Translate this page into:
Effect of multilevel lower-limb botulinum injections & intensive physical therapy on children with cerebral palsy
Reprint requests: Dr Monica Juneja, C-77 South Extension-II, New Delhi 110 049, India e-mail: drmonicajuneja@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Botulinum toxin is considered as an effective treatment for spasticity in children with cerebral palsy (CP). However, there are only a few long-term studies, and the effects on motor function have been inconclusive. Moreover, due to its high cost and need for intensive post-injection therapy, utility in context of developing nations has not been established. This retrospective study was undertaken to assess the long term effects of botulinum toxin-A with physical therapy in children with CP.
Methods:
This retrospective study was conducted at a tertiary care centre in India, where a limited supply of botulinum toxin was introduced in the year 2009. It was used in a selective group of patients with CP along with intensive physical therapies. All children who received lower-limb botulinum injections over a 42-month period were analyzed. For evaluation of treatment effect, the measurement at 1st pre-injection assessment and the last measurements, i.e. 12 wk after last injection received by that child were compared.
Results:
Twenty nine patients (20 males, median age 51 months) received 69 sessions of botulinum toxin injections in the lower limbs over a 42-month period. Thirteen patients were diplegic, 10 were quadriplegic, five were triplegic and one was hemiplegic. There was a significant improvement in pre- and post-injection scores on Observational Gait Scale (right side 7.1±3.6 to 10.7±3.7, left side 6.7±3.5 to 9.9±3.4), Gross Motor Function Measure Scale (47.9±17.7 to 67.6±17.2), Modified Ashworth Scale, passive range of motion and Gross Motor Function Classification System. Most of the patients showed gain in motor milestones as well.
Interpretation & conclusions:
Our results showed that judicious use of botulinum injections along with intensive physio/occupational therapies could yield good results in children with CP.
Keywords
Botulinum
cerebral palsy
intensive therapy
lower limbs
physical therapy
spasticity
Cerebral palsy (CP) is a group of permanent disorders of the development of movement and posture, causing activity limitations attributed to non-progressive disturbances that occurred in the developing foetal or infant brain. It is often associated with disturbances of sensation, cognition, communication, perception, seizure disorder, behaviour and secondary musculoskeletal problems1. CP is a common problem, and it has worldwide incidence of approximately 2-2.5 cases per 1000 live births2. CP is classified physiologically on the basis of predominant tone into spastic, choreoathetoid and ataxic types, with spastic type being the most common accounting for 80 per cent of the cases. Spastic CP is further classified based on the topography, i.e. hemiplegia, quadriplegia, etc3.
A multidisciplinary team is required for the management of CP and its associated problems though physiotherapy remains the mainstay of management in young children with CP3. For controlling spasticity, several pharmacological and surgical treatments are available including oral medications such as baclofen, diazepam, tizanidine and dantrolene; neuromuscular-blocking agents such as botulinum toxins A and B; chemical denervation using phenol injections; selective dorsal rhizotomy and intrathecal baclofen3. However, the treatment is complex and there is no standardized approach, and often, the outcomes are unsatisfactory.
Botulinum toxin-A was introduced around two decades back for controlling localized/segmental spasticity in children with CP. Since then, several studies have been published on this costly intervention from Western countries, showing variable effects on spasticity reduction and functional improvement4. Systematic review of literature has concluded that it results in significant reduction in spasticity scores at two, four weeks and three months after treatment5. With respect to the functional outcomes in lower limbs, additional use of botulinum toxin-A with usual therapy or physiotherapy has been shown to have a positive effect on walking at 12 and 24 week. However, studies comparing botulinum toxin-A alone or botulinum toxin-A plus casting versus casting alone have shown no difference between the groups56. Regarding upper limbs, systematic review and meta-analysis suggest that botulinum toxin-A when used along with occupational therapy is more effective than occupational therapy alone in reducing impairment, improving activity level outcomes and goal achievement measured at three and six months after injections78.
Although botulinum toxin-A has been established as an effective therapy in CP, many questions still need to be answered about this intervention. It has been shown to reduce muscle tone, but the effects on motor function in the lower limbs have been inconclusive7. Most trials on the use of botulinum toxin-A in the lower limb have been published with a relatively shorter follow up (maximum of one year). Studies to assess the efficacy of long-term use of botulinum toxin-A in children with CP are very few9101112131415. Moreover, the feasibility and efficacy of this treatment for children with CP in the developing countries has not been studied; hence, this study was planned to assess the long-term effect of botulinum toxin-A injections with physical therapy in children with CP.
Material & Methods
This retrospective study was conducted (July 2009 - December 2012) at the child development centre of Maulana Azad Medical College and Associated LN Hospital, New Delhi, India. The child development centre is a referral centre for children with developmental and behavioural disorders and has a team of developmental paediatricians, occupational therapists, physiotherapists, speech therapists, special educators and clinical psychologists, to provide comprehensive services to these children. Every year around 800 new cases are being enrolled at this centre, with around 170 patients having CP16. Botulinum injections were introduced in the care of children with CP at this centre in the year 2009; however, due to its high cost, supply was limited.
Selection of patients: A team of two developmental paediatricians and two physio/occupational therapists at child development centre were involved in assessing patients with CP for the selection of patients. The following guidelines were used for selection of cases: (i) presence of spasticity interfering with the functioning of the child, e.g., toe walking, scissoring and crouch gait; (ii) children having reasonably good cognition, so as to actively follow and comply with the physical therapy; (iii) age 2-12 yr; (iv) weight ≥10 kg if child was between 2 and 4 yr of age and ≥14 kg if older; (v) absence of fixed contractures; (vi) power ≥3/5 in the target muscles, by manual muscle charting; and (vii) family willing to visit the centre thrice a week on a long-term basis for regular therapy and periodic assessment and also willing to carry out intensive therapy at home.
Once a child was considered eligible for botulinum injections, he/ she was enrolled for intensive conventional occupational therapy and physiotherapy for a minimum of 1-2 months duration. This was done to see the response to intensive centre-based therapy as well as address the issue of compliance with the intensive therapy regime, which would follow the injections. The study was approved by the Institutional Ethical Committee of Maulana Azad Medical College. Written informed consent was taken from the parents for the treatment.
Pre-injection evaluation: All the children underwent detailed evaluation before the injections, using various subjective and objective measures by a physio/occupational therapist, and findings were discussed and reconfirmed by the team of physio/occupational therapist(s) and developmental paediatrician. Repeat assessments were done by the physio/occupational therapist at two weeks and 12 wk after injections in all children. Assessment was also repeated at 4-6 months or later for planning next injection.
The objective measures included: (i) Modified Ashworth Scale for grading spasticity17; (ii) Gross Motor Function Measure Scale (GMFM) for noting the level of achievement of gross motor milestones18; (iii) Gross Motor Function Classification System (GMFCS)19; (iv) Modified Physician Rating Scale for observation-based gait analysis20; and (v) Range of motion using a goniometer for adductors, hamstrings (popliteal angle) and gastrocnemius (ankle dorsiflexion angle with knee extended)21.
Subjective assessment: A detailed subjective assessment was aimed at functional assessment of the child in various developmental positions. The child was asked to assume developmental positions such as cross-legged sitting, long sitting, short sitting, quadruped, kneeling, standing and walking in parallel bar/walking independently. In addition to these, the child was asked to perform various transitions to note his control over body movements. These included rolling, getting up from supine, quadruped to sitting, crawling patterns, getting up from the floor and getting up from chair. In all these static positions and transition patterns, the child's ability to move in and out of the position, control over movement pattern, abnormal positions or patterns acquired by the child, such as log rolling, bunny hopping, W-sitting, sacral sitting, crouch, talipes equinus and genu recurvatum, associated movements and any asymmetrical movements or patterns were noted. Video recording of the functional movement patterns of the child was also done at the time of each assessment and compared to the previous assessments. The information was entered in a pre-designed proforma.
Injection procedure: Botulinum toxin-A (BOTOX, Allergan, USA) injection was used after diluting it to a concentration of 50 units/ml. The injections were administered in a dose of 2-5 U/kg/muscle with a maximum dose of 50 U/muscle and a maximum total dose of 25 U/kg/session (maximum 400 U/session). The dosages depended on the muscle size, functional assessment and individual treatment goals.
The muscles of the lower limbs, namely, gastrocnemius, soleus, medial hamstrings and adductors were mainly targeted. Other muscles occasionally targeted included iliacus, psoas, tibialis anterior and tibialis posterior, lateral hamstrings and rectus femoris. Injections were given using manual method of muscle localization (except for psoas, for which electromyographic guidance was used). Local anaesthesia in the form of Eutectic Mixture of Local Anesthetics (EMLA) was used in a few initial sessions; however, it was stopped later because of suboptimal response. Subsequently, the injections were given under short dissociative anaesthesia using ketamine.
Post-injection therapy: Immediately, after botulinum toxin-A injections, the injected muscles were stretched to ensure wide dissemination of the injection into the entire muscle belly. This was repeated for next 3-5 days.
After two weeks, Plaster of Paris casting of the lower limb was done if active ankle dorsiflexion was not possible till the neutral position. In general, above knee cast was applied to maintain neutral knee extension with ankle at neutral position, for initial two weeks. Subsequently, serial casting was done with increasing ankle dorsiflexion (5-10° at a time for two weeks), with total duration of cast application being six weeks.
Once the cast was removed, the child was again taken up for regular intensive standardized therapy comprising of stretching, strengthening (especially of the agonist & antagonist muscles and truncal muscles), gait training and balance and coordination training. As the child's status improved or the child achieved a milestone, the therapy was customized to suit the needs of the particular child. The therapy session lasting for 60-90 min each was given thrice a week in the centre. The parents were also trained to administer the same program at home, 2-3 times a day. Orthoses specific to their needs were prescribed to all the children.
Statistical analysis: The patient information was transferred from the proforma to a Microsoft Excel and analyzed using SPSS version 10 (SPSS Inc., Chicago, IL, USA). For evaluation of the treatment effect, the grades/measurement at 1st pre-injection assessment and the last measurements, i.e. 12 wk after last injection were compared. Wilcoxon signed-rank test was used for comparing continuous data.
Results
Overall, 29 patients (20 males, 9 females) received botulinum toxin-A injections in the lower limbs over a 42-month period (July 2009 to December 2012). The median age of the patients was 51 months with range of 27 to 84 months. Thirteen patients were diplegic, 10 were quadriplegic, five were triplegic and one was hemiplegic. Two patients had mixed CP, rest all were spastic. Seven patients had epilepsy, four patients had mild mental retardation, 10 had squint and one patient had hearing impairment. The baseline GMFCS level was I in one, II in seven, III in eight, IV in 12 and V in one patient. Table I shows the baseline grade of tone on Modified Ashworth Scale in various muscle groups.
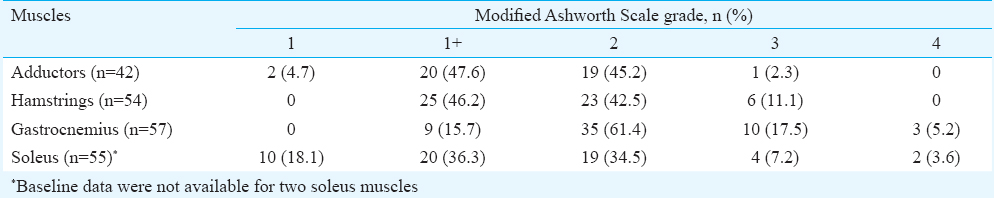
Overall, 69 sessions of injection botulinum toxin-A were held. Twenty two patients had two or more sessions of botulinum toxin-A injections with maximum number of sessions being six for one patient. The median interval between the injection sessions was 7.5 months with a range of 3-24 months. Overall, 42 adductors were injected in 21 patients, 54 medial hamstrings were injected in 27 patients and 57 gastrocnemius and soleus were injected in the 29 patients. Eleven patients received injections in other muscles of the lower limbs on one or more occasions. Eight patients received injections in the upper limbs also (data not shown). The mean dose of botulinum toxin-A injected per session was 17.61±3.81 U/kg. The median interval between the initial and the last assessment (three months after the last dose) was 13 months with range of 3-39 months. The pre- and post-injection scores/levels of various objective measures are shown in Table II. The difference in all the measures was highly significant, except for the range of motion at ankle joint. The gain in milestones is given in Table III.
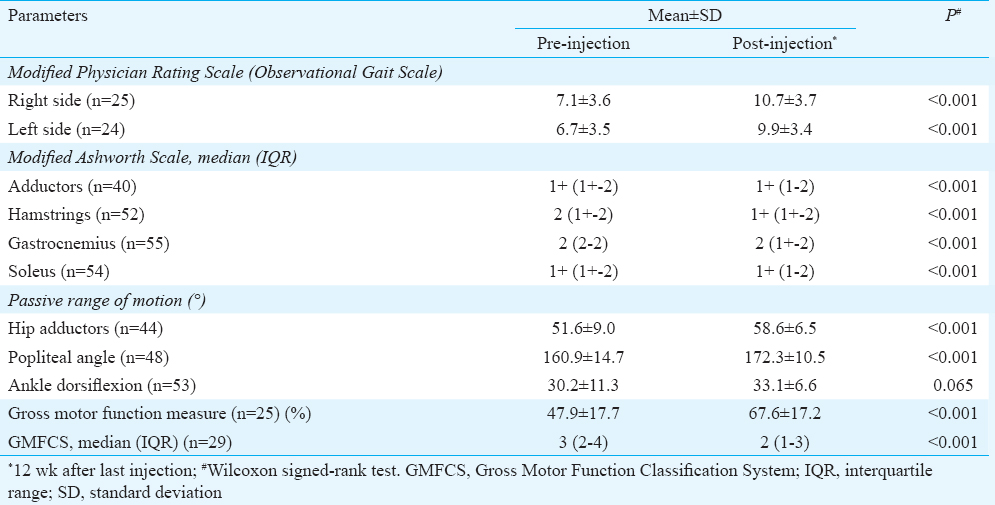
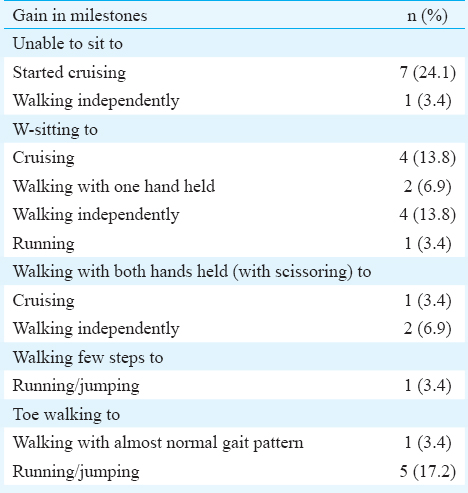
Because of long gap between the last few sessions (due to non-availability of botulinum toxin-A on time), eight patients relapsed repeatedly, especially the crouch gait, and were referred for surgery (lengthening of medial hamstrings and/or Achilles tendon) and were excluded from the study after the surgery. Their pre-surgery readings, i.e. 12 wk after the last injection were used for data analysis. Two patients were lost to follow up four months and 6.5 months after first injection, respectively; their only reading available was the first 12-wk assessment.
Side effects in form of fever with upper respiratory tract infection were seen in three children and two children complained of local weakness for few days, due to sudden decrease of tone after the injection. However, side effects were very common (62%) after casting, in the form of intertrigo, ulceration and contact dermatitis; six patients also developed knee joint or ankle joint swelling that resolved after anti-inflammatory drugs.
Discussion
Injections of botulinum toxin-A into the lower limbs of children with CP have been shown to reduce spasticity, but there are very few studies on long-term clinical gains with this approach. Another issue has been that in most of the studies, botulinum toxin-A has been shown to have good results in children with milder disease, i.e. those who were ambulatory with or without assistance, the response in children with much severe disease has not been studied in many studies. Our study showed that repeated multilevel botulinum toxin-A injections in the lower limbs along with comprehensive management, given to an unselected population of children with CP (44.82% children having GMFCS level IV-V), were effective in improving their gross motor skills. There was also a significant improvement in their muscle tone and range of motion. Majority of patients also showed change in their GMFCS level.
Most of the initial studies of botulinum were aimed to assess its short-term effect on spasticity grades, gait and function, typically 4-12 wk after the injections. In a study from The Netherlands, 46 children with spastic diplegia/hemiplegia were randomized to receive either multilevel botulinum injections and comprehensive rehabilitation or usual physiotherapy. Gait and spasticity scores showed a significant improvement at six weeks after injections, but the effect was not sustained at 24 wk. GMFM-66 scores showed a significant improvement in botulinum group at 12 wk which persisted at 24 wk2223. In a study from Germany, spasticity and GMFM scores were assessed after 4 and 12 wk of botulinum injections in adductors and medial hamstrings. There was a significant reduction in spasticity, without any effect on GMFM scores24.
In a crossover trial from Australia, significant improvement in spasticity scores on Modified Ashworth Scale was noted in calf muscles and hip adductors at six-month post-injection; however, there was no significant change in GMFM scores21. A controlled study from Egypt, involving 40 children with spastic diplegic CP, demonstrated significant reduction in spasticity and improvement in gait function and range of motion at 4-, 8- and 12-wk post-treatment25. Ubhi et al26 from the UK assessed the effect of botulinum toxin-A injections in a randomized placebo-controlled trial on 40 children with spastic diplegia and hemiplegia. Video gait analysis showed a significant improvement in initial foot contact at six weeks and 12 wk following botulinum injections. The GMFM also showed a significant improvement.
All these trials showed short-term effect of botulinum toxin-A injections on muscle tone. Most of these trials also showed significant functional gains up to 24 wk after the injections. However, as the effect of botulinum toxin is typically known to last for around 12-24 wk, there were concerns regarding its long-term effect and the effect of repeated injections. In a two-year follow up study from Israel12, the outcomes of 26 children who received 2-4 sessions of multilevel botulinum toxin-A injections in the lower limbs were prospectively evaluated. The baseline score before the first injection was compared with the score before the last injection. The GMFM score showed a significant improvement from 65.4 to 73.9. There was no significant change in the Ashworth scores, popliteal angle, or ankle dorsiflexion angle. As compared to this study, in the present study, most of the children had change in GMFCS level as well as gain of motor milestones. The better results in the present study, despite including quadriplegic and triplegic children as well, could be because of much rigorous post-botulinum toxin-A therapy including serial casting. Furthermore, in the study from Israel12, only gastrocnemius and hamstring muscles were injected unlike the present study where other muscles were also injected, based on clinical assessment.
In a long-term study by Tedroff et al10, the longitudinal change in the muscle tone and range of motion following botulinum toxin-A injections in gastrocnemius, hamstrings and adductors was studied in 94 children with different subtypes of CP, with different severity as per GMFCS scale. The median follow up time was one year and six months and median two injections were given to a specific muscle. Short leg casting for three weeks was done in some patients after gastrocnemius injections. Reduction in long-term spasticity was seen in all the muscle groups, maximally in gastrocnemius muscle. The range of motion increased initially, however, after some injections; the angles returned to baseline and then showed a decrease. There was no difference in response depending on the type of CP. Furthermore, casting showed no significant benefits. Functional outcomes, however, were not studied. In the present study, significant reduction of tone was seen in all the muscle groups, similar to the results of Tedroff et al10. The range of motion also worsened in gastrocnemius, however, that of hip adductors and hamstrings showed improvement.
Medium long-term functional benefits were also studied by Linder et al14 in an open-label, prospective study on 25 children who were followed up for one year. There was significant gain in GMFM scores with median gain of six per cent and range of 4-26 per cent; the gains were most clearly evident in children with moderate motor impairment, i.e. GMFCS level III. The range of movement increased after 1st injection but decreased to baseline at 12 months. The results were better in children with equinus than in adductor spasm. In the present study, there was an almost 40 per cent increase in mean GMFM score, presumably due to longer study duration and intensive therapies.
One-year follow up was also studied in a large multicentre study ‘BOTULOSCOPE’15 from France, involving 282 children, most of whom underwent 2-3 injection procedures during the study period. Eighty five per cent of patients were able to walk with or without aids at the time of inclusion. Forty three per cent of patients had received botulinum toxin-A before this study. Physiotherapy and serial casting as well as orthoses were prescribed as indicated to the cases. The spasticity significantly decreased for the hip, knee and ankle muscles at one and three months but returned to baseline level for the hip muscles at 12 months. The joint range of motion increased for the hip and ankle but not for the knee at 1, 3 and 12 months. The gait also improved as documented by gain of at least 2 points on Physician Rating Scale in 61 per cent of patients. The GMFM score for ‘standing’ increased from 72 to 78 per cent and for ‘walking, running and jumping’ from 62 to 68 per cent for the 155 children who were assessed after 12 months. In the present study, almost a similar treatment protocol was followed and the changes in spasticity, joint range of motion and gait were almost similar; however, the change in GMFM score was much more impressive.
The main strength of the present study was the detailed assessment of patients on various parameters and long-term effects of a comprehensive management documenting significant functional gains. The major limitations of this study were a small and heterogeneous profile of patients, lack of a control group and long intervals between the injections due to limited availability of botulinum toxin-A.
In conclusion, botulinum injections showed a promising role in the management of patients with CP. Although high cost is a factor in developing countries, judicious use along with intensive pre- and post-injection therapies and casting can yield good results. This intervention should be made available to a selected population of children with CP, along with intensive post-injection therapies, as this will ultimately reduce the burden of disability in these children.
Conflicts of Interest: None.
References
- Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571-6.
- [Google Scholar]
- An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509-19.
- [Google Scholar]
- Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins (Basel). 2015;7:1629-48.
- [Google Scholar]
- Practice parameter: Pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2010;74:336-43.
- [Google Scholar]
- Effects of leg muscle botulinum toxin A injections on walking in children with spasticity-related cerebral palsy: A systematic review. Dev Med Child Neurol. 2011;53:210-6.
- [Google Scholar]
- Efficacy of upper limb therapies for unilateral cerebral palsy: A meta-analysis. Pediatrics. 2014;133:e175-204.
- [Google Scholar]
- Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE) Cochrane Database Syst Rev. 2010;1:CD003469.
- [Google Scholar]
- Botulinum toxin A treatment in toddlers with cerebral palsy. Acta Paediatr. 2010;99:1156-62.
- [Google Scholar]
- Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009;51:120-7.
- [Google Scholar]
- Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev Med Child Neurol. 2009;51:436-45.
- [Google Scholar]
- Long-term effect of repeated injections of botulinum toxin in children with cerebral palsy: A prospective study. J Child Orthop. 2008;2:29-35.
- [Google Scholar]
- Functional outcome of botulinum toxin injection of gastrocnemius and adductors in spastic hemiplegic cerebral palsied children. Eura Medicophys. 2007;43:13-20.
- [Google Scholar]
- Medium-term functional benefits in children with cerebral palsy treated with botulinum toxin type A: 1-year follow-up using gross motor function measure. Eur J Neurol. 2001;8(Suppl 5):120-6.
- [Google Scholar]
- A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE. Eur J Paediatr Neurol. 2011;15:439-48.
- [Google Scholar]
- Referral profile of a child development clinic in Northern India. Indian J Pediatr. 2012;79:602-5.
- [Google Scholar]
- Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206-7.
- [Google Scholar]
- Gross Motor Function Measure (GMFM-66 and GMFM-88) User's Manual. London, UK: Mac Keith Press; 2002.
- Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-23.
- [Google Scholar]
- Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol. 1999;6:s23-35.
- [Google Scholar]
- Functional outcome of botulinum toxin A injections to the lower limbs in cerebral palsy. Dev Med Child Neurol. 2002;44:820-7.
- [Google Scholar]
- The combined effect of lower-limb multilevel botulinum toxin type a and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Arch Phys Med Rehabil. 2006;87:1551-8.
- [Google Scholar]
- Effect of multilevel botulinum toxin a and comprehensive rehabilitation on gait in cerebral palsy. Pediatr Neurol. 2007;36:30-9.
- [Google Scholar]
- Treatment of adductor spasticity with BTX-A in children with CP: A randomized, double-blind, placebo-controlled study. Dev Med Child Neurol. 2006;48:10-3.
- [Google Scholar]
- The effect of botulinum toxin type-A injection on spasticity, range of motion and gait patterns in children with spastic diplegic cerebral palsy: An Egyptian study. Int J Rehabil Res. 2004;27:275-81.
- [Google Scholar]
- Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Arch Dis Child. 2000;83:481-7.
- [Google Scholar]






