Translate this page into:
Effect of galantamine on platelet functions in healthy elderly people
Reprint requests: Dr Ahmet Turan Isik, Professor, Dokuz Eylul University, School of Medicine, Department of Geriatric Medicine, 35340 Balcova Izmir/Turkey e-mail: atisik@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Galantamine, a centrally-acting cholinesterase inhibitor, has been used in the treatment of mild-to-moderate dementia of Alzheimer disease. Increased mortality, mainly due to cardiovascular events, was observed in placebo-controlled trials of galantamine. Several studies have evaluated the efficacy of galantamine in dementia, it is not clear whether it has an effect on platelet function. It is important to clarify this effect, because it may be related to thrombotic tendency or bleeding diathesis. This study was aimed to investigate the effect of galantamine on platelet aggregation in whole blood from healthy, elderly subjects.
Methods:
Fifteen healthy (mean age 76.8 ± 7.2 yr) volunteers were included in the study. Three concentrations of galantamine solution (20, 40 and 80 ng/μl) were prepared. Each concentration of galantamine solution and control diluent without galantamine were incubated with whole blood. After incubation, aggregation responses were evaluated with ADP (5 μM) and collagen (2 μg/ml) in platelet-rich plasma.
Results:
Compared to control, pre-incubation with all dilutions of galantamine had no detectable effect on platelet aggregation response induced by ADP and collagen. Galantamine also had no detectable effect on platelet aggregation in a dose-dependent manner.
Interpretation & conclusions:
This in vitro study suggested that galantamine administration had no effect on platelet aggregation in the clinically relevant doses.
Keywords
Alzheimer disease
elderly
galantamine
platelets
Galantamine, a centrally-acting cholinesterase inhibitor (competitive and reversible) used in the treatment of mild to moderate dementia of Alzheimer's disease (AD), elevates acetylcholine in the cerebral cortex by slowing the degradation of acetylcholine, and modulates nicotinic acetylcholine receptor to increase acetylcholine from surviving presynaptic nerve terminals1234. It has beneficial effects on cognition, function and behavioural symptoms in patients with AD. These effects have also been observed in clinical studies of patients with probable vascular dementia and AD with cerebrovascular disease (CVD)567. However, the GAL-INT Study Group5 reported that recorded mortality due to cardiovascular event was greater in the galantamine group than in the placebo group, and interestingly, about half of the deaths in galantamine appeared to result from various vascular causes (myocardial infarction, stroke, and sudden death). Although these studies were not designed to assess mortality, and the results were highly discrepant with studies of galantamine in other dementia populations8. In addition, consistent with this finding, galantamine-induced mortality due to probable cardiovascular events was reported in the different studies.91011. Though the current literature relationship between platelets and cardiovascular events is well-known, little is known about the effects of galantamine on platelet function.
Due to the high frequency of co-morbidities and age-related changes in the elderly, most of the elderly patients with dementia are taking various medications concomitantly. These drugs may have effects on platelet function12. Therefore, it is important to assay platelet function in response to galantamine because it alone or in combination with other medications may have unknown additive or synergistic effects on platelet function. The aim of the present in vitro study was to investigate the effects of galantamine on platelet function obtained from healthy elderly people. It is difficult to find elderly patients using solely galantamine because of their need of other medications. Consequently, this study was designed to investigate platelet function after incubation with galantamine in the elderly volunteers taking no medications.
Material & Methods
This study protocol was approved by the Ethics Committee of Gulhane School of Medicine, Ankara, Turkey. Written informed consent was obtained from each participant.
Fifteen consecutive healthy volunteers (5 female, 10 male) with a mean age of 76. 8 ± 7.2 (range 67-87 yr), who were referred to our geriatric clinic and did not have abnormal platelet count, anaemia, hypertension, diabetes mellitus, ischaemic heart disease, peripheral arterial disease, renal disease, metabolic disease, a history of thrombosis or abnormal bleeding, active neoplasia, active inflammatory disease, and a history of smoking or alcohol, and who consented to participate were included in this study from August 2009 to February 2010. It was ascertained that no drugs had been taken within two weeks prior to tests.
It is known that galantamine prolonged release of 24 mg (qd) capsule and immediate release of 12 mg (bid) tablet result in 40. ± 8.1 and 43.8 ± 10.0 ng/ml average plasma concentration at steady state, respectively13. Therefore, galantamine solutions in three different concentrations, which would yield 20, 40, and 80 ng/ml galantamine concentrations in the plasma, similar to that observed after clinical therapeutic oral application were prepared. Galantamine (supplied commercially as Galantamine Hydrobromide G1660-10MG; Sigma-Aldrich, USA) test solutions (20, 40, and 80 ng/ml) were prepared by using 5 per cent dextrose solution and galantamine.
Venous blood samples (20 ml) were drawn without a tourniquet from the ante-cubital vein from each volunteer. These samples were anticoagulated with 0.129 molar sodium citrate solution (anticoagulant to blood ratio: 1/9). Complete blood counts were done by using an Abbott Cell-Dyn 4000 cell counter device (Abbott Park, IL, USA). Blood samples were kept at room temperature and tested within 30 min. Hematocrit was determined and the blood sample was divided into four equal parts. According to the amount of plasma (derived from the hematocrit), the calculated volume of galantamine solution and the diluent, as control, without galantamine were added to blood samples (1 μl solution for 1 ml of plasma), which yielded 0, 20, 40, and 80 ng/ml galantamine concentrations in the plasma. Each aggregation study was performed with previously prepared stock solutions of galantamine and the diluent. Each concentration of galantamine and control solutions were incubated with whole blood at 37ºC. After incubation for 15 min, blood samples were centrifuged (100 g, 10 min) to isolate platelet-rich plasma from supernatant. Turbidometric aggregation was performed using a Whole Blood Lumi-Ionized Calcium Aggregometer (Chrono-log Corporation, Model 560-Ca Havertown, PA, USA) according to the manufacturer's protocol. Platelet aggregation responses were evaluated with adenosine diphosphate (ADP) (5 μM final concentration) and collagen (2 μg/ml final concentration). Aggregating reagents were obtained from Chrono-log Corporation. The investigators were blinded to the sequences by which different dilutions were studied in the aggregation. Platelet aggregation curves were calculated automatically by the device. Maximal aggregation (%) was obtained from the aggregation curve.
The study was planned with the assumption that 40 ng/ml galantamine incubation leads to 3 per cent increase in platelet aggregation response and standard deviation of this change is 3.5 per cent. The needed sample size was calculated as 13 samples for 95% confidence interval and 80 per cent power.
Statistical analysis: Aggregation data were presented as mean ± SEM. Friedman repeated measures analysis of variance was used to compare platelet aggregation response to ADP or collagen between control and each galantamine solutions. Wilcoxon test was used to determine differences between paired data. P<0.05 was considered statistically significant.
Results & Discussion
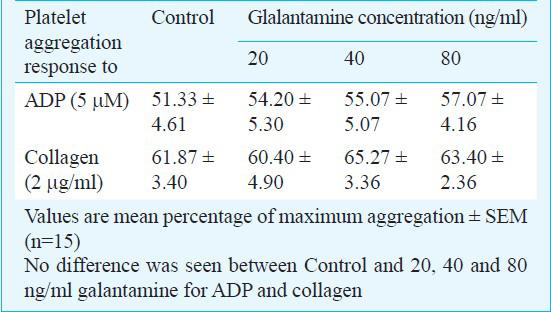
All 15 volunteers had normal haemogram parameters. There was no significant effect on platelet aggregation response to ADP and collagen after incubation with each concentrations of galantamine compared with control (95% CI- 16.00, 11.01 in 20 ng/ml, 18.99, 9.99 in 80 ng/ml of galantamine for ADP, and 95% CI-11.00, 12.00 in 20 ng/ml and 95% CI,-10.00, 8.01 in 80 ng/ml of galantamine for collagen; Table). Platelet aggregation response to ADP or collagen was compared between control and all galantamine concentrations. There was no relationship between galantamine concentration (20-40-80 ng/ml) and platelet aggregation responses in dose-dependent manner.
Recently the therapeutic effectiveness of galantamine, a cholinesterase inhibitor used in the treatment of dementia of AD, on cognitive function in patients with dementia has been extensively investigated56141516. It has been reported that galantamine therapy may be associated with increased cardiovascular mortality891011. Other studies reported that galantamine was associated with lower mortality rate than placebo or had no effect on mortality417. In a meta-analysis of mortality data of randomized 12 trials, no evidence of increased risk of mortality was found to be associated with galantamine treatment18. In our earlier studies, we also did not observe increase in cardiovascular event after the follow up of galantamine-treated elderly patients1415.
Platelet aggregation is believed to contribute significantly to cardiovascular events. Thrombotic conditions are also common in elderly patients and are directly involved in the onset of clinical complications19. However, no data have been reported about the potential effect of galantamine on platelet function. Our study results showed that galantamine compared with control had no significant effect on platelet aggregation response to ADP and collagen. There was no relationship between increased concentration of galantamine and platelet aggregation responses in dose-dependent manner. These results suggested that galantamine related increased cardiovascular events in the elderly patients were not related to platelet function abnormality due to galantamine. It may also be related to other effect of galantamine, which is different from its effect on platelet function. It may also be a result of interaction of galantamine with other drugs and co-morbidities with direct effect on platelet function.
Older adults are particularly vulnerable to side effects of the drugs because they often have multiple co-morbidities requiring multiple drug therapies.2021 Impaired platelet aggregation is one of these side effects and many drugs and systemic diseases can lead to disturb the platelet function. It is also known that an antiplatelet drug is one of the most commonly used medications in elderly patients22. Other drugs given to the elderly population may potentiate antiplatelet effect. This situation might be clinically significant in patients with pre-existing qualitative or quantitative platelet defects. Our study results showed no hypoaggregability related to galantamine.
In conclusion, our results showed that galantamine did not have any effect on the responsiveness of normal platelets to ADP and collagen in vitro. This may indicate that there is no relationship between galantamine and, increased platelet aggregability related thrombotic tendency. Also, there may not be association between galantamine and bleeding diathesis related to impaired platelet function.
References
- Therapeutic continuity in Alzheimer's disease: Switching patients to galantamine. Panel Discussion: Recommendations for Prescribers. Clin Ther. 2001;23(Suppl A):A31-9.
- [Google Scholar]
- APA Work Group on Alzheimer's Disease and other Dementias. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Second edition. APA Work Group on Alzheimer's disease and other Dementias. Am J Psychiatry. 2007;164(Suppl 12):S5-56.
- [Google Scholar]
- Galantamine in AD: A 6-month Randomized, Placebo-Controlled Trial With a 6-month Extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2261-8.
- [Google Scholar]
- A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269-76.
- [Google Scholar]
- An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin Ther. 2003;25:1765-82.
- [Google Scholar]
- Long-term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer's disease with cerebrovascular disease. Eur J Neurol. 2003;10:633-40.
- [Google Scholar]
- Management of patients with Alzheimer's disease plus cerebrovascular disease: 12-month treatment with galantamine. Dement Geriatr Cogn Disord. 2004;17:29-34.
- [Google Scholar]
- Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024-35.
- [Google Scholar]
- Mortality-associated factors in patients with Alzheimer's disease treated with Galantamine. Med Clin (Barc). 2006;127:206-10.
- [Google Scholar]
- Alzheimer's disease: beware of interactions with cholinesterase inhibitors. Prescrire Int. 2006;15:103-6.
- [Google Scholar]
- The clinical importance of acquired abnormalities of platelet function. N Engl J Med. 1991;324:27-39.
- [Google Scholar]
- Pharmacokinetics of extended-release and immediate-release formulations of galantamine at steady state in healthy volunteers. Curr Med Res Opin. 2005;21:1547-1554.
- [Google Scholar]
- Acetylcholinesterase inhibition and insulin resistance in late onset Alzheimer's disease. Int Psychogeriatr. 2009;27:1-7.
- [Google Scholar]
- Trospium and cognition in patients with late onset Alzheimer disease.J Nutr Health Aging. . 2009;13:672-6.
- [Google Scholar]
- Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007;69:448-58.
- [Google Scholar]
- Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): a randomized, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8:39-47.
- [Google Scholar]
- Analyses of mortality risk in patients with dementia treated with galantamine. Acta Neurol Scand. 2009;119:23-31.
- [Google Scholar]
- Prevention of Cardiovascular Events in Elderly People. Drugs Aging. 2005;22:859-76.
- [Google Scholar]
- Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-106.
- [Google Scholar]
- Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52:794-8.
- [Google Scholar]
- Oral anticoagulant and antiplatelet drugs used in prevention of cardiovascular events in elderly people in Poland. BMC Cardiovasc Disord. 2012;12:98.
- [Google Scholar]






