentrated under reduced pressure 22-26 mm Hg at 45°C, and the residue obtained was stored at 4°C.
Preparation of stock and test concentrations: One gram of crude extract was first dissolved in 100 ml of acetone (stock solution). From the stock solution, 1000 and 500 mg/l was prepared. Polysorbate 80 (Qualigens) was used as an emulsifier at the concentration of 0.05 per cent in the final experimental media.
Mosquito culture: An. subpictus larvae were collected from rice field and stagnant water area of Melvisharam and identified in Zonal Entomological Research Centre, Vellore, Tamil Nadu, to start the colony and larvae were kept in plastic and enamel trays containing tap water in Unit of Bioactive Natural Products, Post Graduate & Research Department of Zoology, C. Abdul Hakeem c0 ollege, Melvisharam, Vellore district, Tamil Nadu, India. All experiments were carried out, at 27 ± 2°C and 75-85 per cent relative humidity under 14:10 light and dark cycles. Larvae were fed a diet of brewers yeast, dog biscuits and algae collected from ponds in a ratio of 3:1:1, respectively. Pupae were transferred from the trays to a cup containing tap water and were maintained in our insectary (45 × 45 × 40 cm) where adults emerged. Adults were maintained in glass cages and were continuously provided with 10 per cent sucrose solution in a jar with a cotton wick. On day five the adults were given a blood meal from a pigeon placed in resting cages overnight for blood feeding by females. Glass petridishes with 50 ml of tap water lined with filter paper were kept inside the cage for oviposition. They were maintained and reared in the laboratory as per the method of Kamaraj et al16.
Oviposition - deterrence assay: To study the oviposition- deterrence effect and the number of eggs deposited in the presence of different solvent extracts of experimental plants, a multiple concentration test was carried out. For bioassay test, 20 males and 20 females were separated in the pupal stage (by size of the pupae) and were introduced into screen cages (45×45×40 cm) in a room at 27 ± 2°C and 75-85 per cent relative humidity with a photoperiod of 14:10 h light and dark cycles. The pupae were allowed to emerge into adults in the test cages. Adults were provided continuously with 10 per cent sucrose solution in a plastic cup with a cotton wick. They were blood fed (from pigeon) on day five after emergence. In the multiple concentration test, five cups, each containing 100 ml distilled water with a 9-cm piece of white filter paper for oviposition as well as solvent extracts at a concentration of 31.21, 62.42,124.85, 249.71, and 499.42 mg/l were placed in each cage. A sixth cup without extract served as a control. The control was set up with acetone, water and polysorbate 80. The positions of the plastic cups were alternated between the different replicates so as to nullify any effect of position on oviposition. Five replicates for each concentration were run with cages placed side by side for each bioassay. After 24 h, the number of eggs laid in treated and control cups were counted under a stereomicroscope. The per cent effective repellency for each concentration was calculated using the following formula.
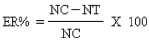
where ER=effective repellency, NC=number of eggs in control, and NT=number of eggs in treatment17. The oviposition experiments were expressed as mean number of eggs and oviposition activity index (OAI), which was calculated using the following formula:
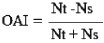
where Nt = total number of eggs in the test solution and Ns = total number of eggs in the control solution. Oviposition active index of +0.3 and above are considered as attractants, while those with -0.3 and below are considered as repellents18. Positive values indicate that more eggs were deposited in the test cups than in the control cups and that the test solutions were attractive. Conversely, negative values indicate that more eggs were deposited in the control cups than in the test cups and that the test solutions were a deterrent.
Ovicidal assay: For ovicidal activity, the freshly laid eggs were collected by providing ovitraps in mosquito cages. Ovitraps were kept in the cages two days after the female mosquitoes were given a blood meal. The eggs were laid on filter paper lining provided in the ovitrap. After scoring, 100 gravids were placed in a screen cage where ten oviposition cups were introduced for oviposition 30 min before the start of the dusk period. Of these ten cups, nine were each filled with test solution of 31.21, 62.42,124.85, 249.71, 499.42 and 998.85 mg/l, and one was filled with 100 ml of water containing acetone and polysorbate 80 that served as a control. A minimum of 100 eggs was used for each treatment, and the experiment was replicated five times. After treatment, the eggs were sieved through muslin cloth, thoroughly rinsed with tap water, and left in plastic cups filled with dechlorinated water for hatching assessment after counting the eggs under microscope19. The per cent egg mortality was calculated on the basis of nonhatchability of eggs with unopened opercula20 and the hatching rate of eggs was assessed 98 h post treatment21.
Repellency activity: The stock solutions of the extracts were diluted with acetone, polysorbate 80 and distilled water to obtain test solutions of 31.21, 62.42, 124.85, 249.71, and 499.42 mg/l. For repellent experiment, 50 laboratory reared blood-starved adult female mosquitoes 3 and 10 days old were placed into separate laboratory cages (45×45×40 cm). Before each test, the forearm and hand of a human subject were washed with unscented neutral soap, thoroughly rinsed, and allowed to dry 10 min before extracts application. After air drying the arm only 25 cm2 of the dorsal side of the skin on each arm was exposed, the remaining area being covered by rubber gloves. The different plant extracts being tested were applied from the elbow to the fingertips. The arm was left undisturbed. An arm treated with acetone and polysorbate 80 served as control. The control and treated arms were introduced simultaneously into the cage. The numbers of bites were counted over 5 min every 30 min, from 18:00 h to 21:00 h2223. The mosquitoes that landed on the hand were recorded and then shaken off before imbibing any blood; making out a 30-min. Protection time was recorded as the time elapsed between repellent application and the observation period immediately preceding that in which a confirmed bite was obtained. If no bites were confirmed at 150 min, tests were discontinued and protection time was recorded as 150 min. An attempt of the mosquito to insert its stylets was considered a bite. If no mosquito attempted to bite the control arm during the observation period, that trial was discarded, and the test was repeated with a new batch of mosquitoes to ensure that lack of bites was due to repellence and not to mosquitoes not being predisposed to get a blood meal at the time. The experiments were conducted five times in separate cages and in each replicate different volunteer were used to nullify any effect of skin differences on repellency. It was observed that there was no skin irritation from the plant extract. The percentage protection was calculated by using the following formula2122:
Protection = ({No. of bites received by control arm}- {No. of bites received by treated arm}) (No. of bites received by control arm) × 100.
Statistical analysis: One-way analysis of variance (ANOVA) was used for the multiple concentration tests and for per cent mortality to determine significant treatment differences24. P<0.05 was considered significant.
Results & Discussion
In the oviposition deterrence assay, gravid An. subpictus preferred to lay eggs in the distilled water control cups than in the cups treated with solvent extracts of three plants (Table I). There was also a marked difference in the number of eggs laid. The results showed that the 499.42 mg/l treated cups received a mean number of 21±1.31, 18±1.46 and 33±3.12 eggs per cup while the control cups received a mean number of 480±2.80, 520±1.34 and 384±2.81 eggs per cup tested the leaf ethyl acetate, acetone and methanol extracts of A. paniculata, respectively. The mean number of eggs laid in acetone and methanol leaf extracts of E. prostrata and T. erecta showed 36±2.16, 48±1.36, 30±2.17 and 34±1.11, respectively, compared with the controls. The present results indicated that the oviposition deterrence was concentration dependent, as 499.42 mg/l of ethyl acetate, acetone and methanol leaf extracts of experimental plants exhibited strong deterrent effect when compared with 31.21 mg/l against oviposition. The solvent leaf extracts strongly deterred oviposition by gravid An. subpictus, with a significantly lower proportion of eggs being laid on ovitraps containing extracts in comparison with control solutions (P<0.05). The number of eggs laid showed the effective repellency against oviposition was 26 noted in 499.42 mg/l followed by 33, 52, 71 and 86 that were noted in 249.71, 124.85, 62.42, and 31.21 mg/l in ethyl acetate extracts of E. prostrata, respectively (Table I). Significantly (P<0.05) less number of eggs were deposited in oviposition bowls treated with ethyl acetate, acetone and methanol extracts of A. paniculata, E. prostrata and T. erecta at all concentrations compared to respective control media. The experimental plant extracts showed effective repellent the gravid females for egg deposition in a concentration-dependent manner. The plant extracts elicited positive oviposition response in the females of An. subpictus, with increasing concentrations the egg deposition was reduced (Table I). It is possible that the compound in the crude extracts, acted as low repellent at a lower dose and showed high oviposition- deterrence effect at higher doses. Accordingly, the ethyl acetate, acetone and methanol extracts of A. paniculata, E. prostrata and T. erecta at 31.21 mg/l received more number of eggs compared with 499.42 mg/l. Though the oviposition response of females of An. subpictus was positive, there is a concentration-dependent reduction in egg deposition, giving evidence that this plant extracts are more attractive at lower dose and act reversely at higher doses (Table I). The OAI value of ethyl acetate, acetone and methanol extracts of A. paniculata, E. prostrata and T. erecta at 499.42 mg/l were -0.91, -0.93, -0.84, -0.84, -0.87, -0.82, -0.87, -0.89 and -0.87 respectively. The OAI values revealed that the solvent plant extracts have deterrent effect and these caused a remarkable negative response resulting in oviposition of very few eggs. Mean per cent hatchability of the ovicidal activity was observed 24 h after treatment. The per cent hatchability was inversely proportional to the concentration of extract and directly proportional to the eggs; 100 per cent mortality (no egg hatchability) with ethyl acetate and methanol extracts of A. paniculata, E. prostrata and T. erecta were exerted at 998.85 mg/l. The maximum repellent activity was observed at 499.42 mg/l in ethyl acetate extracts of A. paniculata, E. prostrata and methanol extracts of T. erecta (Table II), and the mean complete protection time ranged from 120 to 150 min with the different extracts tested. Coria et al25 have reported that the full oviposition deterrency was obtained with Melia azedarach leaf extract at 1 g/l against Ae. aegypti. The benzene, chloroform, ethyl acetate, and methanol extracts of Azadirachta indica showed the highest effective attractancy of 90.09, 94.20, 85.43, and 95.75 per cent at 100 ppm and the lowest effective attractancy of 47.17, 61.94, 49.28, and 68.12 per cent at 25 ppm against An. stephensi, respectively26. The oviposition deterrence effects of ethanolic leaf extract of Cassia obtusifolia at higher concentration (400 mg/l) showed 92.5 per cent effective repellency against oviposition, followed by 87.2, 83.0, and 75.5 per cent, at 300, 200, and 100 mg/l respectively against An. stephensi17. The oviposition deterrent properties against An. stephensi have been observed for various plant extracts including the methanol extract of Pelargonium citrosa, which exhibited 56 and 92 per cent inhibition of oviposition at 1 and 4 ppm, respectively27. The OAI value of methanol extracts of Aegle marmelos, Andrographis lineata, and Cocculus hirsutus at 500 ppm were -0.91, -0.94, and -0.86 respectively28.
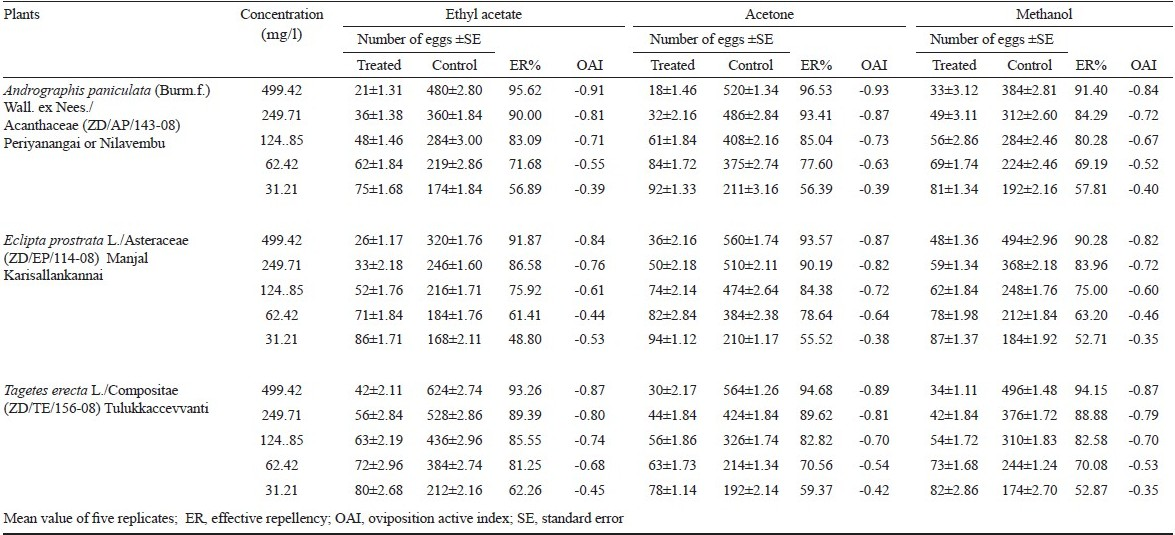
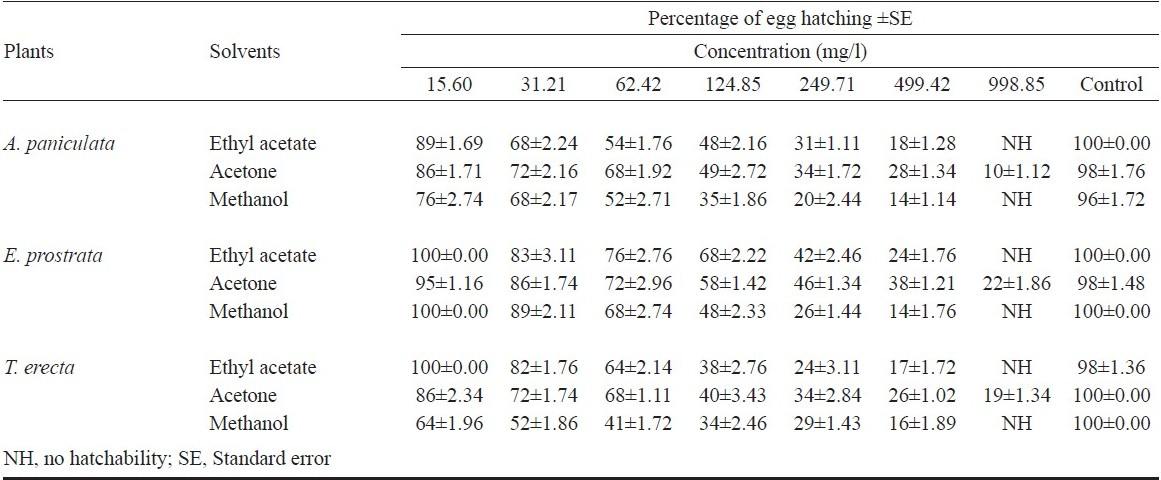
In the present study, the ethyl acetate and methanol extracts of A. paniculata, E. prostrata and T. erecta exerted 100 per cent mortality (no hatchability) at 998.85 mg/l (Table II). Almost 100 per cent hatchability obtained in the control experiments. In the case of ovicidal activity, exposure to freshly laid eggs was more effective than to the older eggs. It has been shown that the age of the embryos at the time of treatment played a crucial role with regard to the effectiveness of the chitin synthesis inhibitor, dimilin to C. quinquefasciatus29. The bioactive compound Azadirachtin isolated from Azadirachta indica showed complete ovicidal activity in eggs of Cx. tarsalis and Cx. quinquefasciatus exposed at 10 ppm30.
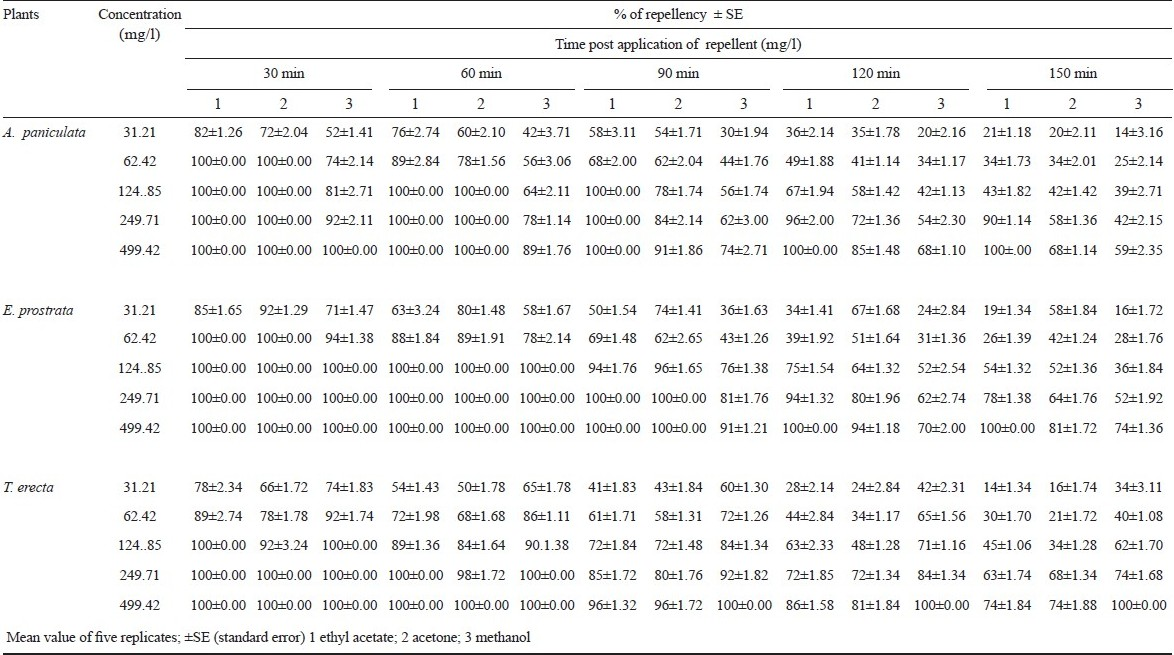
The highest concentrations of 499.42 mg/l over 150 and 120 min protection in leaf ethyl acetate extract of A. paniculata and methanol extracts of E. prostrata and T. erecta against A. subpictus. Lower concentrations provided 30 to 60 min of protection (Table III). The control provided only 3.6±0.63 min of protection. The results showed that repellent activity was dose dependent. The repellent activity of methanol extract of Ferronia elephantum leaves against Ae. aegypti at 1.0 and 2:5 mg/ cm2 concentrations gave 100 per cent protection up to 2:14±0:16 h and 4:00±0:24 h, respectively, and the total percentage protection was 45.8 per cent at 1: 0 mg/cm2and 59.0 per cent at 2:5 mg/cm2 for 10 h22. The five most effective oils were those of Litsea (Litsea cubeba), Cajeput (Melaleuca leucadendron), Niaouli (Melaleuca quinquenervia), Violet (Viola odorata), and Catnip (Nepeta cataria), which induced a protection time of 8 h at the maximum and a 100 per cent repellency against Ae. aegypti, An. stephensi, and Cx. quinquefasciatus31.
In conclusion, the present study revealed the oviposition deterrent, ovicidal, and repellent activities at low concentrations and short exposure time of some Indian medicinal plants. These plant extracts may have potential for the development of new and safe control products for An. subpictus. As naturally occurring insecticides, these plant-derived materials could be useful as an alternative for synthetic insecticides controlling field populations of An. subpictus. Further studies on isolation of bioactive fraction/constituent may provide lead products for field application of mosquito control.
The authors acknowledge C. Abdul Hakeem College Management, Dr S. Mohammed Yousuff, Principal, Dr K. Abdul Subhan, Associate Professor and Head, Zoology Department, and Dr Sait Sahul Hameed, Associate Professor in Zoology for the facilities and support.
References
- Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69-78.
- [Google Scholar]
- Multiple host feeding in field populations of Anopheles culicifacies and An. subpictus in Sri Lanka. Med Vet Entomol. 1999;13:124-31.
- [Google Scholar]
- Indian Anophelines. New Delhi: Oxford & IBH Publishing Co. Pvt. Ltd; 1995. p. :1-416.
- [Google Scholar]
- Variations in ornamentation of wings and palpi of Anopheles (Cellia) subpictus Grassi collected from northwest India. J Vector Borne Dis. 2004;41:37-41.
- [Google Scholar]
- Malaria vectors in a traditional dry zone village in Sri Lanka. Am J Trop Med Hyg. 1999;60:421-9.
- [Google Scholar]
- Evaluation of the larvicidal activity of the leaf extract of a weed plant, Ageratina adenophora, against two important species of mosquitoes, Aedes aegypti and Culex quinquefasciatus. Afr J Biotechnol. 2007;6:631-8.
- [Google Scholar]
- Filaricidal properties of a wild herb, Andrographis paniculata. J Helminthol. 1982;56:81-4.
- [Google Scholar]
- Efficacy of crude extracts of Andrographis paniculata nees. on Callosobruchus chinensis L. during post harvest storage of cowpea. Indian J Exp Biol. 2001;39:715-8.
- [Google Scholar]
- Toxicity of medicinal plant extracts to Lycoriella ingenua (Diptera: Sciaridae) and Coboldia fuscipes (Diptera: Scatopsidae) J Asia Pac Entomol. 2008;11:221-3.
- [Google Scholar]
- Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera:Culicidae) Mem Inst Oswaldo Cruz. 1997;92:565-70.
- [Google Scholar]
- Argentine plants as potential source of insecticidal compounds. J Ethnopharmacol. 1999;67:219-23.
- [Google Scholar]
- Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol Res. 2009;104:1381-8.
- [Google Scholar]
- Botanical derivatives in mosquito control; a review. J Am Mosq Control Assoc. 1991;77:210-37.
- [Google Scholar]
- Bioefficacy of Cassia fistula Linn.(Leguminosae) leaf extract against chikungunya vector, Aedes aegypti (Diptera: Culicidae) Eur Rev Med Pharmacol Sci. 2009;13:99-103.
- [Google Scholar]
- Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J Med Entomol. 2006;43:113-9.
- [Google Scholar]
- Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae) Parasitol Res. 2009;104:1163-71.
- [Google Scholar]
- Larvicidal and oviposition activity of Cassia obtusifolia Linn (Family: Leguminosae) leaf extract against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae) Parasitol Res. 2009;104:337-40.
- [Google Scholar]
- Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol. 1979;8:1111-7.
- [Google Scholar]
- Ovicidal activity of neem products (Azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae) J Am Mosq Control Assoc. 1998;14:204-9.
- [Google Scholar]
- Oviposition deterrent, ovicidal and gravid mortality effects of ethanolic extract of Andrographis paniculata Nees against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae) Entomol Res. 2008;38:119-25.
- [Google Scholar]
- Comparative efficacy of insect repellents against mosquito bites. New Engl J Med. 2002;347:13-8.
- [Google Scholar]
- Repellent activity of Ferronia elephantum Corr. (Rutaceae) leaf extract against Aedes aegypti (L.) Bioresour Technol. 2001;76:287-8.
- [Google Scholar]
- The essential oil of Zingiber officinalis Linn (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol Res. 2008;102:1289-91.
- [Google Scholar]
- Biometry: The principles and practice of statistics in biological research (2nd ed). New York: WH Freeman and Company; 1981.
- Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae) Bioresour Technol. 2008;99:3066-70.
- [Google Scholar]
- Studies on effect of Acalypha indica L. (Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera:Culicidae) Parasitol Res. 2008;103:691-5.
- [Google Scholar]
- Studies on effects of Pelargonium citrosa leaf extracts on malarial vector, Anopheles stephensi Liston. Bioresour Technol. 2003;89:185-9.
- [Google Scholar]
- Oviposition-deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae) Parasitol Res. 2009;105:1567-76.
- [Google Scholar]
- Effects of the insect growth inhibitor, dimilin on hatching of mosquito eggs. J Econ Entomol. 1976;69:655-8.
- [Google Scholar]
- Insecticidal and ovicidal effect of the seed effect of Atriplex canescens against Culex quinquefasciatus. Pharm Biol. 1998;36:69-71.
- [Google Scholar]
- Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res. 2006;99:478-90.
- [Google Scholar]






