Translate this page into:
Effect of the COVID-19 vaccination on feto-maternal outcomes: A prospective cohort study among Indian pregnant women
For correspondence: Dr Tarundeep Singh, Department of Community Medicine and School of Public Health, Postgraduate Institute of Medical Education and Research, Chandigarh 160 012, India e-mail: tarundeep.singh@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Studies on the effects of COVID-19 vaccination among pregnant women in Asian settings, more specifically in India, are scarce. The present study evaluated the feto-maternal outcomes among Indian pregnant women who received the COVID-19 vaccine.
Methods
A prospective cohort study was undertaken among 430 pregnant women from two primary health centres (PHC) in Chandigarh, India during 2021-2022. The feto-maternal outcomes evaluated in the study included abortions, live birth/stillbirth, term/pre-term/post-term delivery, mode of delivery (normal vaginal/caesarean section/forceps), birth weight and intrauterine growth retardation.
Results
Of the 430 study participants, 295 pregnant women received COVID-19 vaccines, with an uptake rate of 68.6 per cent. Majority of vaccinated women (280- who completed the study) were in their second trimester (133, 47.5%), while 92 (32.9%) were in their third, and 55 (19.6%) were in their first trimester when they were enrolled in the study. Neonatal intensive care unit (NICU) admission was significantly lower among the vaccinated pregnant women, while other feto-maternal and neonatal outcomes were similar between vaccinated and unvaccinated pregnant women.
Interpretation & conclusions
The findings of this study suggest COVID-19 vaccination in pregnant women in India might be safe, in terms of feto-maternal outcomes
Keywords
COVID-19 vaccine
Covishield
fetal outcome
India
pregnancy
Vaccines are an effective way of specific protection against diseases and COVID-19 vaccines have reduced the risk and averted mortality caused by this novel infection1,2. It has also reduced the morbidity in terms of hospitalizations and intensive care unit (ICU) admissions owing to COVID-193,4. The safety profile of these vaccines (short-term and long-term) is currently under study, and as data emerges5, the recommendations are being updated accordingly. One group of adults, pregnant women, were not included in the initial COVID-19 vaccine trials. Pregnant women infected with COVID-19 were reported to have significantly higher adverse outcomes than non-pregnant women, indicating the greater vulnerability of this specific population6. The presence of comorbidities and other maternal risk factors compound such risks7. Later, considering the safety and efficacy of these vaccines in other groups of the population, COVID-19 vaccines were recommended for pregnant women by various countries at different time points. Subsequently, studies on the safety profile of the COVID-19 vaccines among pregnant women were undertaken, with most of the studies reporting favourable feto-maternal outcomes7-9.
Vaccine safety has been a major concern in COVID-19 vaccination programme10,11, and a significant determinant for vaccine acceptance among pregnant women12. Studies on the effects of COVID-19 vaccination among pregnant women in Asian settings, more specifically in India, are scarce. India approved COVID-19 vaccination for pregnant women from the first week of July 202113. However, for ChAdOx1 nCoV (COVISHIELD), a recombinant adenovirus vector vaccine, the safety data is limited among pregnant women. This is significant since it is the most widely used and available vaccine in India14. A cohort study from India comparing the adverse events following COVID-19 immunization (AEFIs) till 28 days post-vaccination among the pregnant and non-pregnant women found the vaccine to be safe15. Yet, no study on pregnant women’s long-term feto-maternal outcomes was available from India. Active surveillance is necessary to gauge the feto-maternal outcomes and safety profile of the COVID-19 vaccines among pregnant women16. In light of the above background, the present study was conducted in primary care settings to evaluate the feto-maternal outcomes among pregnant women who were vaccinated with the COVID-19 vaccine from India.
Materials & Methods
A prospective cohort study was undertaken among pregnant women who were registered with two primary health centres (PHC) in Chandigarh, which are the field practice area of the department of Community Medicine and School of Public Health, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. Ethical approval for the study was obtained from the Institutional Ethics Committee of PGIMER, Chandigarh. The study settings of the PHCs have coverage of 46,204 population and a yearly birth cohort of ∼850. The study enrolled all registered and willing pregnant women >18 yr in any trimester who consented to participate in the study. Pregnant women who had taken the first dose of the COVID-19 vaccine before the date of the last menstrual period (LMP), and awaiting the second dose of COVID-19 vaccination, were also included in the study. Pregnant women who had a history of an allergic or anaphylactic reaction to a previous dose of COVID-19 vaccine, allergic to any other vaccines or to injectable therapies, drugs and food items were excluded from the study and vaccination. Women who had COVID-19 infection currently or within the past 12 wk and women who were treated with anti-COVID-19 monoclonal antibodies or convalescent plasma were also excluded17. A written informed consent was obtained from all the pregnant women before including them in the study.
The enrolment period was from July 10 to October 31, 2021. The follow up period was until the pregnancy outcome and four weeks after the delivery for (pregnancies with live births). All pregnant women were counselled and offered the COVID-19 vaccination by the respective area’s Auxiliary nurse midwife (ANM) at the PHC. The exposure group consisted of women who accepted and were vaccinated against COVID-19, while the other women who did not vaccinate against COVID-19 were included as controls. The baseline socio-demographic, clinical and vaccination-related details were recorded when the participants were enrolled in the study. The pregnant women were followed up during the pregnancy by the ANMs for any complications during pregnancy and fetal outcomes.
The feto-maternal outcomes evaluated in the study included abortions, live birth/stillbirth, term/pre-term/post-term delivery, mode of delivery (normal vaginal/caesarean section/forceps), birth weight (low birth weight and small for gestational age), and intrauterine growth retardation. Fetal growth restriction was defined as ‘a fetus with a sonographic estimation of fetal weight below the tenth percentile for a given gestational age, with increasing specificity for adverse perinatal outcomes below the third percentile’18. Further, neonatal outcomes such as congenital anomalies, Appearance, Pulse, Grimace, Activity, and Respiration (Apgar) scores, neonatal intensive care unit (NICU) admission, infections and neonatal mortality were also evaluated till four weeks after delivery. Global alignment of immunization safety assessment in pregnancy (GAIA) tool was used to define the feto-maternal outcomes18. Operational definitions of all the variables and outcomes of the study were compiled and shared with the data collectors to improve data quality and management.
Statistical analysis
Data analysis was undertaken in SPSS v26.0 (IBM Corp., SPSS Statistics, Armonk, USA). Frequencies and proportions were calculated for categorical variables. The median [interquartile range (IQR)] was calculated for the continuous variables. Univariate analysis between the outcomes and the COVID-19 vaccination status was undertaken by chi-square or Fishers exact and Mann-Whitney tests. Multi-variate analysis was undertaken after adjusting for the variables found significant (P<0.05) in the univariate analysis.
Results & Discussion
The total number of eligible pregnant women during the study period was 466 of whom 430 were included in the study. Of the 430 study participants, 295 pregnant women were vaccinated with COVID-19 vaccine during their pregnancy, and 135 did not take up the vaccination, which gave a COVID-19 vaccination uptake rate of 68.6 per cent among the studied pregnant women. Among the 280 pregnant women in the vaccination group, 6 (2.14%) reported receiving the first dose of vaccination before LMP. Due to migration, 21 participants (15 in the exposed group and 6 in the control group) were lost to follow up (4.9%) (Figure).
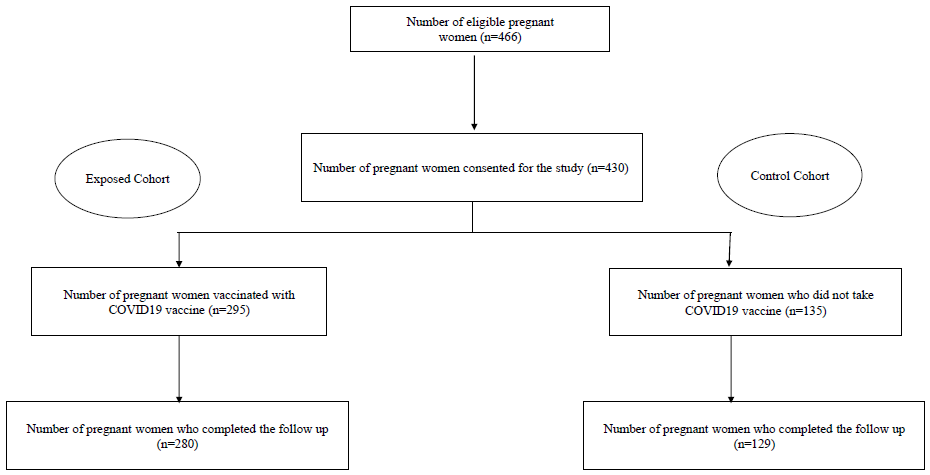
- Enrolment and follow up of the study participants.
The socio-demographic characteristics of the COVID-19 vaccinated and unvaccinated pregnant women are enumerated in Table I. Among the COVID-19 vaccinated women during the study (274), the majority took the vaccine in the second trimester 130(47.4%), while 92 (33.6%) were in the third trimester, and 52 (19%) were in the first trimester of their pregnancy. By the end of their pregnancy, 44.3 per cent (124) completed the two-dose schedule, while 55.7 per cent (156) had one dose of the COVID-19 vaccine. Among the demographic characteristics, education was significantly associated with vaccination status (Table I). Among the women included in the study, 17 women had anemia (9 in vaccinated group and 8 in un-vaccinated group), 5 women had gestational diabetes (3 in vaccinated group and 2 in un-vaccinated group), 1 woman had oligohydramnios (vaccinated group), 12 cases of pregnancy induced hypertension (7 in the vaccinated and 5 in unvaccinated group), 1 case of pre-eclampsia (in the unvaccinated group), which were identified during their ante-natal period and the incidence was not significantly different between the vaccinated and un-vaccinated women (P>0.05). None of the women had ante-partum hemorrhage, fetal growth restriction or other ante-natal complications. Three confirmed cases of breakthrough infection of COVID-19 were reported by the vaccinated pregnant women (1.07%) during the follow up period. However, it is also pertinent to point out that only 55 vaccinated pregnant women had the record of COVID-19 testing for the follow up period of the study.
| Vaccinated pregnant women (Exposed cohort) | Unvaccinated pregnant women (Control cohort) |
Unadjusted P value |
|||
|---|---|---|---|---|---|
| n=280 | % | n=129 | % | ||
| Age (yr); median (IQR) | 25 (22,28) | - | 25 (22,28) | - | 0.781 |
| Education | |||||
| Illiterate | 31 | 11.1 | 25 | 19.8 | 0.014 |
| Higher secondary or below | 203 | 72.5 | 74 | 58.7 | |
| Graduate & above | 46 | 16.4 | 27 | 21.4 | |
| Occupation | |||||
| Home maker | 272 | 97.1 | 121 | 93.8 | 0.105† |
| Private job | 8 | 2.9 | 6 | 4.7 | |
| Government job | 0 | 0 | 2 | 1.6 | |
| Below poverty line (<1479) | 41 | 15.9 | 28 | 23.5 | 0.075 |
| Socioeconomic status | |||||
| I | 14 | 5.4 | 6 | 5 | 0.537 |
| II | 77 | 29.8 | 29 | 24.4 | |
| III | 73 | 28.3 | 35 | 29.4 | |
| IV | 76 | 29.5 | 35 | 29.4 | |
| V | 18 | 7 | 14 | 11.8 | |
| Consanguinity (marriage) | 9 | 3.2 | 0 | 0 | 0.063† |
| Migrant | |||||
| Yes | 152 | 54.3 | 57 | 44.2 | 0.058 |
| No | 128 | 45.7 | 72 | 55.8 | |
| Gravida | n=278 | n=120 | |||
| Primi | 101 | 36.3 | 46 | 38.3 | 0.704 |
| Multi | 177 | 63.7 | 74 | 61.7 | |
| History of abortions | |||||
| Yes | 54 | 19.3 | 18 | 14 | 0.188 |
| No | 226 | 80.7 | 111 | 86 | |
| Tobacco usage | |||||
| Yes | 1 | 0.4 | 0 | 0 | 1 |
| No | 279 | 99.6 | 129 | 100 | |
| Pre-existing diseases | |||||
| Hyperthyroid | 1 | 0.4 | 0 | 0 | 1† |
| Diabetes | 0 | 0 | 1 | 0.8 | 0.315† |
| Epilepsy | 1 | 0.4 | 1 | 0.8 | 0.532† |
| Allergies | 0 | 0 | 1 | 0.8 | 0.315† |
| Hypertension | 16 | 5.7 | 6 | 4.7 | 0.658† |
| COVID-19 disease history | n=278 | n=126 | |||
| Yes | 1 | 0.4 | 2 | 1.6 | 0.231† |
| No | 277 | 278 | 124 | 98.4 | |
| Trimester of COVID-19 vaccination | (n=274) | (n=274) | |||
| First | 52 | 19 | - | - | - |
| Second | 130 | 47.4 | - | - | |
| Third | 92 | 33.6 | - | - | |
| Completed two doses of COVID-19 vaccine | |||||
| Yes | 124 | 44.3 | - | - | - |
| No | 156 | 55.7 | - | - | - |
| Place of delivery | |||||
| Public hospital | 262 | 96 | 116 | 90.6 | 0.07 |
| Private hospital | 10 | 3.7 | 11 | 8.6 | |
| Home | 1 | 0.4 | 1 | 0.8 | |
NICU admissions were significantly higher among neonates of unvaccinated mothers than those vaccinated against COVID-19, which was also maintained in the adjusted analysis (adjusted for education) (R2=0.040, P=0.035). While small for gestational age (SGA) appeared to have higher incidence rate among the unvaccinated mothers than those vaccinated against COVID-19 in univariate analysis, no such association was identified after adjusted analysis. Overall, the other specific feto-maternal outcomes were not significantly different between the vaccinated and unvaccinated pregnant women (Table II).
| Vaccinated pregnant women (Exposed cohort) | Unvaccinated pregnant women (Control cohort) |
Unadjusted P value |
|||
|---|---|---|---|---|---|
| n=280 | % | n=129 | % | ||
| Adverse feto-maternal outcome (any) | 118 | 42.1 | 60 | 46.5 | 0.408 |
| Abortion | 3 | 1.1 | 1 | 0.8 | 1† |
| Outcome | n=277 | n=128 | |||
| Live birth | 273 | 98.6 | 128 | 100 | 0.313† |
| Stillbirth | 4 | 1.4 | 0 | 0 | |
| Delivery | |||||
| Term | 216 | 78 | 92 | 71.9 | 0.405 |
| Pre-term | 45 | 16.2 | 27 | 21.1 | |
| Post-term | 16 | 5.8 | 9 | 7 | |
| Mode of delivery | |||||
| NVD | 198 | 71.5 | 98 | 76.6 | 0.284 |
| LSCS | 79 | 28.5 | 30 | 23.4 | |
| Birth weight (kg); median (IQR) | 2.7 (2.5,3) | - | 2.7 (2.4,3) | - | 0.617 |
| Low birth weight | 50 | 18.4 | 32 | 25 | 0.126 |
| SGA | 0 | 0 | 3 | 2.3 | 0.031† |
| Apgar Score | |||||
| 1 min | 8 (8,8) | - | 8 (8,8) | - | 0.313 |
| 5 min | 9 (9,9) | - | 9 (9,9) | - | 0.355 |
| NICU admission | 3$ | 1.1 | 7# | 5.5 | 0.013† |
| Fever | 12 | 4.3 | 3 | 2.3 | 0.407† |
| Seizure | 2 | 0.7 | 0 | 0 | 1† |
| Shock | 2 | 0.7 | 0 | 0 | 1† |
| Rash | 11 | 4 | 3 | 2.3 | 0.562† |
| Diarrhoea | 9 | 3.2 | 1 | 0.8 | 0.181† |
| Pneumonia | 3 | 1.1 | 0 | 0 | 0.555† |
| Pathological jaundice | 12 | 4.3 | 8 | 6.3 | 0.408 |
| Feed intolerance | 1 | 0.4 | 0 | 0 | 1† |
| Respiratory distress | 1 | 0.4 | 1 | 0.8 | 0.533† |
| Congenital anomaly | 1 | 0.4 | 0 | 0 | 1† |
| Any neonatal infections | 4 | 1.4 | 0 | 0 | 0.313† |
| Neonatal mortality | 3 | 1.1 | 1 | 0.8 | 1† |
| Failure to thrive | 1 | 0.4 | 1 | 0.8 | 0.533† |
| Renal Failure | 1 | 0.4 | 0 | 0 | 1† |
Newer vaccines in pregnancy are a significant concern in terms of acceptability and safety. The current study, which is the first prospective cohort study on the feto-maternal safety profile of the Covishield vaccine among pregnant women from India, revealed no significantly different rates of adverse feto-maternal outcomes. This is in line with the findings of previous studies from other parts of the world and vaccines9. Previous studies have also reported that the pregnancy outcomes were not exceeding the background rates7. In the present study, prematurity and SGA were not significantly different between the vaccinated and unvaccinated participants after adjustment for multiple factors. This was also echoed in the previous studies19-21. Studies reporting lower rates of pre-term and adverse Apgar scores among the COVID-19 vaccinated pregnant women, indicating the protective nature of the COVID-19 vaccines against adverse feto-maternal outcomes, are also available22. The risk of abortions was reported to be similar between the vaccinated and non-vaccinated pregnant women8, which was also observed in the present study. An analysis of the Individual Case Safety Reports (ICSRs) from the EudraVigilance database revealed that mRNA vaccines had significantly lower odds of miscarriage than the Oxford-AstraZeneca vaccines23. Covishield vaccines used in the present study are closer to the Oxford-AstraZeneca vaccines in Europe. In a previous study, the 28-days AEFIs following the COVID-19 vaccination was reported to be 76.5 per cent among the pregnant women (belonging to the current cohort)15, which was found to be statistically similar to the AEFI rate reported among the non-pregnant women15.
The incidence of neonatal mortality and stillbirths was 1.1-1.4 per cent among the vaccinated pregnant women of the index study. In the current settings, COVID-19 vaccination might be protective against NICU admissions among the study population, while others reported no such association9,22. Tripathy et al22 in their retrospective study from Odisha reported a significantly higher proportion of preterm deliveries and vaginal deliveries among the unvaccinated pregnant women, than the (COVID-19) vaccinated women, while other obstetric outcomes were similar24. Fetal and neonatal outcomes such as SGA and NICU admissions were not affected by vaccination in their study. In a retrospective study from Gujarat, pre-maturity and NICU admission were significantly higher among the unvaccinated pregnant women25. Overall maternal complications rate of 2.53 per cent and 6.57 per cent with Astra-Zeneca and Janssen vaccinees were reported from Brazil, while neonatal mortality was 0.06 per cent and 0 per cent, respectively26. In a study from Romania, no significant difference in the maternal and neonatal outcomes was reported among the pregnant women who were vaccinated with Ad26.COV2.S (Janssen) vaccine (33.6% of the total vaccinees) when compared with the unvaccinated pregnant women27. Other studies which included low proportion of the pregnant women vaccinated with viral vector vaccines also reported no significant increase in adverse effects on SGA, prematurity, NICU admissions20, and spontaneous abortions28,29. Estimates from the previous studies had revealed that neonatal mortality and stillbirths were significantly lower among the COVID-19 vaccinated women with mRNA vaccines19. In contrast, the present study reported no such relationship between Covishield and neonatal mortality.
A per our knowledge, this is the first analytical, prospective study on the feto-maternal outcomes of the COVID-19 vaccine in a pragmatic, primary care setting in India. However, the following limitations warrant a cautious interpretation of the findings of the study including inadequate power (∼13%) owing to small sample size [for a risk of any adverse feto-maternal outcome of 42.1 per cent in the exposed (vaccinated) and 46.5 per cent in the unexposed (non-vaccinated) pregnant women, at 95 per cent confidence interval, the power came to be 12.96%], disproportionate exposed and control cohorts with higher pregnant women in the exposed cohort than the control group, administration of only Covishield vaccines (policy decision) in the public health settings of the study area hence the findings do not apply to BBV152 (Covaxin) beneficiaries, selection bias (since participants were only from two PHCs selected by non-probability sampling) and lack of generalizability to other States of India. A probable significant and positive association between the vaccination status and the NICU admission must be interpreted cautiously owing to the following:(i) wherever documentation was not available, NICU admissions and clinical manifestations were recorded based on the history of mother/primary informant;(ii) limited sample size and inadequate power of the study. Outcomes were studied only for a maximum period till 28-days following delivery, hence, long term effects of the vaccination on the children (e.g., post-neonatal hospital admissions, COVID-19 infections, infant mortality rate, growth, development) and the women (fertility, future pregnancy) could not be assessed, which is another limitation.
Overall, no significant difference in the feto-maternal and neonatal outcomes was reported among the covishield-vaccinated pregnant women from Chandigarh, India. Covishield might be protective against NICU admissions in the study settings. However, further multi-centric studies with adequate sample size and including other vaccines in India, such as the Covaxin administered to pregnant women and the impact of the booster doses in pregnant women, must be undertaken to improve the validity of the findings from the current study. Also, a study on the follow up data from the current vaccinated cohort may be undertaken to shed light on the long-term effects of the COVID-19 vaccination during pregnancy on the women and the children.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Impact of vaccination on risk of COVID-19–related mortality. Available from: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/science/data-review/vaccines.html, accessed on October 2, 2023.
- Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect Dis. 2022;22:1293-302.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10:873596.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The impact of vaccination on Coronavirus Disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73:2257-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 advice for the public: Getting vaccinated. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice, accessed on October 29, 2024.
- Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;370:m3320.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety of COVID-19 vaccines during pregnancy: A systematic review and meta-analysis. Vaccine. 2023;41:3688-700.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The risk of miscarriage following COVID-19 vaccination: A systematic review and meta-analysis. Hum Reprod. 2023;38:840-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Associations of COVID-19 vaccination during pregnancy with adverse neonatal and maternal outcomes: A systematic review and meta-analysis. Front Public Health. 2023;11:1044031.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnant women’s perceptions of the COVID-19 vaccine: A French survey. PLoS One. 2022;17:e0263512.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197-211.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Factors affecting COVID-19 vaccine acceptance among pregnant women: A cross sectional study from Abha City, Saudi Arabia. Vaccines (Basel). 2023;11:1463.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnant Women now eligible for COVID-19 Vaccination. Available from: https://www.pib.gov.in/PressReleasePage.aspx?PRID=1732312, accessed July 10, 2024.
- Effectiveness of Covishield vaccine in preventing Covid-19 - A test-negative case-control study. Vaccine Netherlands. 2022;40:3294-7.
- [Google Scholar]
- COVID-19 vaccination uptake and adverse events following COVID-19 immunization in pregnant women in Northern India: A prospective, comparative, cohort study. J Rural Med. 2022;17:228-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety of COVID-19 vaccines, their components or their platforms for pregnant women: A rapid review. Vaccine. 2021;39:5891-908.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ministry of Health and Family Welfare. Government of India. Operational Guidance for COVID-19 Vaccination of Pregnant Women. 2021. Available from: https://www.mohfw.gov.in/pdf/OperationalGuidanceforCOVID19vaccinationofPregnantWoman.pdf, accessed on October 2, 2023.
- Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA). Available from: https://brightoncollaboration.org/global-alignment-of-immunization-safety-assessment-in-pregnancy-gaia/#, accessed on October 29, 2024.
- Effect of COVID-19 vaccination and booster on maternal-fetal outcomes: A retrospective cohort study. Lancet Digit Health. 2023;5:e594-e606.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327:1469-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176:470-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Systematic review and meta-analysis of neonatal outcomes of COVID-19 vaccination in pregnancy. Pediatr Res. 2023;94:34-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Maternal, fetal and neonatal outcomes among pregnant women receiving COVID-19 vaccination: The preg-co-vax study. Front Immunol. 2022;13:965171.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of Covid-19 Vaccination during pregnancy on the obstetric and neonatal outcomes in a tertiary health care center. J Mother Child. 2023;27:72-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How safe is COVID-19 vaccination among pregnant women and its outcome - A hospital-based retrospective study in Indian population. J Family Med Prim Care. 2023;12:2140-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adverse events of COVID-19 vaccines in pregnant and postpartum women in Brazil: A cross-sectional study. PLoS One. 2023;18:e0280284.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunogenicity following administration of BNT162b2 and Ad26.COV2.S COVID-19 vaccines in the pregnant population during the third trimester. Viruses. 2022;14:307.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385:2008-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






