Translate this page into:
Effect of inherited red cell defects on growth of Plasmodium falciparum: An in vitro study
For correspondence: Dr Kanjaksha Ghosh, Surat Raktadan Kendra & Research Centre, Udhna Khatodara Urban Health Centre, Udhna Magdalla Road, Surat 395 002, Gujarat, India e-mail: kanjakshaghosh@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
High prevalence of certain polymorphic alleles of erythrocytes in malaria endemic area has been linked to the resistance provided by these alleles against parasitic infestations. Numerous studies undertaken to demonstrate this correlation have generated conflicting results. This study was undertaken to investigate the abilities of various polymorphic erythrocytes to support in vitro growth of Plasmodium falciparum parasites.
Methods:
In this study under in vitro condition the ability of P. falciparum parasites to grow was assessed in the erythrocytes obtained from a total of 40 patients with various haemoglobinopathies, such as β-thalassaemia (β-Thal), sickle cell anaemia, erythroenzymopathy-like glucose-6-phosphate dehydrogenase deficiency and membranopathy-like hereditary spherocytosis.
Results:
Significantly reduced in vitro invasion and growth of parasites was seen in the cultures containing abnormal erythrocytes than in control cultures containing normal erythrocytes (P< 0.05). The mean per cent parasitaemia comparison was also carried out among the three polymorphic erythrocyte groups, i.e. β-Thal, sickle cell anaemia and enzyme-membranopathies.
Interpretation & conclusions:
Erythroenzymopathies and membranopathies were found to provide a more hostile environment for parasites, as the least parasitaemia was observed in these erythrocytes. The present in vitro study showed that P. falciparum did not grow well and did not invade well in erythrocytes obtained from common inherited red cell disorders.
Keywords
In vitro culture
inherited red cell disorders
Plasmodium falciparum
resistance
Plasmodium falciparum malaria is believed to be the strongest known selective force in the recent history of human genome selection and modulation1. Erythrocytes play a crucial role in the asexual development of malarial parasites. Thus, many erythrocyte-related polymorphisms are thought to have occurred during the parallel evolution of P. falciparum and its human host which provides a survival advantage in severe infection. In 1949, Haldane hypothesized that natural selection must have acted in malaria-endemic regions over generations by encouraging the development of highly protective red cell variant alleles to high prevalence2, thus developing the concept of balanced polymorphism. Haldane's so-called ‘malaria hypothesis’ was based on the observations that many red cell disorders such as sickle cell anaemia and thalassaemia are prominent in malaria-endemic regions. The geographic distribution of several other red cell disorders which are also linked to malaria resistance including α-thalassaemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency correlates to malaria endemicity345. ABO blood group distribution is also found to be consistent with malaria's tropical distribution. A high prevalence of ‘O’ blood group has been observed in the geographic regions where malaria is currently or was earlier endemic6. This results in ‘balanced polymorphism' where the homozygote's haematological disadvantage (disease) is balanced by the resistance to malaria displayed by hugely over-represented heterozygotes in the community.
Development of in vitro malaria parasite cultivation techniques has allowed experimental assessment of the ability of the parasite to grow in various types of abnormal red cells. In this study, some of the most common red cell polymorphisms such as ABO blood groups, haemoglobinopathies such as β thalassaemia (β-Thal) and sickle cell anaemia, a frequently found enzymopathy-like G6PD deficiency and a red cell membranopathy-like hereditary spherocytosis (HS) were selected and the in vitro growth of P. falciparum parasites was assessed in the cultures containing erythrocytes obtained from individuals with these conditions.
Material & Methods
The study was performed at the ICMR-National Institute of Immunohaematology, Mumbai, India, during the six months study period from February 2015 to July 2015. The sample size was determined with the help of observed data. From our previous studies on red cells from more than 100 healthy volunteers without alpha-thalassaemia mutations7, we established the infection mean and standard deviation of 16±3.2 taking a standard deviate Z at 1.96 and minimum difference to be correctly detected at 2. The following formula was used to get a minimum number to be tested for statistical significance8.
n=(zα/2σ/E)2, i.e. [1.96×3.2/2]2, which gives n=9.83, i.e. 10 at the statistical power level of 80 per cent.
Thus, 10 samples were collected for each group. In case of enzymo-membranopathy (En-mem) group, the sample size could not be achieved due to low frequency of these diseases910 and subsequent inadequate number of patients was referred to the study centre during the desired time period. Samples were tested for haematological parameters and included in the respective category. Samples dual positive for different polymorphisms were excluded from the study.
A total of 70 blood samples were collected for this study, ten each from normal healthy individuals of blood groups A, B and O which constituted control group (n=30 for control), ten from β-Thal trait and five from non-transfused β-Thal major patients (n=15 for β-Thal group), ten from sickle cell trait (HbAS) and seven from non-transfused sickle cell anaemia (HbSS) patients (n=17 for sickle cell group) and five from G6PD-deficient and three samples from red cell membrane deficient (HS) patients [n=8 for enzymo-membranopathy (En-mem) group]. These patients were investigated and confirmed in the Haematogenetics Department at the ICMR-National Institute of Immunohaematology, Mumbai, India. Five millilitres venous blood was collected in EDTA tube from all subjects.
No distinction of race and gender was considered. Patients' past malarial infections were not taken into account as only isolated erythrocytes were used for the study and the plasma was discarded.
The study was approved by the Institutional Ethics Committee Review Board, Institutional Committee for Research on Human Subjects, and written informed consent was obtained from all participants.
Haematological investigations: Blood grouping was done for all the samples. Complete blood count was done on an automated cell counter (Sysmex K-1000; Sysmex Corporation, Kobe, Japan). Haemoglobin A2, foetal haemoglobin (HbF) and haemoglobin S (HbS) levels were estimated by high-performance liquid chromatography (HPLC) using the Variant Hb Testing System (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the β-globin gene mutations were confirmed by molecular analysis11. Screening for G6PD deficiency was done by dichlorophenol-indophenol dye decolourization test12. Membrane defect in red blood cells was detected by flow cytometry using eosin-5'-maleimide as described by Kedar et al13.
α-Thalassaemia mutations were not taken into consideration, but all the red cells that were used in this study were free from eight common α-thalassaemia deletional mutations as was confirmed by the standard multiplex Gap polymerase chain reaction routinely done in our laboratory14.
Plasmodium falciparum parasites: P. falciparum laboratory strains 3D7 and 7G8 parasites were maintained in complete Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, USA). 3D7 is a chloroquine-sensitive strain while 7G8 is resistant. At about 10 per cent parasitaemia, when majority of the parasites were in the ring stage, synchronization of parasite stages was done with the help of five per cent D-sorbitol (Sigma, USA). Five volumes of five per cent D-sorbitol solution was added to the cell pellet and incubated for five min at 37°C. Cells were washed twice with complete RPMI medium [RPMI 1640 (Gibco, USA) supplemented with 25 mM HEPES buffer, 2.1 g/l sodium bicarbonate, 200 μg/ml gentamycin, 5% Albumax (Gibco) and 0.05% hypoxanthine (Sigma-Aldrich)] and the cell pellet was used as an inoculum for the assay.
In vitro parasite cultures: The in vitro cultures were performed in six-well sterile tissue culture plates (Nunclon, Denmark) with the help of modified Trager and Jensen's15 method as described earlier16. Cultures were initiated with both the strains at 0.5 per cent parasitaemia and five per cent haematocrit by co-culturing 107 parasitized erythrocytes containing synchronized P. falciparum parasites of both the strains with 200 μl of packed cells of different polymorphisms in 4 ml complete RPMI medium. The cell mixture was further distributed equally in two wells of the tissue culture plate. Thus, each sample had two replicates and parasite density per well was maintained at 108 cells/μl. The parasites were allowed to grow for five days in a fixed amount of erythrocytes. The cultures were maintained at 37°C in an atmosphere of five per cent CO2 with daily replacement of medium. The degree of overall haemolysis as judged by the colour of the exhausted medium was negligible.
Determination of parasitaemia: Per cent parasitaemia and the intra-erythrocytic growth stages of the parasite were monitored by observing Giemsa-stained thin blood films. Per cent parasitaemia was calculated for each sample after every 24 h. The thin films were fixed with absolute methanol and stained in 10 per cent Giemsa stain (Sigma-Aldrich) for 40 min and examined under ×1000 magnification. Parasitaemia was measured as the percentage of infected erythrocytes among 1000 erythrocytes counted. Slides were blinded before counting to eliminate examiner bias. Pyknotic as well as necrotic appearance of nuclear material (of parasite) was observed in the parasitized cells as an index of nonviable parasites in these red cell type cultures.
Study design and statistical analysis: The analysis was done using Microsoft Excel (Edition 2007) software (Microsoft, USA). The mean and standard deviation and 95 per cent confidence intervals of per cent parasitaemia for all cultures were calculated. Paired Student's t test was used to compare the means between control and treated groups, whereas unpaired Student's t test was used to compare the means among the polymorphic groups.
Results
Of the 70 samples collected, 30 were from normal healthy individuals of blood groups A, B and O having all the haematological parameters within normal range, while the other 40 were from individuals diagnosed with six different inherited red cell defects. Among those, 15 cases were of β-Thal major and traits, 17 were of sickle cell anaemia and traits and remaining eight were of En-mem (n=5 for G6PD and n=3 for HS due to band 3 protein defect). None of them had any history of receiving blood transfusions. Of the 15 β-Thal samples, four were from β-Thal intermedia cases requiring no transfusions while one case was previously transfused four weeks back. Only three samples of red cell membrane disorders were collected due to low frequency of these patients referred. The group of normal individuals with different blood groups (n=30) was considered as the control group for analyzing the difference in parasite growth between normal and three groups of haematologically abnormal red cells.
During the five day study period, parasites of both the strains completed their two growth cycles. On the fourth day (96 h), peak parasitaemia was observed for all cultures. Initial parasitaemia in all groups was essentially the same. Similar invasion (measured as per cent parasitaemia) was detected in all four group cultures, irrespective of the red cell type. The most profound differences were observed in the invasion of parasites after 96 h, i.e. at the end of the second growth cycle for strains 3D7 (Fig. 1) and 7G8 (data not shown). Mean per cent parasitaemias in all defective red cell groups, i.e. β-Thal, sickle cell and En-mem, were significantly lower than control group. En-mem group demonstrating the lowest parasitaemia among them.
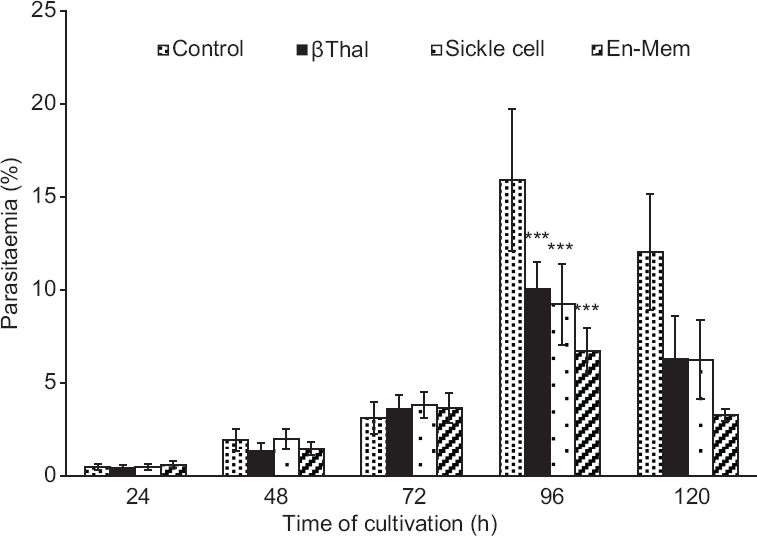
- Growth of Plasmodium falciparum strain 3D7 parasites in cultures containing different polymorphic erythrocytes; Control (n=30), β-Thal (n=15), Sickle cell (n=17) and En-mem (n=8). Error bars represent standard deviation. ***P<0.001, compared to control. β-Thal, β-thalassaemia; En-mem, enzyme-membranopathies.
In the control group, mean per cent parasitaemia of O group cultures was significantly higher (P<0.05) than A and B group cultures for both the strains. All normal samples of A, B and O groups were pooled together to calculate the mean per cent parasitaemia for control cultures which were 15.92±3.83 and 16.6±3.4 for strain 3D7 and 7G8, respectively. In both the haemoglobinopathies groups, i.e. β-Thal and sickle cell, mean per cent parasitaemias were found to be pared down in the homozygous red cell cultures. Amidst all defective red cell cultures, G6PD exhibited the lowermost mean per cent parasitaemia (Fig. 2).
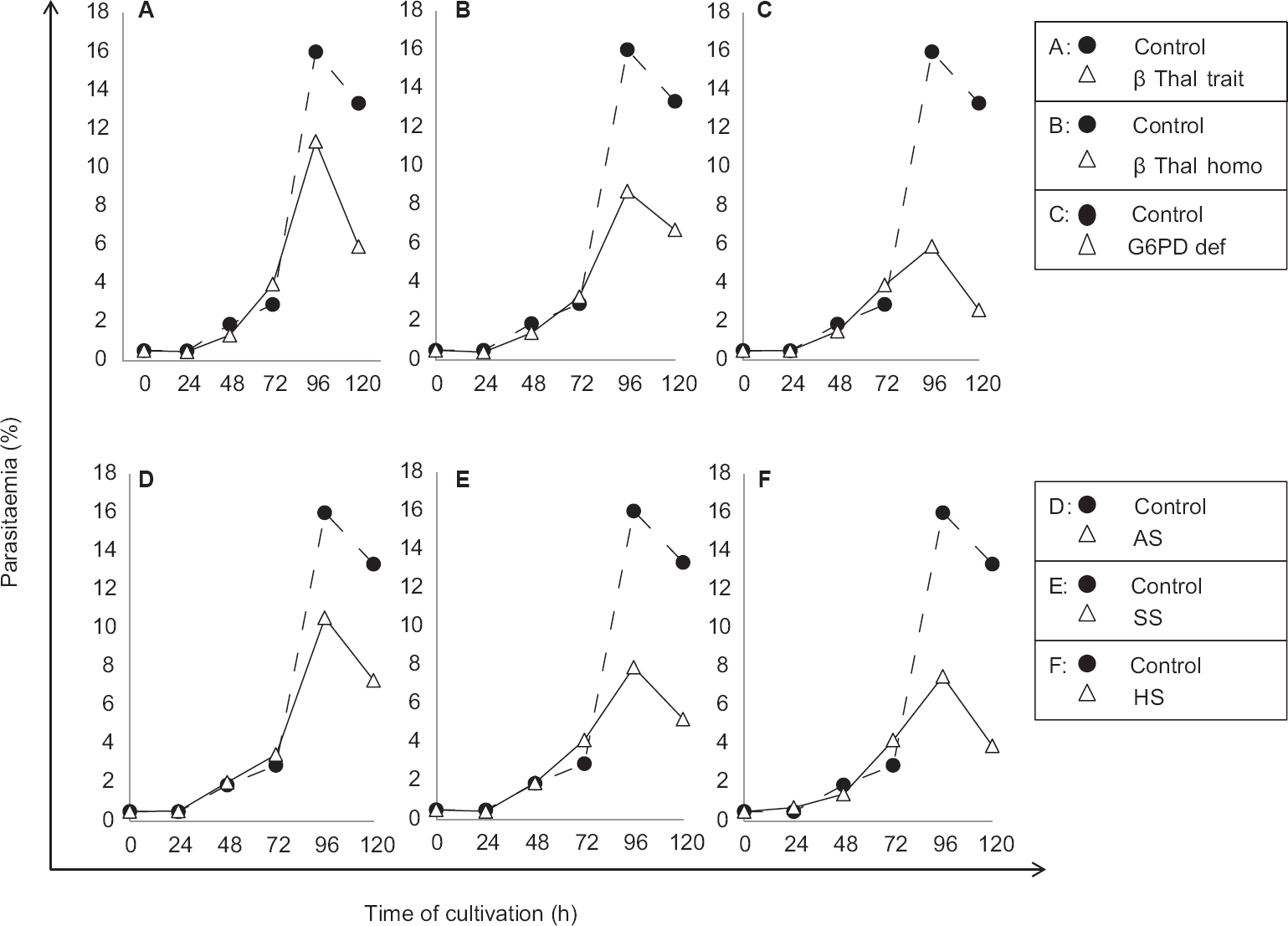
- Parasite growth curves for Plasmodium falciparum strain 3D7 in the cultures containing different types of polymorphic erythrocytes compared to control. β-thal trait (A), β-thal homo (B), G6PD def (C), AS (D), SS (E), and HS (F). β-Thal trait, β-thalassaemia trait; β-Thal homo, β-thalassaemia homozygous; G6PD def, glucose-6-phosphate dehydrogenase deficiency; AS, sickle cell trait; SS, sickle cell homozygous; HS, hereditary spherocytosis.
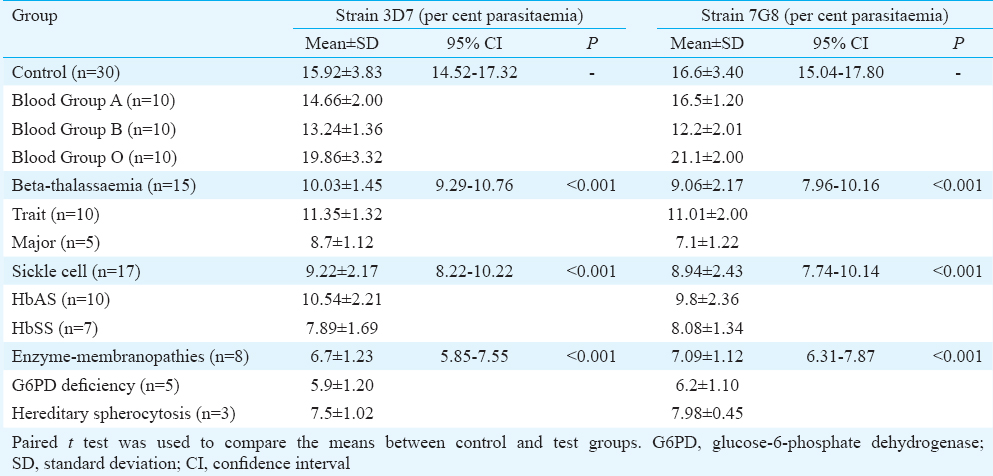
The mean per cent parasitaemias for both the P. falciparum strains in cultures containing nine different types of red cells categorized in four groups are given in the Table.
Parasite cultures of ABO blood groups were equally viable at 72 and 96 h of cultivation, i.e. on the third and fourth day, but for all other red cell type cultures, parasite growth was found to be impaired (Fig. 3). Pyknotic as well as necrotic appearance of the nuclear material (of parasite) was observed in the parasitized cells as an index of non-viable parasites in these red cell type cultures. Changes in parasite morphology occurred by day three (72 h) in the cultures containing G6PD-deficient cells and by day four (96 h) in other abnormal red cell cultures.

- Micrograph of Giemsa-stained thin films depicting growth of Plasmodium falciparum parasites on day four in cultures containing nine different polymorphic erythrocytes (×1000). Inset shows a magnified image of an infected erythrocyte (×2000), scale bar=10 μm. Pyknotic and necrotic nuclear material (blue) can be seen in abnormal red cell types (AS, SS, G6PD def, β thal trait, β thal homo, and HS). A, blood group A; B, blood group B; O, blood group O; AS, sickle cell trait; SS, sickle cell homozygous; G6PG def, glucose-6-phosphate dehydrogenase deficient; β-Thal trait, β-thalassaemia trait; β-Thal homo, β-thalassaemia homozygous; HS, hereditary spherocytosis.
Discussion
In 1949, Haldane hypothesized the protective effect conferred by red cell disorders2. After that, numerous clinical and epidemiological and in vitro studies have been done for the demonstration of the protective effect conferred by variant alleles171819202122. In the present study, though absolute distinction between defects in invasion and growth was not possible, the retarded invasion (measured as lower per cent parasitaemia than controls) and impaired growth (as gauged by high frequency of dead forms) were observed in cultures containing erythrocytes of all the red cell disorders for two different strains of P. falciparum. Thus, it is possible that these disorders provide protection against malaria by affecting both quality and rate of parasite growth.
Since sophisticated tests on viability of the parasites in different types of parasitized red cells using mitochondrial fluorescent dyes such as JC1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethyl-benzimidazolylcarbocyanine iodide) or MitoTracker Red requiring either a fluorescent microscope23 or flowcytometer24 could not be used, our viable parasite rate could be an underestimate using Giemsa smear morphology alone.
Distinct mechanisms conferring protection against severe and complicated malaria have been proposed for the different red cell disorders. These include increase in redox stress and membrane damage in thalassaemic erythrocytes2526, increase in HbS polymerization which may physically damage parasites2728, impairment of antioxidant defence, leading to membrane damage in the absence of G6PD enzyme and early phagocytosis of parasite-infected G6PD-deficient erythrocytes29. This genetically based resistance is involved in either altering erythrocyte invasion by merozoites, in lowering parasite growth or in impairing merozoite viability after being released from schizonts and selective removal of parasitized cells (parasite suicide)30.
Alpha-thalassaemia has also been shown to provide extremely good protection from severe falciparum malaria in epidemiological studies31, and it would have been pertinent to include a group of alpha-thalassaemic red cells bereft of any other significant red cell genetic defect to see the nature of in vitro parasite growth in such type of red cells.
Among ABO blood group cultures, parasites of both the strains were found to be viable after the second growth cycle and significant differences were observed in parasite invasion. It has been suggested that during infection with P. falciparum, O group offers a survival advantage, Group ‘A’ confers disadvantage and Group ‘B’ has an intermediate effect6. In view of these assumptions, one would expect lower parasitaemia in O group cultures than other blood group cultures, but conversely here, in the absence of immune pressure, P. falciparum parasites could achieve the highest parasitaemia in O group cultures than in other blood group cultures. It indicated that the O group erythrocyte itself was not resistant towards parasite entry. Perhaps, the in vivo protective effect of O group individuals is due to enhanced immune responses such as reduced rosetting potential32 and increased phagocytosis33 of infected O erythrocytes.
In case of β-Thal homozygous individuals, frequent transfusions may complicate the interpretation of findings. Thus, only pre-transfused homozygous and thalassaemia intermedia individuals requiring no transfusion were included in the study. However, it is not clear whether increased amounts of HbF also played a role in the protection against parasite invasion and growth in β-Thal homozygotes. The HbF has been shown to exhibit inhibitory effect on the growth of malaria parasites previously34.
Lowest parasitaemia was observed in the cultures containing G6PD-deficient and spherocytic red cells, indicating that these alterations in host cells have more deleterious effects on parasite growth and developmental pathways. Similar parasitaemia in the β-Thal and sickle cell group suggests that these haemoglobinopathies provide equal resistance towards parasite growth. However, the alleles of β-Thal and sickle cell anaemia may provide more resistance in homozygous condition than heterozygous condition with the cost of the deleterious effects on health inflicted by the disease.
In the present in vitro study, it was demonstrated that erythrocytes harbouring heterozygous or homozygous alleles of haemoglobinopathies, enzymopathies and membranopathies provided resistance towards malaria parasite growth and development. These findings indicated the protective advantage of red cell polymorphisms in malaria-endemic regions and supported the continued investigations of mechanisms underlying it. It also may help in developing new strategies to combat against the malaria.
Acknowledgment
Authors thank Dr Shobhana Sharma from the Department of Biological Sciences, Tata Institute of Fundamental Research, Mumbai, and to Dr Paushali Mukherjee from International Centre for Genetic Engineering and Biotechnology, New Delhi, for providing frozen P. falciparum strain 3D7 and 7G8 parasites, respectively, and acknowledge Dr Praveen Sahu from Ispat General Hospital, Rourkela, Odisha, for statistical assistance.
Financial support & sponsorship: The first author (VP) received junior research fellowship (JRF) from Indian Council of Medical Research, New Delhi
Conflicts of Interest: None.
References
- How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171-92.
- [Google Scholar]
- G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: A geostatistical model-based map. PLoS Med. 2012;9:e1001339.
- [Google Scholar]
- World Distribution, Population Genetics, and Health Burden of the Hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a011692.
- [Google Scholar]
- The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110:2250-8.
- [Google Scholar]
- HLA associations in P.falciparum malaria patients from Mumbai, western India. Indian J Malariol. 2002;39:76-82.
- [Google Scholar]
- How to Determine Sample Size, Determining Sample Size. Available from: http://www.isixsigma.com/tools-templates/sampling-data/how-determine-samplesize-determining-sample-size/
- [Google Scholar]
- Hemolytic anemias and anemia due to acute blood loss. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison's principles of internal medicine (17th ed). New York: McGraw-Hill Medical; 2010. p. :110-27. Ch. 106
- [Google Scholar]
- Application of covalent reverse dot blot hybridisation for rapid prenatal diagnosis of the common Indian thalassaemia syndromes. Indian J Hematol Blood Transfus. 1997;15:10-3.
- [Google Scholar]
- A rapid screening dye test for the detection of glucose-6-phosphate dehydrogenase deficiency in red cells. Nature. 1962;194:192-3.
- [Google Scholar]
- Experience with eosin-5'-maleimide as a diagnostic tool for red cell membrane cytoskeleton disorders. Clin Lab Haematol. 2003;25:373-6.
- [Google Scholar]
- Laboratry manual: Screening, diagnosis and molecular analysis of hemoglobinopathies and red cell enzymopathies. Mumbai: Bhalani Publishing House; 2008.
- [Google Scholar]
- Correlation between ‘H’ blood group antigen and Plasmodium falciparum invasion. Ann Hematol. 2016;95:1067-75.
- [Google Scholar]
- Associations between red cell polymorphisms and Plasmodium falciparum infection in the middle belt of Ghana. PLoS One. 2014;9:e112868.
- [Google Scholar]
- Malaria parasites and red cell variants: When a house is not a home. Curr Opin Hematol. 2014;21:193-200.
- [Google Scholar]
- Thalassemic erythrocytes inhibit in vitro growth of Plasmodium falciparum. J Clin Microbiol. 1987;25:56-60.
- [Google Scholar]
- Glucose-6-phosphate dehydrogenase deficiency inhibits in vitro growth of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983;80:298-9.
- [Google Scholar]
- The interaction between sickle haemoglobin and the malarial parasite Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1980;74:701-5.
- [Google Scholar]
- Growth of Plasmodium falciparum in human erythrocytes containing abnormal membrane proteins. Proc Natl Acad Sci U S A. 1990;87:7339-43.
- [Google Scholar]
- A novel live-dead staining methodology to study malaria parasite viability. Malar J. 2013;12:190.
- [Google Scholar]
- Flow cytometric readout based on Mitotracker Red CMXRos staining of live asexual blood stage malarial parasites reliably assesses antibody dependent cellular inhibition. Malar J. 2012;11:235.
- [Google Scholar]
- Genetic resistance to malaria, oxidative stress and hemoglobin oxidation. Parassitologia. 1999;41:203-4.
- [Google Scholar]
- Effect of heterozygous beta thalassemia on the phosphorylative response to Plasmodium falciparum infection. J Proteomics. 2012;76:251-8.
- [Google Scholar]
- Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet. 1970;1:319-21.
- [Google Scholar]
- Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701-3.
- [Google Scholar]
- Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood. 1998;92:2527-34.
- [Google Scholar]
- High frequencies of alpha-thalassaemia are the result of natural selection by malaria. Nature. 1986;321:744-50.
- [Google Scholar]
- Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci U S A. 2007;104:17471-6.
- [Google Scholar]
- ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathog. 2012;8:e1002942.
- [Google Scholar]
- Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977;270:171-3.
- [Google Scholar]






