Translate this page into:
Effect of a polyherbal formulation cream on diabetic neuropathic pain among patients with type 2 diabetes – A pilot study
Reprint requests: Dr Vijay Viswanathan, M. V. Hospital for Diabetes & Prof. M. Viswanathan Diabetes Research Centre (WHO Collaborating Centre for Research, Education & Training in Diabetes), No. 4, West Madha Church Road, Royapuram, Chennai 600 013, Tamil Nadu, India e-mail: drvijay@mvdiabetes.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Painful diabetic neuropathy is a common complication of diabetes and can severely limit patients’ daily functions. The aim of this pilot study was to evaluate the safety and effect of using a polyherbal formulation in reducing the symptoms of diabetic neuropathic pain in comparison with placebo among patients with type 2 diabetes.
Methods:
A total of 50 (M:F = 33:17) consecutive type 2 diabetes patients with painful diabetic neuropathy were enrolled in this study. All these patients had either two or more symptoms of diabetic neuropathy such as pain, burning and pricking sensations and numbness in their feet. They were randomly assigned to two groups: group 1 (n = 26) patients were treated with polyherbal formulation cream and group 2 (n = 24) patients were administered placebo. The patients were followed up for six months. Changes in the symptoms of painful diabetic neuropathy of each patient were recorded at baseline, third and sixth month using the Diabetic Neuropathic Score.
Results:
The mean age of the patients, duration of diabetes and glycated haemoglobin (HbA1c) were similar in both groups at baseline. During follow up visits, there was a decrease in the HbA1c levels in the study and control groups. The symptoms of painful diabetic neuropathy were also similar in both groups at baseline. A significant decrease in symptoms of neuropathic pain was observed among the group of patients treated with polyherbal formulation cream (76.9 per cent) compared to the placebo-treated group (12.5 per cent) (P<0.001), at the end of the final follow up.
Interpretation & conclusions:
In this pilot study polyherbal formulation cream was found to be effective as well as safe to treat painful diabetic neuropathy. However, its long term use needs to be evaluated for any further effectiveness and side effects.
Keywords
Painful diabetic neuropathy
polyherbal formulation
type 2 diabetes
Diabetic foot infection is a leading cause for hospital admission among patients with diabetes in India12. The median expenditure for hospital admissions of patients with foot infection was highest indicating the associated huge economic burden in developing countries3. Painful diabetic peripheral neuropathy (DPN), a significant complication of diabetes, afflicts more than 15 per cent of diabetic patients and has an established negative impact on quality of life456. Painful symptoms such as burning, tingling (’pins and needles’ or paraesthesia), shooting (like electric shock) or lancing (stabbing) are present in around one-third of patients with DPN and about 20 per cent of all patients with diabetes78. DPN starts in the toes and gradually moves proximally. Once it is well established in the lower limbs, it affects the upper limbs, with sensory loss following the typical ‘glove and stocking’ pattern of distribution. Neuropathic pain can be extremely distressing, resulting in poor quality of life and depression9.
The management of diabetic painful neuropathy is a challenge to the physician, and there are various strategies including control of hyperglycaemia, pharmacotherapy using anticonvulsants to reduce the intensity of pain, electrical spinal cord stimulation and other topical and physical treatments using medicinal gels and creams10111213.
A few studies have shown the efficacy and benefits of herbal formulation creams in treating diabetic foot ulcers141516. In our previous report, the diabetic wound cream prepared using polyherbal formulation was found to be effective as well as safe in healing diabetic foot ulcers as compared to standard silver sulphadiazine cream16. NGX-4010, a capsaicin (8%) dermal patch, has been licensed in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults and in the United States for the treatment of neuropathic pain associated with post-herpetic neuralgia17. A multicentric, randomized, open-label study conducted by Martini et al18 to evaluate the effect of capsaicin (8%) in 91 patients with painful diabetic neuropathy showed that pain score reduced in capsaicin treated patients. Another study evaluated the efficacy of the application of lidocaine (4%) or Topicaine® gel or Betacaine Enhanced Gel 4 along with the NGX-4010 application for 12 wk and reported that the treatment was safe, well tolerated and resulted in approximately 30 per cent reduction in neuropathic pain19. However, there is limited published research on the beneficial effects of herbal formulation creams in the management of diabetic neuropathic pain from developing countries. Hence, the present pilot study was conducted to evaluate the efficacy of external application of a polyherbal formulation cream in reducing the painful symptoms of diabetic neuropathy in comparison with a placebo among patients with type 2 diabetes.
Material & Methods
This study was conducted in a tertiary care centre for diabetes in Chennai, Tamil Nadu, India (M. V. Hospital for Diabetes and Prof. M. Viswanathan Diabetes Research Centre). Patients with type 2 diabetes with more than 10 years duration and aged between 40 and 70 yr, who had symptoms of peripheral neuropathic pain, were enrolled during August 2011 to February 2012. Those with diabetic foot ulcers, deformed/contracted foot, congenital deformities, any other neurological complications/diseases, type 1 diabetes and those on neuropathy drugs were excluded. The study protocol was approved by the Ethics Committee of the institution. The patients were fully informed regarding the composition of the cream and its role in treating neuropathic symptoms. All those patients included provided written informed consent for participation in the study.
A total of 54 consecutive patients with type 2 diabetes and neuropathic pain were enrolled in this study. Four of them dropped out (two due to transfer to a different place and two withdrew due to non-response to treatment) and the remaining 50 patients (M:F = 33:17) were finally included. Neuropathy was diagnosed by vibration perception threshold, and a value of >25 V was considered abnormal20. All patients had two or more symptoms of diabetic neuropathy pain. Diabetic Neuropathic Score (DNS) was used to assess the severity of neuropathy21. The symptoms were as follows: numbness in the legs/feet, pricking sensation in the legs/feet, unsteadiness in walking and pain/burning/aching in the legs/feet. Patients having the presence of any of these symptoms in one leg and not the other were rounded to a whole score. Symptom scores were divided into three categories: score 0 (no symptoms), score 1 (one symptom) and score 2 (two or more symptoms). Neuropathic pain was assessed by a trained physiotherapist.
The patients were randomly assigned to two groups: group 1 or study group (n = 26) patients were treated with the polyherbal formulation pain cream (prepared by Sanjeevanam Product and Services, Chennai, India) and group 2 (n = 24) or control group patients were treated with a placebo cream. Age and duration of diabetes were recorded for all patients. Glycated haemoglobin (HbA1c) percentage was estimated by high-performance liquid chromatography method using the Bio-Rad variant turbo equipment (Hercules, CA, USA). The polyherbal neuropathic pain cream was applied externally twice a day by the patients in group 1. Each time, the tube containing the formulation cream was replaced by a new tube by the patients. The study patients were followed up for six months with an interim visit at three months. The effect of the polyherbal formulation cream was determined by assessing the reduction in symptoms of diabetic neuropathy using DNS among the patients in group 1 and compared with that in group 2 patients. During the study, alternative treatments were not given to the patients.
Active ingredients of the polyherbal formulation pain cream
Glycyrrhiza glabra 0.20 per cent: Glycyrrhizin is the active compound, a natural anti-inflammatory and antiviral triterpene in clinical use. It acts as an anti-inflammatory in painful neuropathy.
Musa paradisiaca 19.42 per cent: It possesses analgesic activity, which relieves neuropathic pain.
Curcuma longa 2.43 per cent: Curcumin, the principle compound with anti-inflammatory and antibacterial activities, protects the skin from infections in diabetic neuropathy. Curcumin also has the potential to attenuate hyperalgesia in peripheral diabetic neuropathy.
Pandanus odoratissimus 9.70 per cent: The essential oil of the plant relieves pain, and it contains anti-inflammatory, anti-viral and anti-allergic activities to prevent infections of the diabetic foot.
Aloe vera 4.85 per cent: It contains analgesic activity and reduces pain; it is anti-oedemic and has antidiabetic activity. The coolant effect of the gel in the leaf produces soothing effect on the skin, especially with the symptoms of burning and aching sensations.
Cocos nucifera oil (coconut milk): It nourishes the skin combined with Pandanus odoratissimus and Shorea robusta. It forms a fine protective layer and reduces dryness, scaling and cracking of the skin.
Statistical analysis: SPSS package (Version 16.0, Chicago, USA) was used for performing statistical analyses. Each symptom was entered in a binary score indicating the presence or absence of symptom for each foot (zero meaning absent and one meaning present), and then a total score was calculated by adding these symptom scores. Student t-test and Mann Whitney U tests were used to compare the groups with respect to the variables tested and score of the symptoms experienced by them, for three visits. The baseline visits and the follow ups were compared using repeated measures ANOVA with a Greenhouse-Geisser correction, and Chi-square test was performed to compare the presence and absence of pain/symptoms in the study and control groups.
Results
All patients in both the groups had two or three symptoms of neuropathic pain before enrolment into the study. Table I shows the general characteristics of the study patients. It was observed that mean age, HbA1c levels and duration of diabetes were similar among both the groups. In the first follow up, there was a significant difference in the presence and absence of pain/symptoms between the study and control groups (Chi-square value = 24.693, P<0.005) showing that the study group patients’ symptoms decreased to a large extent than the control group. The second follow up also showed a significant difference between both groups wherein the difference between the study and control groups was significant, with less symptoms present in group 1 (Chi-square value = 20.852, P<0.005).
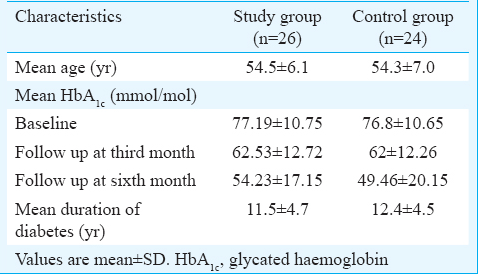
Table II shows the distribution of the patients based on their neuropathic scores during the baseline and follow up visits. At baseline, patients in both the groups had neuropathic symptoms (score 2). During the follow up, the study group patients who applied the polyherbal formulation cream showed a decrease in symptom scores during the first and the second follow up visits. It was observed that 73.1 per cent of them showed a significant reduction in neuropathic pain (with no symptoms), and 3.8 per cent came down to a lesser score of symptoms (score = 1). During the final visit, 76.9 per cent of patients who used this cream had a significant reduction in neuropathic pain. However, 23.1 per cent of the study group patients who had score 2 during the second follow up still had no change in their symptom score at the end of the study, indicating the positive effect of the cream during the first three months follow up itself. The reduction in neuropathic pain was significant among the study group patients compared to that in the control group during both the first and second follow up visits (P<0.001). No adverse events were reported in both groups during the study.
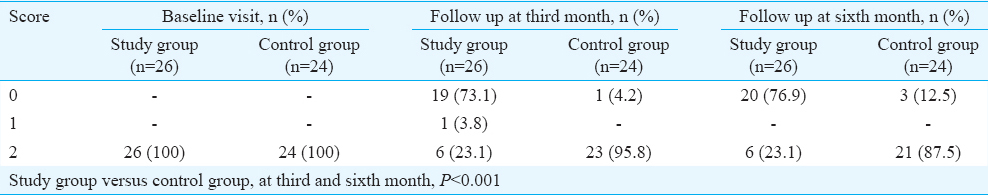
When using a repeated measures ANOVA with a Greenhouse-Geisser correction, in study group, the mean HbA1c values were significantly different through the baseline, until the final follow up (F = 15.734, P<0.005). There was a significant difference between baseline and first follow up visit HbA1c values in the study group, but not so when comparing the first and the second follow up visits. The mean differences between the first and second, second and third and first and third visits were 1.168 (P = 0.003), 0.368 (P = 0.273) and 1.1537 (P = 0.001), respectively.
In the control group, the mean HbA1c values were not significantly different through the baseline, until the final follow up (F = 3.76, P<0.068). There was no significant difference between baseline and first and second follow up visits. The mean differences between first and second, second and third and first and third visits were 0.950 (P = 0.354), 0.470 (P = 0.493) and 1.420 (P = 0.180), respectively.
Discussion
Painful diabetic neuropathy has debilitating consequences with a significant impact on quality of life and cost of management. Many herbal combinations of external remedy, which are cost-effective, are used to reduce the severity of neuropathic pain. The results of the present study showed that neuropathic pain cream prepared using polyherbal formulation was effective as well as safe in reducing symptoms of diabetic neuropathy along with a reduction in neuropathic pain.
Various studies have shown that herbal products are safe and effective for the treatment of diabetes and its complications1617. The previous report from India on a polyherbal formulation showed that daily application of polyherbal cream could reduce the wound size significantly approximately in a mean period of six weeks without any adverse side effect16. Studies on similar herbal formulation, capsaicin cream, derived from chillies, showed a reduction in diabetic neuropathic pain after the first week of its usage1819. However, a few adverse effects such as burning, sneezing or coughing during application were observed. No adverse effects were observed in the present study, indicating the safety of this herbal formulation. Besides, the active ingredients present in this herbal formulation are known to possess anti-inflammatory and analgesic actions, which aid in alleviating the increased inflammation associated with painful diabetic neuropathy.
There were some limitations in the present study. First, it was a pilot study with small sample size. Therefore, the findings have to be confirmed by further evaluating the effect of this cream in a large sample size. Second, the prolonged use of this herbal cream which may have beneficial or probable side effects, was not assessed in this study.
In conclusion, our results showed that daily application of polyherbal cream significantly reduced the symptoms of painful diabetic neuropathy without any adverse side effect. The polyherbal cream may be effective in treating painful diabetic neuropathy along with routine standard care. It must, however, be emphasized that intensive hyperglycaemic control also plays an important role in reducing neuropathic pain in patients with diabetes.
Acknowledgment
Authors thank Sanjeevanam Product and Services, Chennai, India, for providing the polyherbal cream with their formulation, Dr Sonitha Sarathy for her help in preparing the manuscript and Ms. Brindha Manickavasakan for statistical analysis.
Conflicts of Interest: None.
References
- International Diabetes Federation. In: Time of Act: Diabetes and Foot Care. Brusseles: International Diabetes Federation; 2005. p. :1-198.
- [Google Scholar]
- Soci-cultural practices that may affect the development of the diabetic foot. IDF Bull. 1997;42:10-2.
- [Google Scholar]
- The costs of treating long-term diabetic complications in a developing country: a study from India. J Assoc Physicians India. 2013;61:102-9.
- [Google Scholar]
- The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817-24.
- [Google Scholar]
- Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350-4.
- [Google Scholar]
- Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123-8.
- [Google Scholar]
- Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig. 2011;2:33-42.
- [Google Scholar]
- The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518-22.
- [Google Scholar]
- Psychological aspects of diabetic peripheral neuropathy. Diabetes Rev. 1999;7:387-94.
- [Google Scholar]
- Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348:1698-701.
- [Google Scholar]
- The effectiveness of topically applied capsaicin. A meta-analysis. Eur J Clin Pharmacol. 1994;46:517-22.
- [Google Scholar]
- Increased contact heat evoked potential stimulation latencies in responders to spinal cord stimulation for painful diabetic polyneuropathy. Neuromodulation. 2015;; 18:126-32.
- [Google Scholar]
- Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815-23.
- [Google Scholar]
- Pharmacology of medicinal plants and natural products. Indian J Pharmacol. 2000;32:S81-118.
- [Google Scholar]
- A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers. Indian J Med Res. 2011;134:168-73.
- [Google Scholar]
- Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J Pain Res. 2011;4:385-92.
- [Google Scholar]
- Pharmacodynamic analysis of the analgesic effect of capsaicin 8% patch (Qutenza™) in diabetic neuropathic pain patients: detection of distinct response groups. J Pain Res. 2012;5:51-9.
- [Google Scholar]
- Tolerability of NGX-4010, a capsaicin 8% patch, in conjunction with three topical anesthetic formulations for the treatment of neuropathic pain. J Pain Res. 2012;5:7-13.
- [Google Scholar]
- The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557-60.
- [Google Scholar]
- Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med. 2002;19:962-5.
- [Google Scholar]






