Translate this page into:
Effects of infliximab on bacterial translocation in experimental acute necrotizing pancreatitis
Reprint requests: Dr. M Refik Mas, Department of Internal Medicine & Geriatrics, Dokuz Eylül University, Faculty of Medicine, Balcova, 35340, Izmir, Turkey e-mail: refikmas@yahoo.com
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Translocation of bacteria from the gut is an important factor in the development of septic complications and mortality in acute pancreatitis (AP). The present study was designed to assess the effects of infliximab treatment on bacterial translocation (BT) in experimental acute necrotizing pancreatitis.
Methods:
Male Sprague-Dawley rats (n=45) were allocated into three groups. AP was induced in group II (positive control, n=15) and group III (Infliximab; n=15) by retrograde injection of taurocholate into the common biliopancreatic duct. Group I rats (Sham; n=15) received normal saline infusion into the common biliopancreatic duct as placebo. Groups I and II were treated by normal saline and group III was treated with infliximab intraperitoneally on 6, 30 and 54 h after induction of pancreatitis. All surviving animals were killed 60 h after the induction of pancreatitis, and specimens were collected for amylase measurement as well as histopathologic and microbiologic examinations.
Results:
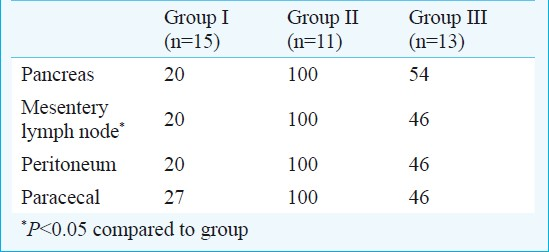
Oedema, acinar cell necrosis, inflammatory infiltration, haemorrhage, fat necrosis and perivascular inflammation in group III rats were decreased with infliximab treatment when compared with group II (P<0.001). BT to mesentery lymph node in groups I, II and III were 20, 100 and 46 per cent, respectively. BT to peritoneum and pancreas in group III was lower than group II (P<0.05).
Interpretation & conclusions:
Infliximab administration resulted in beneficial effects on BT and histopathologic changes in the experimental necrotizing pancreatitis. Whether anti-TNF therapy has a role in prevention of complications of ANP needs to be established.
Keywords
Acute necrotizing pancreatitis
bacterial translocation
infliximab
In acute pancreatitis (AP), inflammatory cells migrate towards the pancreatic tissue after the initial acinar cell injury and release inflammatory mediators such as interleukins (ILs) and tumour necrosis factor alpha (TNF-α)12, a key pro-inflammatory cytokine in the cascade. The resultant events also contribute to the severity of AP and development of multiple organ failure3.
Infliximab, a chimeric anti-TNF-a monoclonal antibody (mAb) that blocks the interaction of this cytokine, induces the lysis of the TNF-α producing cells. It is used widely for the treatment of various inflammatory diseases4. The present study was aimed to investigate the effect of blockage of TNF-α on histopathological changes in pancreas, and whether bacterial translocation to pancreatic tissue can be prevented in AP in an animal model.
Material & Methods
Animals: The study was carried out at Department of Animal Laboratory, Gulhane School of Medicine, Ankara, Turkey. Male Sprague-Dawley rats weighing from 280 to 350 g were obtained from Gulhane School of Medicine Research Center, Ankara, Turkey. Before the experiment, the animals were fed with standard rat chow and water ad libitum and housed in metabolic cages at controlled temperature and 12-h light/dark cycles for at least 1 wk. Food was withdrawn 12 h before experiment. The experiment was approved by the Institutional Animal Use and Care Committee of the Gulhane Medical Academy, Ankara, Turkey.
Experimental model of acute pancreatitis: Anaesthesia was induced with Sevoflurane (Sevorane® Liquid 250 ml, Abbott, Istanbul, Turkey) inhalation. Laparotomy was performed through a midline incision. The common biliopancreatic duct was cannulated with a 28 gauge ½-inch, micro-fine catheter. One microaneurysm clip was placed on the bile duct below the liver and another around the common biliopancreatic duct at its entry into the duodenum to avoid reflux of enteric contents into the duct. Then, 1 ml/kg of 5 per cent sodium taurocholate (Sigma, USA) was slowly infused into the common biliopancreatic duct, and the infusion pressure was kept below 30 mmHg, as measured with a mercury manometer56. After the infusion, the microclips were removed, and the abdomen was closed in two layers. All procedures were performed using sterile techniques.
Study protocol: After the stabilization period, 45 male rats were randomly divided into three groups. Group I (sham plus saline, n=15) underwent laparotomy with only manipulation of the pancreas. Groups II and III underwent induction of acute pancreatitis. Group II (AP plus saline, n=15) received saline injection (10 ml/kg, ip) whereas group III (AP plus infliximab, n=15) received infliximab (Sigma, USA) (10 mg/ml injection (0.125 ml, ip) after the induction of pancreatitis. Both saline and infliximab were started six hours after the induction of AP and repeated every 24 h for two days. Six hours after the third dose blood was drawn from the surviving animals and they were killed under anaesthesia to collect tissue samples.
Laboratory tests: Amylase was measured in plasma by autoanalyzer (Hitachi 917, Japan) using a commercial kit (Boehringer Mannheim, Mannheim, Germany).
Grading of morphological severity of acute necrotizing pancreatitis: Tissue samples were fixed in 10 per cent neutral buffered formalin and embedded in paraffin. One paraffin section stained with hematoxylin and eosin was examined for each pancreas. Two pathologists blinded to the treatment protocol scored the tissues for oedema, acinar cell necrosis, inflammatory infiltrate, haemorrhage, fat necrosis, and perivascular inflammation in 20 fields. The scores of each histological examination were summed up, with a maximum score of 24 as defined by Schmidt et al7.
Quantitative cultures and bacterial identification: The portion of the pancreas with macroscopic necrosis, caecum, peritoneum and mesentery lymph node were excised, weighed, and homogenized. The homogenate was diluted serially, quantitatively plated in duplicate on phenylethyl alcohol and MacConkey II agar and incubated aerobically at 37°C for 24 h. Bacterial counts were expressed as colony forming units (cfu/g tissue), and counts of 1,000 cfu/g and higher were considered to be indicative of a positive culture. Gram-negative bacteria were identified with the API-20E system (BioMerieux Vitek, Hazelwood, MO, USA). Gram-positive bacteria were identified to the genus level by means of standard microbiologic methods8.
Statistical analysis: Results were expressed as mean ± SEM. Translocation incidence was evaluated by Fisher's exact test where appropriate. The significance of differences in histopathologic scores and serum amylase levels were assessed by One way ANOVA test. Subgroup analyses were assessed by Tukey HSD test, Kruskal Wallis test and Mann-Whitney U test. P<0.05 was considered significant. All statistical measurements were made by using SPSS PC ver. 11.0 (SPSS Inc. USA).
Results
Groups II and III rats developed acute pancreatitis, demonstrated by macroscopic parenchymal necrosis and abundant turbid peritoneal fluid. All animals except four in group II and two in group III survived the experiment period of 54 h.
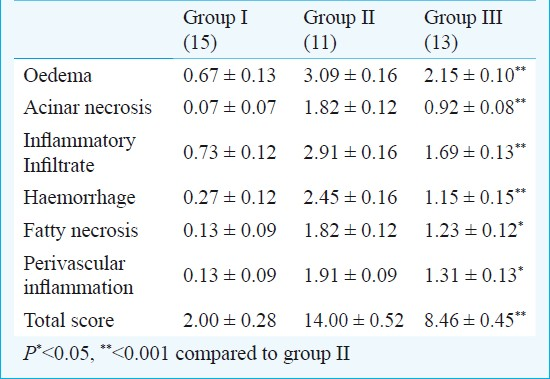
Serum amylase levels were significantly (P<0.01) reduced in group III compared with group II. Total histopathologic score was significantly reduced in the group III (8.46 ± 0.45) compared with the rats in the group II (14.0 ± 0.52), (P<0.001). Oedema, acinar cell necrosis, inflammatory infiltration, haemorrhage, fatty necrosis and perivascular inflammation in group III were also decreased with infliximab treatment when compared with group II (Table 1; Fig.).
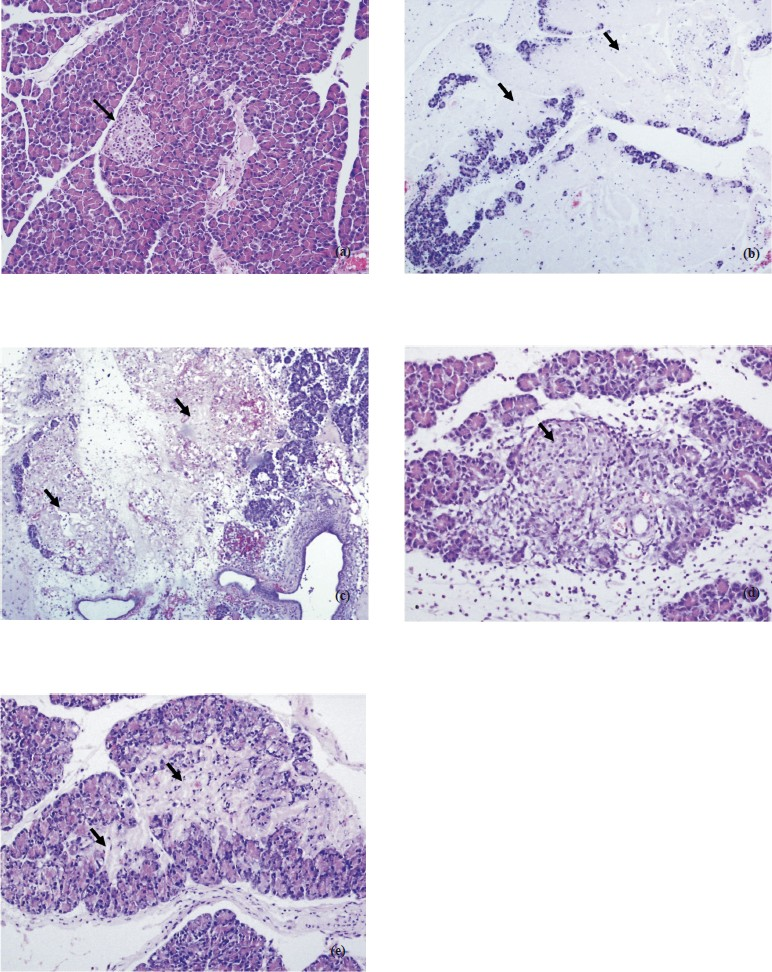
-
(a): Normal pancreas panceatic islet close to center area and normal acinar around (arrow). H&E × 200. (b). Group I extensive parancyhmal necrosis of acinar (arrows). H&E × 100. (c). Group II extensive parancyhmal necrosis o Acinar. H&E × 100. (d). Group III recovery-slightly fibrosis-parancyhma islet in the center and replacement of aciner structures with fibrosis in the inferior area (arrow). H&E × 200. (e). Group III Parancyhmal injury-recovery with slightly fibrosis replacement of acinar structures with fibrosis in the inferior area (arrows). H&E × 200.
BT to pancreatic tissue was detected in 6 of 13 (54%) rats treated with infliximab (group III) whereas all pancreatic tissue was infected in rats treated with saline (group II). Decrease in BT to pancreas in group III was significant (P<0.05) compared with that in group II Table II. Bacteria isolated from pancreas of rats with AP included Escherichia coli, Enterococcus spp., Staphylococcus spp., Klebsiella oxytoca and Proteus spp. The most common bacteria were E. coli.
Discussion
AP is the inflammation of the pancreas, a serious event with no specific treatment. The pancreas can become inflamed for many reasons, but mainly as a complication from gallstones or excess alcohol intake. If severe, the organ may lose its blood supply, a complication called pancreatic necrosis that can be detected by computed tomography (CT) scanning, and death can occur either early in the progress in association with uncontrolled inflammatory responses due to multiple organ- system failure (MOSF), or late when the necrotic tissue becomes infected, which might necessitate major surgery to remove the infection, with the risk of death rising from 10 to over 40 per cent9.
In AP, pancreatic infections are caused by translocation of enteric bacteria via lymphatic route; therefore, therapies preventing or inhibiting BT are likely the most feasible approaches theoretically10–12. Although mechanism of BT is not known completely, it has been linked to various factors. Previous studies have confirmed that mucosal injury, cecal bacterial overgrowth, decreased gut motility and compromised host immune function are underlying mechanisms of BT13–15.
It was demonstrated by leukocyte scintigraphy that, as the process progresses, macrophages and granulocytes migrate into the inflamed pancreas in the early phase16. Activation of complement and subsequent release of C5a are further enhanced by accumulated inflammatory cells. Leukocyte activation leads to increased leukocyte aggregation and tissue infiltration within the microcirculation where these cells increase production of cytokines and other inflammatory mediators including prostaglandins, leukotrienes, thromboxanes, platelet activating factor, free radicals, nitric oxide and proteases. These substrates not only increase vascular permeability by interfering with the pancreatic microcirculation, but also cause pancreatic necrosis by causing thrombosis and haemorrhage. TNF-α and IL-1 are known to be the most prominent and first-line cytokines. TNF-α regulates cell apoptosis by increasing intercellular adhesion molecule-I production and pancreatic leukocyte sequestration, promoting activation of cytokines such as mitogen activated protein kinase, nuclear factor kappa β and pancreas associated peptide-I that are essential for an aggravated inflammatory process1718.
In the intestine, besides regulation of cellular apoptosis, TNF-α was shown to increase paracellular permeability by affecting on tight junctions, indicating a specific role on intestinal epithelial barrier function19. Moreover, this cytokine was also suggested to be a facilitator of bacterial translocation from the epithelium20. Blockage of TNF-α was previously demonstrated to correct intestinal permeability in people with Crohn disease21, suggesting this effect may occur in other types of disorders with altered permeability such as acute pancreatitis. Accordingly, a potential therapeutic role for infliximab in AP was first reported by Oruc et al22 in animal model. The authors demonstrated benefits of infliximab administration in acute oedematous and necrotizing pancreatitis; however, they did not consider BT as a septic complication. On the other hand, since local manifestations of Crohn's disease are not fully present in experimental or human pancreatitis, one pathophysiological explanation cannot suffice for both conditions.
In the present study, infliximab therapy was started 6 h after the induction of pancreatitis, although it was reported that a prophylactic design starting the treatment before the induction is superior23. Development of AP was confirmed by both increase in blood amylase level and histopathologic examination in group II, and the total histopathologic score in group III was better than group II. When a subgroup analysis was performed for these two groups, improvements in oedema, acinar cell necrosis, inflammation and haemorrhage were significantly higher in group II. These findings were similar to earlier results2223.
The present study confirms the previous reports that BT to mesentery lymph node, peritoneum and pancreas is a known event in experimental acute necrotizing pancreatitis in rats1224, though it does not directly show the infection. Moreover, BT to the pronounced sites was diminished by more than 50 per cent, and translocated bacteria to pancreas were demonstrated to be of colonic origin as described by others1224. TNF-α and IL-I related cytokine activation that plays a central role in the pathogenesis of AP, results in microcirculatory damage both in pancreatic or intestinal epithelium. Ischaemia-reperfusion injury in intestines is one of the most important mechanisms resulting in BT1718. In the present study, it was demonstrated that blocking TNF-α receptor with infliximab was able to prevent endotoxaemia related intestinal damage and BT. BT and severity of pancreatitis in group III were significantly different from that in group II. However, though the study was not designed to measure the survival effect, animal deaths in group III were less than those in group II. The data suggest that TNF-α blockage improves the animal survival as well.
In conclusion, the present study showed that blocking TNF-α exerted beneficial effects on blood amylase levels and histopathologic changes in experimental necrotizing pancreatitis. It significantly decreased the degree of BT, a prominent factor in pancreatitis related mortality. Whether TNF-α blockers have a role in prevention of septic and other complications of ANP in the clinical setting deserves attention.
References
- Pathophysiology of acute experimental pancreatitis: lessons from genetically engineered animal models and new molecular approaches. Digestion. 2005;71:162-72.
- [Google Scholar]
- Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-10.
- [Google Scholar]
- Role of cytokines and oxidative stress in the pathophysiology of acute pancreatitis: therapeutical implications. Curr Drug Targets Inflamm Allergy. 2002;1:393-403.
- [Google Scholar]
- Inhibitors of TACE and caspase-1 as anti-inflammatory drugs. Curr Med Chem. 2005;12:2963-77.
- [Google Scholar]
- Hyperbaric oxygen therapy attenuates pancreatic microcirculatory derangement and lung edema in an acute experimental pancreatitis model in rats. Pancreas. 1998;17:44-9.
- [Google Scholar]
- The effect of combination therapy of hyperbaric oxygen, meropenem, and selective nitric oxide synthase inhibitor in experimental acute pancreatitis. Pancreas. 2004;28:53-7.
- [Google Scholar]
- A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56.
- [Google Scholar]
- Quantitative cultures of wound tissues. In: Garcia LS, ed. Garcia LS, ed. Clinical microbiology procedures handbook, 2 (3rd ed). Washington DC: ASM Press; 2010. p. :3-13.
- [Google Scholar]
- Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;5:CD002941.
- [Google Scholar]
- The role of antibiotic prophylaxis in severe acute pancreatitis. Arch Surg. 1997;132:487-93.
- [Google Scholar]
- Plasmid labeling confirms bacterial translocation in pancreatitis. Am J Surg. 1994;167:201-7.
- [Google Scholar]
- Obstructed intestine as a reservoir for systemic infection. Am J Surg. 1990;159:394-401.
- [Google Scholar]
- Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35:1306-10.
- [Google Scholar]
- Scintigraphic assessment of leukocyte infiltration in acute pancreatitis using technetium-99m-hexamethyl propylene amine oxine as leukocyte label. Dig Dis Sci. 1991;36:65-70.
- [Google Scholar]
- Early NF-kB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;175:1402-14.
- [Google Scholar]
- Early changes of serum proinflammatory and anti-inflammatory cytokines after endoscopic retrograde cholangiopancreatography. Pancreas. 2003;26:375-80.
- [Google Scholar]
- Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-72.
- [Google Scholar]
- Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut. 2003;52:224-30.
- [Google Scholar]
- Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000-4.
- [Google Scholar]
- Infliximab: A new therapeutic agent in acute pancreatitis. Pancreas. 2004;28:e1-e8.
- [Google Scholar]
- Timing of tumor necrosis factor antagonism is critical in determining outcome in murine lethal acute pancreatitis. Surgery. 1996;120:515-21.
- [Google Scholar]






