Translate this page into:
Effects of infections on the pathogenesis of cancer
For correspondence: Dr Antonio Biondi, Department of General Surgery & Medical-Surgical Specialties, University of Catania, University Hospital ‘Policlinico G. Rodolico – San Marco’, Via S. Sofia 78, 95123 Catania, Italy e-mail: abiondi@unict.it
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Several studies have shown an inverse relationship between acute infections and cancer development. On the other hand, there is a growing evidence that chronic infections may contribute significantly to the carcinogenesis. Factors responsible for increased susceptibility to infections may include modifications of normal defence mechanisms or impairment of host immunity due to altered immune function, genetic polymorphisms, ageing and malnourishment. Studies have demonstrated that children exposed to febrile infectious diseases show a subsequent reduced risk for ovarian cancer, melanoma and many other cancers, while common acute infections in adults are associated with reduced risks for melanoma, glioma, meningioma and multiple cancers. Chronic inflammation associated with certain infectious diseases has been suggested as a cause for the development of tumours. Mechanisms of carcinogenesis due to infections include cell proliferation and DNA replication by mitogen-activated protein kinase pathway, production of toxins that affect the cell cycle and lead to abnormal cell growth and inhibition of apoptosis. This review was aimed to summarize the available evidence on acute infections as a means of cancer prevention and on the role of chronic infections in the development and progression of cancer.
Keywords
Bacterial
cancer
carcinogenesis
immune system
infections
MAPK pathway
pathogenesis
virus
The complex association between infectious diseases and cancer has been widely discussed since the beginning of the twentieth century. Around 2.2 million new cases of cancer diagnosed in 2012, were attributable to carcinogenic infections. Among the most important infectious agents involved in cancer development and progression, Helicobacter pylori (HP) (770,000 cases), human papillomavirus (HPV) (640,000), hepatitis B virus (HBV) (420,000), hepatitis C virus (HCV) (170,000) and Epstein–Barr virus (EBV) (120,000) have been indicated to play a prominent role1. Infectious agents and related biochemical species are able to modulate a broad range of host immune responses and in turn may affect tumour development and progression. However, the factors related to infection-mediated immunity that influences carcinogenesis has not been completely understood. Acute and chronic infections may play different roles in carcinogenesis; epidemiological studies have shown that acute infections antagonize the onset of cancer while chronic infections are able to favour carcinogenesis2. Acute infections are characterized by rapid onset and short duration and are usually associated with fever, synthesis of hepatic acute-phase proteins and production of cytokines and other inflammatory mediators. In some cases, chronic infections could be a consequence of failed or deficient immune response3. Factors responsible for increased susceptibility to infections include alterations of normal defence mechanisms or impairment of host immunity, abnormal immune function, genetic polymorphisms, ageing and malnourishment. Other factors that may determine whether infections occur are the biological characteristics of viruses, bacteria and parasites such as pathogenicity, virulence, invasiveness infective load, toxinogenesis, contagiousness, tenacity and vitality4. In industrialized countries, some tumours such as melanoma, colorectal, breast, kidney, testicular and prostate cancers are more common among people of higher socio-economic status (SES), while the prevalence of lung, liver, cervical and stomach cancers is higher in people of low SES5. In low SES groups, there is a higher concurrent use of tobacco and alcohol, responsible for lung and liver cancers, and a higher prevalence of chronic infections such as HPV, HP, HBV and HCV, which have been associated with uterine cervix, stomach and liver cancers67. There could be a possible inverse relationship between the decrease of incidence and mortality from infectious diseases and the increase in death rates due to cancer28. Mastrangelo et al8 observed the simultaneous reduction of infections and growth of tumours in Italy. This trend could be explained through a decrease in the activation of immunological mechanisms against transformed cells in early phases of carcinogenesis8. There is evidence that West Nile virus, mumps virus, reovirus, Newcastle disease virus and other viruses may act as oncolytic viruses, causing lysis of infected cells and activation of antitumoural immunity, thus leading to possible spontaneous cancer remission9. Epidemiological studies reported a protective role of childhood mumps against ovarian cancer; the protective mechanism could involve the expression of mucin 1 (MUC1), a cell surface glycoprotein and tumour-associated antigen, which is able to generate immune surveillance of ovarian cancer cells10. Mink et al11 showed an increased incidence of ovarian cancer mediated by anti-MUC1 antibodies among White females. These data supported the hypothesis of an association between the increased incidence of ovarian cancer and the decrease of mumps parotitis infections through vaccination12. Lehrer and Rheinstein13 pointed out that polio virus infection in colon epithelial cells might provide protection against the development of colon cancer. The reduction of death rate from colon cancer due to polio virus infection seems to be mediated by CD155 and polio virus cellular receptor13. Here we summarize the available knowledge on acute infections as a means of cancer prevention and on the role of chronic infections in the development and progression of cancer.
Acute infections
There is evidence that certain acute infections may inhibit neoplastic growth14. Infectious agents and cell damage are able to trigger inflammatory signalling pathways, such as nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways15. Inflammatory cells can produce pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) and prostaglandin E2. The creation of new blood vessels during acute inflammation may have detrimental effects such as the increase of oedema and tissue damage mediated by neutrophils. Some infection-induced factors (TNF-α, IFN-α/β/γ, IL-12, TGF-β and acute-phase proteins) are critical regulators of acute inflammation and prevent neo-angiogenesis16. Pathogen-associated molecular patterns (PAMPs), which include lipopolysaccarides (LPS), a constituent of bacterial cell walls, play a key role in anti-tumour response through the binding with Toll-like receptors (TLRs) on different immune cells, such as neutrophils, T lymphocytes and dendritic cells. During a bacterial infection, PAMPs stimulate the production of pro-inflammatory cytokines (such as IL-1β, TNF-α and IL-12), thus leading to maturation of dendritic cells and initiation of a cytotoxic T-cell–mediated anticancer response17 (Table 1A and B). Hoption Cann et al20 observed that acute infectious diseases of childhood were associated with a reduced risk of future development of melanoma, ovarian cancer and multiple tumours. Acute infections seem to provide protection against cancer also after neonatal and infant life, but this effect decreases with age, consistently with the decline of the immune response2. In adult individuals, there is a reduced risk of developing gliomas, meningiomas, melanomas and multiple tumours after acute infections2021. Spontaneous regression in cancer associated with bacterial, fungal, viral or protozoan infections has been observed in the majority of cancers and most frequently in embryonal and breast cancer, melanoma, neuroblastoma, renal adenocarcinoma, lymhomas/leukaemias and sarcoma or carcinoma of the urinary bladder22. However, spontaneous regression is a rare event; for example, the rate of spontaneous regression for melanoma has been estimated around 1/400 patients, and spontaneous regression of other tumours such as haematologic cancers, is even rarer23. Many microorganisms, such as Vibrio cholerae, Salmonella spp., Escherichia coli, Clostridium spp., Bifidobacterium spp. and Listeria spp., have been evaluated as potential anticancer agents or for the production of vaccines24. Other bacterial infections associated with spontaneous cancer regression include syphilis, tuberculosis, gonorrhoea and diphtheria, whereas viral infections include influenza, rubella, smallpox and hepatitis virus. Protozoa such as Toxoplasma gondii and Besnoitia jellisoni may also cause tumour regression25. In some cases of superficial bladder cancer, live bacillus Calmette–Guèrin (BCG) was administered into the bladder after surgical resection, as it led to a local immune response with production of cytokines such as IL-2, TNF-α and IFN-γ that may destroy residual cancer cells and thereby prevent recurrence24.
| Acute infections | Chronic infections |
|---|---|
| Inhibition of neoplastic growth | Risk factor for cancer development and progression |
| Prevention of neo-angiogenesis through infection-induced factors (TNF-α, IFN-α/β/γ, IL-12, TGF-β and acute-phase proteins) | Promotion of tumour angiogenesis, survival, proliferation and dissemination |
| Anti-tumour response of LPS through the binding with TLRs on different immune cells (T-cells, neutrophils and dendritic cells) | Inhibition of T-cell activity Expression of immune checkpoint regulators (CTLA-4 and PD-1) |
| Acute infections | Chronic infections |
|---|---|
| Production of pro-inflammatory cytokines (IL-1β, TNF-α and IL-12) in response to receptor stimulation by PAMPs | Production of anti-inflammatory cytokines |
| Maturation of dendritic cells | Activation of NF-κB and induction of different genes responsible for cell proliferation, survival and invasion |
| Initiation of cytotoxic T-cell-mediated anticancer response | Inhibition of growth regulators (e.g. tumour suppressor p53) |
Chronic infections
Some microorganisms are able to cause chronic infections and to induce DNA mutations; this in turn, can be correlated with tumour development. Chronic infections and cancer development can be associated with immunological mechanisms, such as induction of regulatory T-cells, production of anti-inflammatory cytokines and expression of immune checkpoint regulators [cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein-1 (PD-1)]. CTLA-4, expressed on T-cells, can interact with CD80 and CD86 to inhibit T-cell activation, thus leading to anergy18. PD-1, mostly expressed on T-cells, can interact with programmed death-ligand 1 and ligand 2, expressed on antigen-presenting cells (APCs) and tumour cells, inhibiting T-cell activity19. Inflammation may act as a promoter on the ‘initiated cells’ accumulated during life driving the progression of cancer. Biosynthesis of biochemical and metabolic intermediates during tumour growth and inflammatory process may damage DNA and other biomolecules leading to the development of malignant lesions26. Macrophages are a major component of the tumour microenvironment, where these generally play a pro-tumour role by promoting tumour survival, angiogenesis, proliferation and dissemination27. In patients with chronic infections, NF-κB may lead to the initiation and progression of cancer by the induction of different specific genes responsible for cell proliferation, survival and invasion while antagonizing growth regulators such as tumour suppressor p5328. There is evidence of regression of low-grade mucosa-associated lymphoid tissue (MALT) lymphomas as well as lymphoma of the stomach, bowel, bladder, larynx, lung, salivary glands, spleen and thyroid after antibiotic therapy29. Some types of lymphoma may be associated with HP, Campylobacter jejuni, HCV or other microorganisms, and, HP eradication therapy is considered as the first therapeutic approach for low-grade gastric MALT lymphoma30. The regression of benign and malignant lesions caused by HPV has been reported after treatment with antiviral drugs3132. Topical treatment with imiquimod, an immune response modifier, or cidofovir, an inhibitor of viral replication, has been shown to be effective (~50-60%) for both anogenital warts and high-grade vulvar intraepithelial neoplasia32. In a phase II randomized controlled trial, topical treatment with the antiviral drug AV2® showed promising results in reducing the size of cervical lesions associated with HPV31. However, these findings need to be confirmed in further well-designed trials.
Viral infections
Viruses have the capability to reverse physiological functions of host cell and pathways regulating growth arrest and apoptosis. Transforming viruses carry oncogenes derived from cellular genes that are involved in mitogenic signalling, cell proliferation and regulation of programmed cell death33. The process of carcinogenesis consists of multiple steps, and viruses associated with cancer such as HBV, HPV and EBV may share common pathways including the functional inactivation of p53 by virally encoded oncoproteins34. Host immunity may influence tumour progression, as many years may pass between the onset of infection and the development of cancer, and carcinogenesis process can be affected by direct and indirect mechanisms such as possible synergy between viruses and environmental cofactors35. So far, seven viruses [EBV, Kaposi's sarcoma-associated human herpes virus (KSHV), HPV, MCPV, HBV, HCV and human T-cell leukaemia virus type 1 (HTLV1)] have been shown to be associated with various types of human cancer36. Tables II and III show the DNA and RNA viruses associated with cancer.
| Virus | Tumour | Odds Ratio (OR) |
|---|---|---|
| EBV | NPC, BL, HD | NPC: Polymorphism at EBV-encoded gene, RPMS1 OR=5.2737 |
| BL: In association with malaria OR=13.238; EBV-alone OR=4.5039 | ||
| HD: Prior history of infective mononucleosis OR=2.43; EBV-positive OR=9.1640 | ||
| HBV | HCC, NHL | HCC: OR=5.38941 |
| NHL: OR=2.5042 | ||
| HPV | Cervical cancer, squamous carcinoma of the vulva, penis and anus, head and neck cancer, oesophageal cancer, breast cancer | Cervical cancer: HPV 16 OR=2770; |
| HPV 18 OR=95043 | ||
| Oropharyngeal cancer: HPV-16 OR=14.644 | ||
| Carcinoma of the head and neck: HPV-16 OR=2.245 | ||
| Anal and perianal skin cancer: HPV 16 OR=3.0; and HPV 18 OR=4.446 | ||
| Oesophageal squamous cell carcinoma: HPV 16 OR=1.0; and HPV 18 OR=0.547 | ||
| Adenocarcinoma of the oesophagus: HPV 16 OR=1.2; and HPV 18 OR=0.247 | ||
| Breast cancer: Mixed genotypes, HPV 18, 16, 33, 6 and 11 OR=4.9248 | ||
| HHV8 or KSHV | KS, BCBL, CL | KS: OR 8.649 |
| MCV | MCC | MCC: OR 4.450 |
EBV, Epstein-Barr virus; NPC, nasopharyngeal carcinoma; BL, Burkitt lymphoma; HD, Hodgkin’s disease; HBV, hepatitis B virus; HPV, human papillomavirus; HCC, hepatocellular carcinoma; HL, Hodgkin’s lymphoma; NHL, non-HL; HHV8, Human herpesvirus 8; KS, Kaposi’s sarcoma; KSHV, KS associated human virus; BCBL, body cavity-based lymphoma; CL, Castleman lymphadenopathy; MCC, Merkel cell carcinoma; MCV, Merkel cell polyomavirus
| Virus | Tumour | Odds ratio (OR) |
|---|---|---|
| HTLV-1 | ATL | ATL: Rate ratio=2.50, Cumulative risk: 6.6% for men and 2.1% for women51 |
| Positive anti-HTLV-I OR=1.6 per two-fold dilution52 | ||
| HIV-1 | NHL, KS, head and neck cancer, testicular cancer, melanoma, anal cancer, HD | NHL: OR=4.453 |
| KS: OR=93.553 | ||
| SIRs: anal (SIR=28), liver (SIR=5.6) HL (SIR=11), laryngeal cancer (SIR=1.5)54 | ||
| HCV | HCC, pancreatic cancer, skin cancer, myelodysplastic syndrome, DLBCL | HCC: OR=31.5; Intrahepatic bile duct tumour: OR=3.40; extrahepatic bile duct tumour: OR=1.90; pancreatic cancer: OR=1.23; anus: OR=1.97; Non-melanoma non-epithelial skin cancer: OR=1.53; myelodysplastic syndrome: OR=1.56; DLBCL: OR=1.5755 |
HTLV-1, human T leukaemia virus type 1; ATL, adult T-cell leukaemia; HIV-1, human immunodeficiency virus-1; HL, Hodgkin’s lymphoma; NHL, non-HL; KS, Kaposi’s sarcoma; HD, Hodgkin disease; SIRs, standardized incidence ratios; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; DLBCL, diffuse large B-cell lymphoma
Epstein–Barr virus (EBV)
EBV was discovered in the cultured cancer cells obtained from a Burkitt lymphoma (BL) biopsy56. EBV is considered, along with other factors, a causal agent for the development of BL, undifferentiated nasopharyngeal carcinoma, immunoblastic lymphomas and lymphoproliferative disorders in immunocompromised patients57. Furthermore, EBV infection represents a risk factor for Hodgkin's disease, non-Hodgkin's lymphoma, thymic lymphoepithelioma, salivary gland and urogenital carcinoma in immunocompetent hosts58 (Fig.1). In patients with autoimmune diseases, with HIV infection or treated with immunosuppressive therapy after an organ transplant, EBV is strongly associated with leiomyomas, leiomyosarcomas and other smooth muscle neoplasms59. A high worldwide prevalence of EBV (around 8.29%) has been reported in patients with gastric adenocarcinoma60. However, it is unclear whether EBV infection is involved in gastric cancer development or is a consequence of gastric inflammation due to immunosuppressive therapies60.
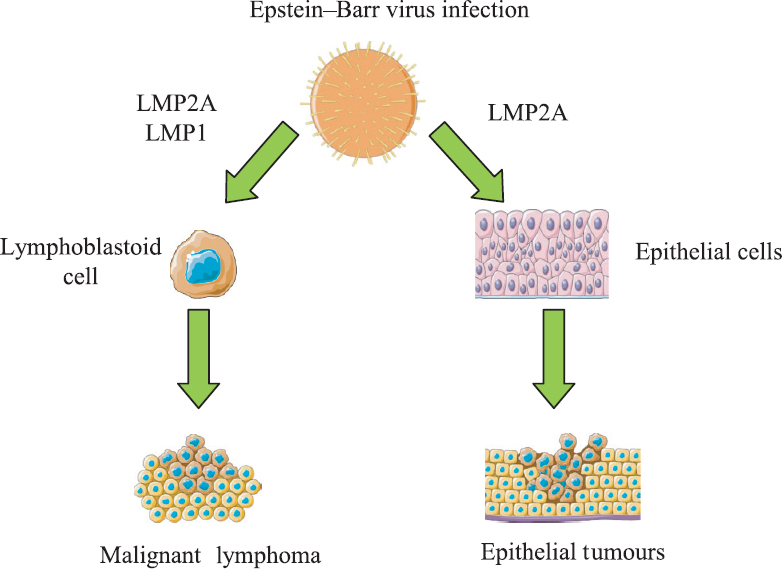
- Pathogenetic mechanisms of Epstein–Barr virus on carcinogenesis. EBV is associated with different human tumours. EBV may infect B lymphocytes, leading to a malignant transformation into a lymphoma. Also, EBV may transform epithelial cells into epithelial tumours, LMP: EBV latent membrane protein; EBV, Epstein–Barr virus. Source: Ref 56.
Hepatitis B virus (HBV)
Chronic infections caused by HBV and HCV represent the main risk factor for the development of hepatocellular carcinoma (HCC). HBV is a non-cytopathic, partially double-stranded hepatotropic DNA virus which belongs to Hepadnaviridae family. There are eight HBV genotypes (designated A-H) and four serotypes (adv, ayw, adr and ayr) characterized by different geographic distribution and clinical differences. Studies on HBV infection showed that genetic characteristics, including viral genotypes and specific mutations, may contribute to the development of HCC through direct and indirect pathways61. HBV can integrate its genome into the human genome, leading to alterations of transcription and cell growth. Integrated HBV sequences have been observed in established hepatoma cell lines and in about 80 per cent of human HBV-related HCCs62. The integration of HBV DNA does not play an essential role in viral replication but may cause viral genome persistence and poses high risk for hepatic cancer. The risk for HCC is increased in patients with active hepatitis and in cirrhotic patients, although HBV may cause cancer even in the absence of cirrhosis63. Continuous injury and regeneration of cirrhotic liver may lead to increased liver cells turnover, favouring genome critical mutations, chromosomal rearrangements, activation of oncogenes and inactivation of tumour suppressor genes. The T-cells immune response, elicited to contrast viral infection, contributes to hepatocytes necrosis, inflammation and regeneration64. HBV can replicate within the pancreas, and higher serum and urinary levels of pancreatic enzymes are frequently observed in patients with HBV chronic infection65. A meta-analysis of eight studies showed an association between past exposure to HBV and increased risk for pancreatic cancer development [odds ratio (OR)=1.24] mainly among individuals without natural immunity [anti-hepatitis B core (HBc)-positive/anti-hepatitis B surface antibody (anti-HBs) negative], with an OR of 1.6766. Chronic hepatitis B- and HBsAg-positive patients had a significantly increased risk for pancreatic cancer (OR=1.20); past exposure to HBV with natural immunity (anti-HBc positive/anti-HBs positive) had no association with pancreatic cancer development with an OR=0.98. Anti-HBs-positive patients showed a lower risk of pancreatic cancer compared to anti-HBs-negative patients (OR=0.54)6768. Further research is needed to explain the pathogenetic mechanisms involved in the progression from active hepatitis virus infection to chronic inflammatory response associated to pancreatic carcinogenesis.
Human papillomavirus (HPV)
HPV, a virus belonging to the Papovavirus group, is responsible for common infections contracted through sexual, oral and skin contact. It is estimated that over 70 per cent of women contract genital HPV infection during their lives, but a large number of infections disappears spontaneously over a few months due to the activity of immune system69. There are more than 100 types of HPV, and most of these do not cause serious disease; however, about 15 genotypes are known to be carcinogenic principally in the transformation zone between different types of epithelium70. This process has been observed in almost all uterine cervix cancers, in which HPV is able to induce different grades of cervical intraepithelial neoplasia (CIN). HPV-16 and HPV-18, the two most carcinogenic genotypes, are responsible for about 50 per cent of CIN3 and 70 per cent of cervical cancer71 (Fig. 2). The use of prophylactic vaccination against HPV infection is efficient and represents the most useful way of cervical cancer prevention. Other cancers associated with HPV infection, are breast, anus, prostate, penis, vagina, vulva, oropharynx, conjunctiva and non-melanoma skin cancers72. Potential cofactors involved in CIN development may include HIV, Chlamydia trachomatis, herpes simplex virus type 2 and immunosuppression72.

- Effects of human papillomavirus (HPV) on the development of cervical cancer. The oncogenic proteins E6 and E7 affect control of the cell cycle by their interaction with p53 and retinoblastoma protein. HPV induces cervical intraepithelial neoplasia (CIN), which may develop into cervical cancer through changes in cytokine levels and reduction of apoptosis. CIN, cervical intraepithelial neoplasia; pRB, retinoblastoma protein; IL-10, interleukin 10; IFN-α, interferon alpha; IFN-β, interferon beta. Source: Ref. 71.
Merkel cell polyomavirus
Merkel cell polyomavirus is found in tumour tissues of Merkel cell carcinoma (MCC). MCC is a rare and aggressive neuroectodermal cancer frequently observed in transplant recipients, immunocompromised those, patients with B-cell lymphoid tumours and patients with acquired immune deficiency syndrome (AIDS)73. Clonal integration of viral DNA into tumour cell genome has been observed in patients with MCC. Treatment protocols include the administration of bleomycin, a drug with direct antiviral effects against HCV and HIV74.
Kaposi's sarcoma-associated human herpes virus (KSHV)
KSHV, also known as human herpes virus 8 (HHV8), is considered the cause of various types of Kaposi's sarcoma (KS), such as AIDS-associated, classic, endemic, African and iatrogenic. HHV8 encodes for homologues of several cytokines and their receptors, but the exact role of virus in cancer induction is not yet clear75. The viral genome of HHV8 has been found in primary effusion lymphoma, multicentric Castleman disease, angioimmunoblastic lymphadenopathy and in some cases of reactive lymphadenopathies. HHV8 is also considered as a cause of body cavity-based lymphomas76.
Parvovirus B19
Parvovirus B19 infection may play a role in the pathogenesis of rheumatic diseases and acute lymphoblastic and myeloblastic leukaemia77. Parvovirus DNA and viral proteins have been detected in neoplastic epithelium of patients with papillary thyroid carcinoma (PTC)78. The possible role of B19 in pathogenesis of thyroid tumours is suggested by the involvement of immune system, NF-κB, IL-6 and viral proteins. The tax- and tat-mediated NF-κB activation plays a crucial role in the HTLV-1-induced acute leukaemia and HIV-induced KS; NF-κB is also significantly increased and co-localized with viral DNA in PTC tissues79. One of the non-structural proteins (NS1) encoded by the viral genome is cytotoxic to the host cells and linked to NF-κB and IL-680. This association is probably due to the fact that non-structural viral proteins resemble tax protein of HTLV-1 and tat protein of type 1-HIV. These all play a part in the viral propagation and activate IL-6 production through the NF-κB-binding site in the IL-6 promoter81. These findings suggested a link between parvovirus B19 and thyroid carcinoma.
Human T-cell leukaemia virus type 1 (HTLV-1)
HTLV-1 is a single-stranded RNA retrovirus predominantly associated to adult T-cell leukaemia (ATL), an aggressive malignancy characterized by uncontrolled growth of CD4+ T-lymphocytes, lymphadenopathy, hypercalcaemia, immunodeficiency and unfavourable prognosis82. HTLV-1 may have a long latent period of several decades after the initial infection. Mechanisms leading to ATL are not well understood; a multi-step leukaemogenic process has been hypothesized, in which the main roles are played by viral genes, their products and host immune status83. High levels of NF-κB, a regulator of immune response against infections, strongly associated with carcinogenesis, have been observed in ATL cells75.
Human immunodeficiency virus (HIV)
HIV is a RNA virus of the Lentivirus genus divided into two strains: HIV-1 and HIV-2. The viral infection is responsible for the AIDS, a disease defined as a set of clinical manifestations due to the depletion of T lymphocyte. HIV is also able to infect other cell types such as lymphocytes, macrophages, microglia and dendritic cells. Patients with HIV showed an increased risk to develop cancers such as KS, non-Hodgkin's lymphoma B-cell, primitive brain lymphoma B-cells and invasive carcinoma of the uterine cervix84. Other HIV/AIDS-related cancers include Hodgkin's disease and cancers of the lung, mouth and digestive system85. Opportunistic infections caused by viruses, bacteria, fungi and parasites are the leading causes of deaths in patients with AIDS (Table IV). The higher risk of developing opportunistic infections, as well as the increased incidence of cancer in HIV-infected patients, is mainly associated with immunosuppression88. The use of highly active antiretroviral therapy (HAART) has led to an increase of life expectancy, progressively transforming HIV into a chronically manageable infection. However, patients treated with HAART showed an increased risk of cancer such as lung, digestive system and liver cancers not necessarily associated with HIV infection89.
| System/organ | Opportunistic pathogen |
|---|---|
| Respiratory system | Pneumocystis carinii |
| Mycobacterium avium | |
| Candida and C. neoformans | |
| Cytomegalovirus (S. pneumoniae, H. influenzae, M. pneumoniae, L. pneumophila and respiratory viruses)* | |
| Digestive system | Candida |
| Herpes simplex | |
| Cytomegalovirus | |
| Central and peripheral nervous system | Cytomegalovirus |
| JC polyomavirus | |
| Toxoplasma gondii | |
| Cryptococcus neoformans | |
| Skin and mucosal surface | Candida |
| Varicella-zoster virus | |
| Herpes simplex | |
| Poxvirus | |
| HPV |
*Patients suffering from AIDS are also subject to infections by micro-organisms that may cause pneumonia and bronchopneumonia in immunocompetent individuals. JC, John Cunningham; C. neoformans, Cryptococcus neoformans; S. pneumonia, Streptococcus pneumonia; H. influenza, Haemophilus influenza; M. pneumonia, Mycoplasma pneumonia; L. pneumophila, Legionella pneumophila; HPV, human papillomavirus. Source: Refs 8687
Hepatitis C virus (HCV)
HCV is a non-cytopathic RNA virus of the family Flaviviridae, and is an important risk factor for HCC development in Western countries. To date, at least six major genotypes of HCV have been identified90. HCV is not able to integrate its genome into host cells because of the absence of reverse transcriptase activity and may cause HCC by indirect pathways such as severe chronic hepatitis, cell death and proliferation which usually progress towards cirrhosis. Furthermore, HCV can evade host immune responses to cause a persistent infection in the liver91. Continuous cycles of death and regeneration in hepatocytes may favour the accumulation of mutations. HCV-infected patients have a higher risk of developing HCC compared with HCV-negative individuals, also depending on the degree of fibrosis and duration of viral infection92.
Bacterial infections and cancer
The effects of bacterial infections on carcinogenesis may depend on many factors, such as the host immune response, promotion of cell proliferation, suppression of apoptosis and production of bacterial toxins93. Some bacterial infections may cause hyperplasia or promote inflammatory response releasing reactive oxygen and nitrogen intermediates which can directly lead to DNA damage94. Bacterial infections may interfere with the fine regulation of host cells signal pathways, which have a central role in cancer development or inhibition. Bacteria generate several effector molecules that interact with the host cells regulating adhesion, modulating cytoskeletal or junctional function and activating specific eukaryotic signalling pathways. These virulence factors can act on cancer promotion, by their direct action on cellular processes93. Suppression of apoptosis is an important step for the development of neoplastic cells, especially during infections caused by intracellular pathogens. This is usually the result of many extracellular stimuli represented by the release of serine protease granzyme B and TNF-α by CD8+ T-cells. High levels of NF-κB can inhibit TNF-α-induced apoptosis95. Bacteria, through the blockage of apoptosis, could facilitate the escape of transformed cells from the destruction mechanisms, thus favouring carcinogenesis96. Escherichia coli cytotoxic necrotizing factor is able to activate Rho family proteins, and Pasteurella multocida toxin, which acts as potent mitogen for quiescent cells and as inducer of anchorage-independent growth, may activate cyclooxygenase-2 (COX-2), an enzyme involved in different stages of carcinogenesis, including the suppression of apoptosis97. Histological alterations (e.g., metaplasia) have been observed before the onset of gastric cancer in individuals with HP infection98. The presence of bacteria could be utilized as a diagnostic marker for cancer: for example, high salivary levels of Capnocytophaga gingivalis, Prevotella melaninogenica and Streptococcus mitis, have been found in cases of oral squamous cell carcinoma, showing diagnostic sensitivity and specificity ≥80 per cent99. The link between bacteria and cancer could be further explained by the regression of lesions after antibiotic treatment, as observed after eradication of HP in patients with gastric cancer100. The association between bacteria and different types of cancer is reported in Table V.
| Bacteria | Tumour | Odds ratio (OR) |
|---|---|---|
| Helicobacter pylori | Gastric cancer | Non-cardia cancers: OR=3.0; 10+ yr before cancer diagnosis OR=5.9 Cardia cancer: OR=1.0101 |
| Streptococcus bovis (Biotype 1) | Colorectal cancer | OR=5.1102 |
| Chlamydia trachomatis | Cervical cancer | OR=2.21103 |
| C. pneumonia | Lung cancer | OR=1.30104 |
| Salmonella Typhi | Gallbladder cancer | OR=4.0105 |
Helicobacter pylori (HP)
Although gastric malignancies may have multifactorial causes (e.g., use of tobacco, low vegetable intake, high dietary salt and nitrate intake), but HP plays a main role in the carcinogenesis as shown by the increased risk of developing gastric cancer in infected individuals106. In 1994, the International Association for Research on Cancer classified HP as Group 1 carcinogen107. The duration of infection and the type of bacterial strain may influence the risk of cancer, and the presence of cagA-positive strains of HP is associated with an increased risk of gastric carcinoma. The cagA gene encodes for one of the major HP virulence proteins, and infection from cagA-positive strains is characterized by increased virulence and inflammation108. The combination of environmental factors, bacterial factors and host immune response may drive the initiation and progression of carcinogenesis (Fig. 3). The precancerous cascade is a progressive process that usually lasts for several decades, but the mechanisms underlying initial DNA damage are still not known. The most plausible hypothesis is that cancer onset could be the result of oxidative stress, as represented by inducible NOS expression due to HP infection109. A reduction in the incidence of gastric cancer and a regression of precancerous lesions have been observed in patients with HP infection after treatment with antioxidant supplements (such as β-carotene, vitamin E and C and selenium)110. Other antioxidants showed protective effects on cancer inhibiting reactive oxygen species production and MAPK signal transduction pathways111112.

- Role of Helicobacter pylori in the pathogenesis of gastric cancer. H. pylori infection may cause a number of diseases, ranging from superficial gastritis to gastric carcinoma. The progression to gastric cancer is mediated by cytokines, genetic polymorphisms, oxidative stress, gastric commensal organisms and alteration of epithelial cells response to acid. Source: Ref 91.
COX-2 is a regulator in immune response and it is often over-expressed in many cancers, especially in colorectal cancer. Chronic induction of COX-2 could favour the survival of transformed cells that otherwise would become apoptotic and die. Therefore, cancer invasiveness could be reduced by inhibition of COX-2 activity113. The use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with a reduced risk of gastric cancer, through both inhibition of COX-2 and NF-κB activity. These findings underlined the role of NF-κB activation as a crucial component in bacteria-associated cancer formation114. Treatment of HP infection may reduce mucosal inflammation, DNA damage and cell turnover and improve gastric acid secretion in patients with previous hypochloridia115. The detection of high levels of HP DNA in liver biopsies of patients with liver chronic diseases, such as HCC, biliary cirrhosis and sclerosing cholangitis, suggested a promoting role of HP in hepatic carcinogenesis116.
Streptococcus infantarius (formerly S. bovis II/1)
Streptococcus infantarius is commonly reported in infections of the central nervous system, osteomyelitis, endocarditis and colon cancer117. However, the link between S. infantarius and cancer is still not clear; the bacterium is occasionally detectable in the normal colonic bacterial flora, but it is increased in faecal material of patients with colorectal cancer. A high percentage of patients with S. infantarius infection also show colorectal adenomatous polyps or carcinoma118. IL-8, a neutrophil and T-cells chemoattractant cytokine, plays a key role in the immune response to S. infantarius and other bacteria. IL-8 can lead to mucosal damage through the production of free radicals and proteolytic enzymes from neutrophils119. S. infantarius infection could be involved in the development of many cancers such as metastatic melanoma lymphoma, pancreas, endometrium, oesophagus, stomach and colon cancer117. However, further research is needed to clarify the link between S. infantarius infection and carcinogenesis.
Chlamydia trachomatis
The consequences of genital C. trachomatis infection are generally represented by urethritis, cervicitis, ectopic pregnancy, infertility, pelvic inflammation, epididymitis, prostatitis and increased risk of HIV infection120. Chlamydial infection causes chronic inflammation with epithelial damage, cytologic cervical atypia and consequent metaplasia, which in turn can increase the risk of cervical cancer121. Invasive squamous cervical cancer has been shown to be associated with serum detection of chlamydial antibodies121.
Chlamydia pneumoniae
Infection with C. pneumoniae, a Gram-negative bacillus, has been associated with an increased risk of lung carcinoma122. A case–control study of chronic C. pneumoniae infection as lung cancer risk factor in smokers showed a significant difference between male patients and their controls for specific C. pneumoniae antibodies, but there were no significant differences between female patients and their control groups123. Chronic C. pneumoniae infection was significantly higher among male patients with lung carcinoma below age 55 than controls, and even in this case, there were no age-related variations to the female sex123. Impairment of lung and bronchial immunity, increased levels of IL-4 and suppression of cellular immunity could facilitate C. pneumoniae lung localization and invasion in smoking subjects. IL-8, also produced by smokers, along with IL-1β and TNF-α, may cause genetic damage124.
Salmonella Typhi
Chronic Salmonella Typhi infection has been found to be strongly associated with gallbladder cancer. Lazcano-Ponce et al125 observed that individuals who became carriers of S. Typhi showed more than eight-fold increased risk of developing gallbladder carcinoma as compared to those who had acute typhoid and cleared the infection. Strom et al126 reported a 12-fold increase in risk of gallbladder cancer in patients with a history of typhoid fever (CI 95% 1.5-598), which unfortunately could not be supported with serological assays. Data from the epidemics of typhus in 1922 in New York and in 1964 in Aberdeen showed that people who contracted typhoid, but did not become carrier, were at lower risk for cancer and chronic typhoid carriers died of hepatobiliary cancer six times more often than the matched controls127.
Parasitic infections and cancer
Opisthorchis viverrini and Schistosoma haematobium can cause liver cholangiocarcinoma and urinary bladder cancer, respectively128. Other reported associations include Clonorchis sinensis and cholangiocarcinoma129, Schistosoma japonicum and HCC or colorectal cancer and Schistosoma mansoni and HCC130 (Table VI).
| Parasites | Tumour | Odds ratio (OR) |
|---|---|---|
| Opisthorchis viverrini | Cholangiocarcinoma | OR=4.39131 |
| Schistosoma haematobium | Urinary bladder cancer | OR=From 2 to 14106 |
| Clonorchis sinensis | Cholangiocarcinoma | OR=6.12131 |
| S. japonicum | HCC, colorectal cancer | Rectal cancer relative risk=8.3132 |
| HCC: OR=3.7; Colon cancer: OR=3.3133 | ||
| Strongyloides stercoralis | Gastrointestinal cancers | OR=6.7134 |
HCC, hepatocellular carcinoma
Strongyloides stercoralis
Strongyloides stercoralis infection is estimated to affect around 100 million people per year worldwide and remains often neglected135. A case–control study showed a high prevalence of S. stercoralis infection in the patients with biliary tract cancer compared to controls136. The presence of S. stercoralis in the duodenum, including the duodenal papilla, may induce various patterns of inflammation, while chronic inflammation of the biliary tract is associated with invading larvae137.
Future treatment and prevention
The focus of research has now moved towards the development of anti-tumour therapeutic vaccines using recombinant viruses such as retroviruses, adenoviruses, lentiviruses (including HIV-1), poxviruses, herpesviruses and adeno-associated viruses. These viruses could be used to transform cells genetically, activate the expression of cancer-specific antigens and infect APCs to increase anti-tumour immune response or for transgene delivery in many cancers138. Some antibiotics and antifungals, able to target specific pathways and genes implicated in cancer, could lead to possible treatment benefit. Antibiotics act trough several mechanisms that include inhibition or regulation of cell wall synthesis, cell metabolism and protein synthesis. Ampicillin, streptomycin sulphate and puromycin dihydrochloride are molecules that specifically target and kill cells. Other anti-tumour antibiotics include bleomycin, enediynes and mitomycin. After oral administration, cefazolin, ciprofloxacin, nitrofurantoin or trimethoprim-sulphamethoxazole showed significant dose-dependent cytotoxicity against bladder cancer cells139. Compounds from filamentous fungi such as Aspergillus, Penicillium and Talaromyces, Macrocyclic trichothecenes, Argemone mexicana L. and a phenazine compound produced by Lactococcus BSN307 showed both antifungal and antitumoural activities. The anti-cancer properties are due to a number of different mechanisms including inhibition of protein, DNA and RNA syntheses, impairment of mitochondrial function and effects on cell division and membranes140.
Conclusions
In general, acute infections may antagonize the development of malignant diseases, whereas chronic infections may induce specific cell changes in the host, increasing the risk of cancer. Mechanisms of carcinogenesis due to infections include cell growth and DNA replication by MAPK pathway, abnormal cell proliferation, inhibition of apoptosis and production of toxins that affect the cell cycle. Acute infectious diseases of childhood were associated to a reduced risk of future development of many types of cancer. Moreover, spontaneous regression associated with bacterial, fungal, viral or protozoan infections has been observed in many types of cancers such as breast cancer, melanoma, neuroblastoma, renal cancer and lymphomas. The research area is wide and further studies are needed to fill the knowledge gap on the link between the infections and the development and progression of cancer. Promising fields of investigation are represented by the development of anti-tumour therapeutic vaccines using recombinant viruses and the assessment of antibiotics and antifungals as possible therapeutic options for cancer.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health. 2016;4:e609-16.
- [Google Scholar]
- Infections and cancer: The “fifty shades of immunity” hypothesis. BMC Cancer. 2017;17:257.
- [Google Scholar]
- Fever and the thermal regulation of immunity: The immune system feels the heat. Nat Rev Immunol. 2015;15:335-49.
- [Google Scholar]
- The consequences of human actions on risks for infectious diseases: A review. Infect Ecol Epidemiol. 2015;5:30048.
- [Google Scholar]
- Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. 2017;2017:2819372.
- [Google Scholar]
- Socioeconomic inequality in concurrent tobacco and alcohol consumption. Asian Pac J Cancer Prev. 2017;18:1913-7.
- [Google Scholar]
- The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-33.
- [Google Scholar]
- Cancer increased after a reduction of infections in the first half of this century in Italy: Etiologic and preventive implications. Eur J Epidemiol. 1998;14:749-54.
- [Google Scholar]
- Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2005;14:1125-31.
- [Google Scholar]
- Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978-1998. Cancer. 2002;95:2380-9.
- [Google Scholar]
- Mumps and ovarian cancer: modern interpretation of an historic association. Cancer Causes Control. 2010;21:1193-201.
- [Google Scholar]
- Inverse relationship between polio incidence in the US and colorectal cancer. In Vivo. 2018;32:1485-9.
- [Google Scholar]
- Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204-18.
- [Google Scholar]
- Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563-82.
- [Google Scholar]
- Pathogen-associated molecular pattern in cancer immunotherapy. Crit Rev Immunol. 2008;28:95-107.
- [Google Scholar]
- PD-L1 and PD-L2 expression correlated genes in non-small-cell lung cancer. Cancer Commun (Lond). 2019;39:30.
- [Google Scholar]
- Acute infections as a means of cancer prevention: Opposing effects to chronic infections? Cancer Detect Prev. 2006;30:83-93.
- [Google Scholar]
- Bacteria and cancer: Cause, coincidence or cure? A review. J Transl Med. 2006;4:14.
- [Google Scholar]
- Spontaneous regression of tumour and the role of microbial infection--Possibilities for cancer treatment. Anticancer Drugs. 2016;27:269-77.
- [Google Scholar]
- The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 1990;29:545-50.
- [Google Scholar]
- Bacteria in cancer therapy: A novel experimental strategy. J Biomed Sci. 2010;17:21.
- [Google Scholar]
- Immunity over inability: The spontaneous regression of cancer. J Nat Sci Biol Med. 2011;2:43-9.
- [Google Scholar]
- Adaptation to HIF1α deletion in hypoxic cancer cells by upregulation of GLUT14 and creatine metabolism. Mol Cancer Res. 2019;17:1531-44.
- [Google Scholar]
- Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8:1596004.
- [Google Scholar]
- Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J Inflamm (Lond). 2014;11:23.
- [Google Scholar]
- The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood. 2016;127:2082-92.
- [Google Scholar]
- Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi144-8.
- [Google Scholar]
- Efficacy of antiviral drug AV2 in the treatment of human papillomavirus-associated precancerous lesions of the uterine cervix: A randomized placebo-controlled clinical trial in Kinshasa, DR Congo.(KINVAV study) Contemp Clin Trials Commun. 2017;8:135-9.
- [Google Scholar]
- Molecular mechanisms of viral oncogenesis in humans. Nat Rev Microbiol. 2018;16:684-98.
- [Google Scholar]
- Effect of hepatitis B virus X gene on the expression level of p53 gene using Hep G2 cell line. Avicenna J Med Biotechnol. 2014;6:3-9.
- [Google Scholar]
- Viral carcinogenesis: Revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405-26.
- [Google Scholar]
- A single nucleotide polymorphism in the Epstein-Barr virus genome is strongly associated with a high risk of nasopharyngeal carcinoma. Chin J Cancer. 2015;34:61.
- [Google Scholar]
- Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS One. 2008;3:e2505.
- [Google Scholar]
- Risk factors for Burkitt lymphoma: a nested case-control study in the UK clinical practice research datalink. Br J Haematol. 2018;181:505-14.
- [Google Scholar]
- Risk factors for Hodgkin's disease by Epstein-Barr virus (EBV) status: prior infection by EBV and other agents. Br J Cancer. 2000;82:1117-21.
- [Google Scholar]
- Analysis of the risk factors of hepatocellular carcinoma in cirrhotic patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi Zhonghua Ganzangbing Zazhi Chin J Hepatol. 2015;23:512-6.
- [Google Scholar]
- Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat. 2018;25:894-903.
- [Google Scholar]
- The risk of cervical cancer associated with specific types of human papillomavirus: a case-control study in a UK population. Int J Cancer. 2011;128:1676-82.
- [Google Scholar]
- Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-56.
- [Google Scholar]
- Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-31.
- [Google Scholar]
- Human papillomavirus infection as a risk factor for anal and perianal skin cancer in a prospective study. Br J Cancer. 2002;87:61-4.
- [Google Scholar]
- Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. J Natl Cancer Inst. 1999;91:156-62.
- [Google Scholar]
- Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer. 2019;19:61.
- [Google Scholar]
- Risk factors for Kaposi's sarcoma in men seropositive for both human herpesvirus 8 and human immunodeficiency virus. AIDS Lond Engl. 2003;17:215-22.
- [Google Scholar]
- Prospective study of Merkel cell polyomavirus and risk of Merkel cell carcinoma. Int J Cancer. 2014;134:844-8.
- [Google Scholar]
- Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int J Cancer. 2000;85:319-24.
- [Google Scholar]
- Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood. 1998;92:3557-61.
- [Google Scholar]
- Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS One. 2008;3:e2505.
- [Google Scholar]
- A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611-22.
- [Google Scholar]
- Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer. 2017;123:1202-11.
- [Google Scholar]
- Epstein-Barr virus from Burkitt Lymphoma biopsies from Africa and South America share novel LMP-1 promoter and gene variations. Sci Rep. 2015;5:16706.
- [Google Scholar]
- EBV-Associated Smooth Muscle Neoplasms: Solid Tumors Arising in the Presence of Immunosuppression and Autoimmune Diseases. Sarcoma. 2008;2008:859407.
- [Google Scholar]
- Risk factors for Hodgkin's lymphoma by EBV status and significance of detection of EBV genomes in serum of patients with EBV-associated Hodgkin's lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S27-32.
- [Google Scholar]
- Epstein-Barr Virus+ Smooth Muscle Tumors as Manifestation of Primary Immunodeficiency Disorders. Front Immunol. 2018;9:368.
- [Google Scholar]
- Epstein-Barr virus is associated with gastric carcinoma: The question is what is the significance? World J Gastroenterol. 2008;14:4347-51.
- [Google Scholar]
- Host and viral genetic variation in HBV-related hepatocellular carcinoma. Front Genet. 2018;9:261.
- [Google Scholar]
- Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15(Suppl):E25-30.
- [Google Scholar]
- The genetic and environmental basis of hepatocellular carcinoma. Discov Med. 2006;6:182-6.
- [Google Scholar]
- Immune cell regulation of liver regeneration and repair. J Immunol Regen Med. 2018;2:1-10.
- [Google Scholar]
- Pancreatic involvement in chronic viral hepatitis. World J Gastroenterol. 2005;11:3508-13.
- [Google Scholar]
- Hepatitis B or C viral infection and risk of pancreatic cancer: A meta-analysis of observational studies. World J Gastroenterol. 2013;19:4234-41.
- [Google Scholar]
- Hepatitis B and C virus infections as possible risk factor for pancreatic adenocarcinoma. Med Hypotheses. 2012;79:678-97.
- [Google Scholar]
- Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621-32.
- [Google Scholar]
- The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-4.
- [Google Scholar]
- Association of Chlamydia trachomatis infection with human papillomavirus (HPV) & cervical intraepithelial neoplasia - A pilot study. Indian J Med Res. 2013;137:533-9.
- [Google Scholar]
- Human herpesvirus type 8-associated large B-cell lymphoma: A nonserous extracavitary variant of primary effusion lymphoma in an HIV-infected man: A case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2016;16:311-21.
- [Google Scholar]
- Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis. 2000;59:672-83.
- [Google Scholar]
- Parvovirus B19 infection in Hashimoto's thyroiditis, papillary thyroid carcinoma, and anaplastic thyroid carcinoma. Thyroid. 2011;21:411-7.
- [Google Scholar]
- HTLV deregulation of the NF-κB pathway: An update on tax and antisense proteins role. Front Microbiol. 2018;9:285.
- [Google Scholar]
- Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species. J Virol. 2010;84:5909-22.
- [Google Scholar]
- High prevalence of human parvovirus infection in patients with malignant tumors. Oncol Lett. 2012;3:635-40.
- [Google Scholar]
- Leukaemogenic mechanism of human T-cell leukaemia virus type I. Rev Med Virol. 2007;17:301-11.
- [Google Scholar]
- HTLV-1 infection and adult T-cell leukemia/lymphoma-a tale of two proteins: Tax and HBZ. Viruses. 2016;8:161.
- [Google Scholar]
- The role of the tumor microenvironment in HIV-associated lymphomas. Biomark Med. 2015;9:473-82.
- [Google Scholar]
- Malignancies in HIV/AIDS: From epidemiology to therapeutic challenges. AIDS. 2014;28:453-65.
- [Google Scholar]
- Opportunistic diseases among HIV-infected patients: a multicenter-nationwide Korean HIV/AIDS cohort study, 2006 to 2013. Korean J Intern Med. 2016;31:953-60.
- [Google Scholar]
- Clinical profile of human immunodeficiency virus patients with opportunistic infections: A descriptive case series study. Int J Appl Basic Med Res. 2015;5:119-123.
- [Google Scholar]
- Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939-45.
- [Google Scholar]
- Hepatitis C virus genotype 6: Virology, epidemiology, genetic variation and clinical implication. World J Gastroenterol WJG. 2014;20:2927-40.
- [Google Scholar]
- Hepatitis C virus and hepatocellular carcinoma: A narrative review. J Clin Transl Hepatol. 2018;6:79-84.
- [Google Scholar]
- Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-7.
- [Google Scholar]
- Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001;171:1-10.
- [Google Scholar]
- The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54.
- [Google Scholar]
- The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27.
- [Google Scholar]
- Efficacy and long-term safety of H.pylori eradication for gastric cancer prevention. Cancers (Basel). 2019;11:593.
- [Google Scholar]
- Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7:59-64.
- [Google Scholar]
- Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347-53.
- [Google Scholar]
- Association between bacteremia due to Streptococcus gallolyticus subsp.gallolyticus (Streptococcus bovis I) and colorectal neoplasia: a case-control study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55:491-6.
- [Google Scholar]
- 2016. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer. Medicine (Baltimore). 95 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4998531/
- Chlamydia pneumoniae infection and risk for lung cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2010;19:1498-505.
- [Google Scholar]
- Salmonella enterica serovar Typhi and gallbladder cancer: a case–control study and meta-analysis. Cancer Med. 2016;5:3310-3235.
- [Google Scholar]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans.Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241.
- [Google Scholar]
- Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815-21.
- [Google Scholar]
- The immune battle against Helicobacter pylori infection: No offense. Trends Microbiol. 2016;24:366-76.
- [Google Scholar]
- Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488-92.
- [Google Scholar]
- Non-steroidal anti-inflammatory drugs in prevention of gastric cancer. World J Gastroenterol. 2006;12:2884-9.
- [Google Scholar]
- Alteration of histological gastritis after cure of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16:1923-32.
- [Google Scholar]
- Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323-4.
- [Google Scholar]
- Group G Streptococcus infective endocarditis in association with colon cancer. Cardiol Res. 2018;9:59-62.
- [Google Scholar]
- Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis) Carcinogenesis. 2004;25:1477-84.
- [Google Scholar]
- Production of cytokines by monocytes, epithelial and endothelial cells activated by Streptococcus bovis. Cytokine. 2000;12:26-31.
- [Google Scholar]
- Chlamydia trachomatis and invasive cervical cancer: A pooled analysis of the IARC multicentric case-control study. Int J Cancer. 2004;111:431-9.
- [Google Scholar]
- Chlamydia pneumoniae infection and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1624-30.
- [Google Scholar]
- Chronic Chlamydophila pneumoniae infection in lung cancer, a risk factor: a case-control study. J Med Microbiol. 2003;52:721-6.
- [Google Scholar]
- Exposure to cigarette smoke and Chlamydia pneumoniae infection in mice: Effect on infectious burden, systemic dissemination and cytokine responses: A pilot study. J Immunotoxicol. 2016;13:77-83.
- [Google Scholar]
- Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349-64.
- [Google Scholar]
- Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer. 1995;76:1747-56.
- [Google Scholar]
- Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet Lond Engl. 1994;343:83-4.
- [Google Scholar]
- Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93-6.
- [Google Scholar]
- Parasite Infection, carcinogenesis and human malignancy. E Bio Medicine. 2017;15:12-23.
- [Google Scholar]
- Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210-21.
- [Google Scholar]
- Schistosoma japonicum and colorectal cancer: an epidemiological study in the People's Republic of China. Int J Cancer. 1984;34:315-8.
- [Google Scholar]
- A matched, case-control study of the association between Schistosoma japonicum and liver and colon cancers, in rural China. Ann Trop Med Parasitol. 2005;99:47-52.
- [Google Scholar]
- Parasitological and immunological diagnosis of Strongyloides stercoralis in patients with gastrointestinal cancer. Scand J Infect Dis. 2008;40:154-8.
- [Google Scholar]
- Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:263-73.
- [Google Scholar]
- Association between Strongyloides stercoralis infection and biliary tract cancer. Parasitol Res. 2007;101:1345-8.
- [Google Scholar]
- Epidemiology of Strongyloides stercoralis infection in Bolivian patients at high risk of complications. PLoS Negl Trop Dis. 2019;13:e0007028.
- [Google Scholar]
- Gene therapies for cancer: Strategies, challenges and successes. J Cell Physiol. 2015;230:259-71.
- [Google Scholar]
- Antitumor activity of common antibiotics against superficial bladder cancer. Urology. 2004;63:457-60.
- [Google Scholar]
- The mechanistic targets of antifungal agents: An overview. Mini Rev Med Chem. 2016;16:555-78.
- [Google Scholar]






