Translate this page into:
Drug-induced diseases (DIDs): An experience of a tertiary care teaching hospital from India
Reprint requests: Dr Vishal R. Tandon, Postgraduate Department of Pharmacology and Therapeutics, Government Medical College, Jammu 180 001, Jammu & Kashmir, India e-mail: dr_vishaltandon@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Drug-induced diseases (DIDs) are well known but least studied. Data on DIDs from India are not available. Hence, this retrospective cross-sectional study was undertaken using suspected adverse drug reaction (ADR) data collected form Pharmacovigilance Programme of India (PvPI) to evaluate profile of DIDs over two years, in a tertiary care teaching hospital from north India.
Methods:
The suspected ADRs in the form of DID were evaluated for drug and disease related variables and were classified in terms of causality.
Results:
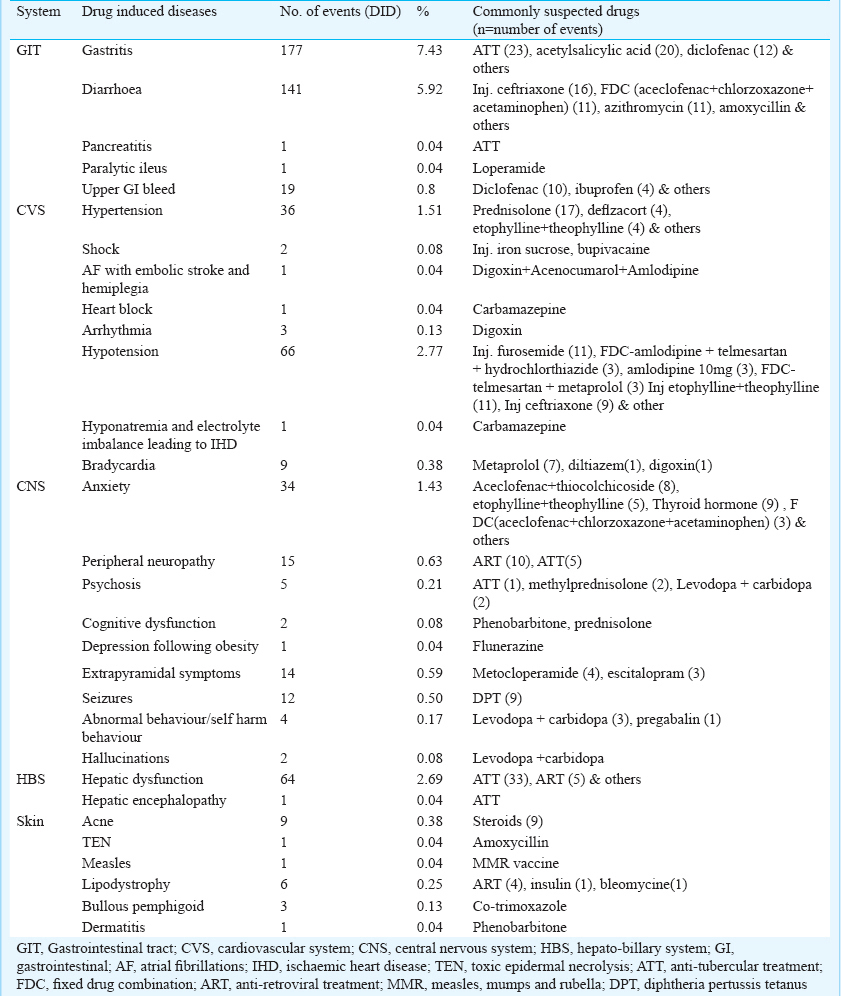
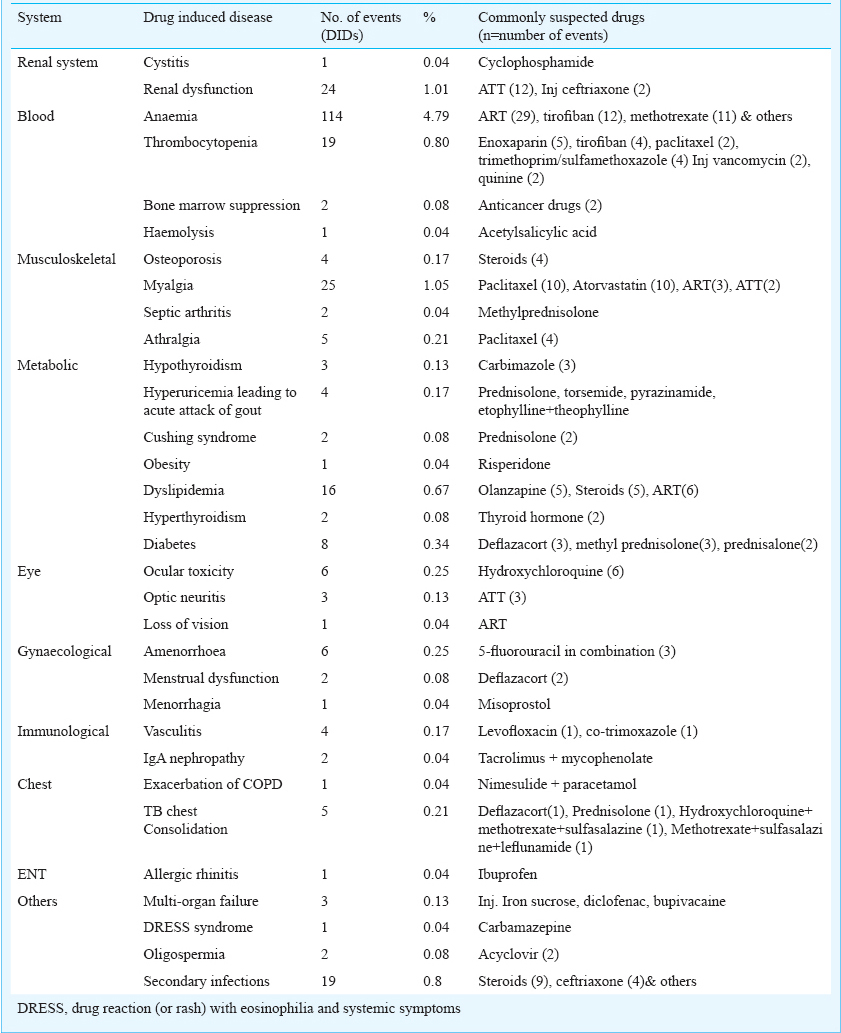
DID rate was 38.80 per cent. Mean duration of developing DIDs was 26.05 ± 9.6 days; 25.16 per cent had more than one co-morbid condition. Geriatric population (53.99%) accounted for maximum DIDs followed by adult (37.79%) and paediatric (8.21%). Maximum events were probable (93.98%) followed by possible (6.04%). All DIDs required intervention. Gastritis (7.43%), diarrhoea (5.92%), anaemia (4.79%), hypotension (2.77%), hepatic dysfunction (2.69%), hypertension (1.51%), myalgia (1.05%), and renal dysfunction (1.01%) were some of the DIDs. Anti tubercular treatment (ATT), anti retroviral treatment (ART), ceftriaxone injection, steroids, non-steroidal anti-inflammatory drugs, antimicrobials and anticancer drugs were found as commonly offending drugs.
Interpretation & conclusions:
Our findings show that DIDs are a significant health problem in our country, which need more attention.
Keywords
Adverse drug reaction
drug-induced disease
iatrogenic
pharmacovigilance
Adverse drug reaction (ADR) has been implicated as a leading cause of considerable morbidity and mortality worldwide. The prevalence rate of ADRs has been reported to range from 0.16 to 15.7 per cent1. Morbidity related to ADRs is also well known and causes a large number of hospital admissions2. Further, ADR related hospitalization in emergency and intensive care units (ICU) is very high among high risk population like elderly population with multiple co-morbidities3. Morbidity related to ADRs can be permanent sometimes to the extent of 20.4 per cent of admissions in ICU4. Besides, ADRs are known to pose huge economic burden on individual, society and nation at large5.
Drug-induced diseases (DID) also called as iatrogenic diseases, are well known but least studied entity. Some of the risk factors of DIDs are multiple chronic diseases, multiple physicians, hospitalization, medical or surgical procedures, long duration of medicine use, advancing age, female sex and a particular class of drugs678. Most of these DIDs are largely preventable9, if strict vigilance and proper periodic clinical and diagnostic monitoring are undertaken. There are studies from the West regarding DIDs910111213141516171819202122, however information from India is lacking. Hence, the current study was undertaken to analyze the profile of DIDs in a tertiary care teaching hospital at Jammu, India.
Material & Methods
A retrospective observational cross-sectional analysis was carried out for the data collected from November 2010 to November 2012 to evaluate the prevalence and profile of DIDs in Adverse Drug Reaction Monitoring (ADRM) Centre, working under Pharmacovigilance Programme of India (PvPI)23 in a tertiary care teaching hospital from north India (Government Medical College, Jammu) using suspected drug reactions monitoring data collection form used under PvPI.
Institute Ethics Committee (IEC) permission was taken prior to commencement of the study.
The ADRs are defined and categorized as per the definition of Edwards and Aronson24, as any response to a drug that is noxious, undesireable and unintended and that occurs at doses normally used in humans for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic function. A drug-induced disease is defined as the unintended effect of a drug that results in mortality or morbidity with symptoms sufficient to prompt a patient to seek medical attention and/or to require hospitalization and may persist even after the offending drug has been withdrawn25.
Information about patient, suspected ADRs in the form of DID, suspected medication, reporter, date of reaction, date of recovery and presentation of problem was recorded. Under suspected medication, name of the drug, brand and generic name of manufacturer (if known), expiry date, dose used, route, frequency and therapy dates as well as reason for prescribing suspected drug were also assessed. The information about de-challenge and re-challenge, concomitant medical treatment record, the relevant biochemical abnormality and use of any diagnostic tool was recorded separately. Other relevant history including pre-existing medical conditions like allergy, pregnancy, smoking and alcohol used and any organ dysfunction was noted. The severity and seriousness of reaction, the outcome of reaction were recorded for every suspected ADR in the form of DID as recommended under PvPI. The suspected ADRs in the form of DIDs were classified in term of causality using WHO-UMC (Uppsala Monitoring Centre) scale26. Types of reaction were classified as Type A (augmented); Type-B (bizarre), Type C (continuous use); Type D (delayed); and Type E (end of use as per recommended standard operating procedure of PvPI26.
Inclusion & exclusion criteria: Any ADR in the form of DID reported from OPD or inpatient of any severity, duration, and any type of reaction was included pertaining to drugs and vaccines. Any case of poisoning, medication error, over dosage, over/non-compliance, natural products/alternate medicines and unidentified drugs were excluded from the analysis.
Statistical analysis: Analysis was carried out with the help of computer software SPSS Version 15 for windows (SPSS, Inc., Chicago, USA). Chi-square test was applied for statistical comparison.
Results
The total number of ADR events reported during the two years study period was 2381 and of these 926 (38.89%) were the drug induced disease rate (Table I). Total number of ADRs was 2242. Mean duration of appearance of DIDs was 26.05±9.6 days. Overall, 10.79, 15.11, 73.98 and 0.10 per cent DIDs were mild, moderate, severe and fatal, respectively; 15.11, 10.79 and 74.08 per cent, respectively were sub acute, acute and latent in nature. Further, 80.99 per cent DIDs were serious and 19.00 per cent non serious in nature. Maximum events were probable (93.95%), followed by possible (6.04%). Overall, 94.60 per cent of DIDs recovered and 5.37 per cent continued in similar mode at the time of report collection (Table I).
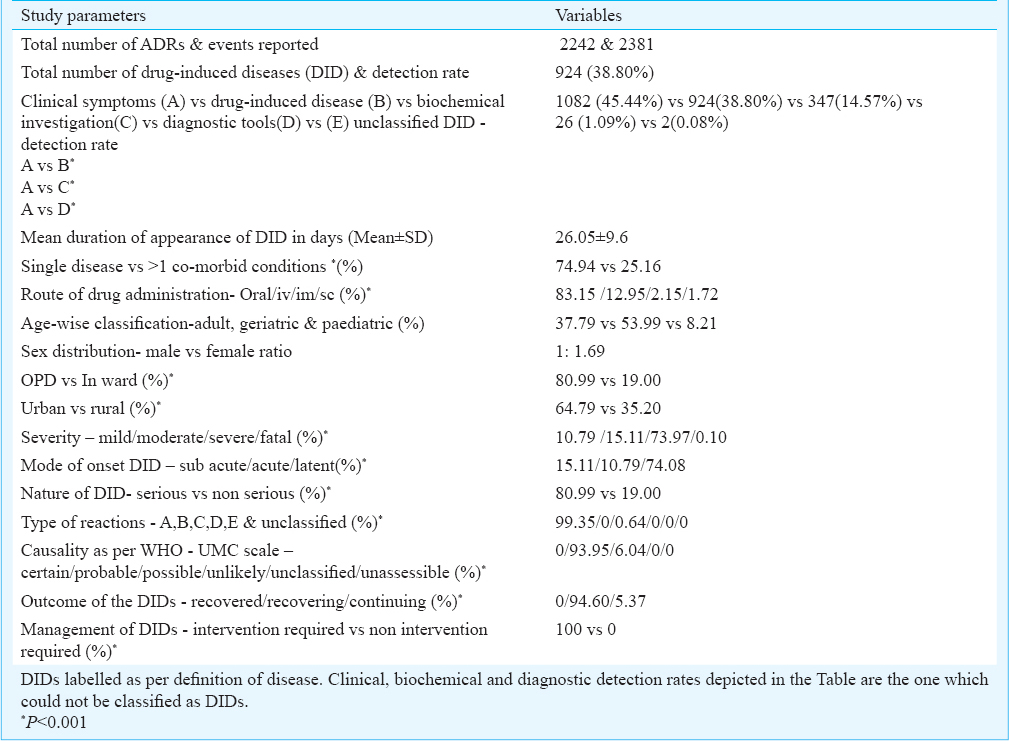
Gastritis (7.43%), diarrhoea (5.92%), anaemia (4.79%), hypotension (2.77%), hepatic dysfunction (2.69%), were some of the common DIDs in the current study. The list of other DID and the common suspected drugs are depicted in Table II a, b.
Discussion
In the current study the DID rate was 38.9 per cent suggesting DIDs to be a significant health problem. The present results were comparable with those of Atiqi et al9 depicting incidences of DIDs between 3.4 and 33.9 per cent. However, the current study largely depended on spontaneous nature of ADR reporting. The prevalence rate of 10.3 per cent of DIDs as reported in a French study10 is far low in comparison to our study. This may be because their study focused on DIDs mainly reported from medicine department, unlike our study which was largely a cross-sectional study.
The females predominated in the current study with male: female ratio of 1: 1.69 and these results were in accordance with a study by Zopf et al6. Geriatric population (53.99%) accounted for maximum DIDs, similar to a study by Permpongkosol7 where elderly patients were shown to encounter more DIDs as a result of multiple chronic diseases, multiple physicians, hospitalization, and medical or surgical procedures. Mean duration of developing DID in the current study was 26.05 days, 25.16 per cent had more than one co-morbid condition and 99.35 per cent of the total events were type A reaction. This clearly indicated that most of the DIDs could have been prevented if strict vigilance, proper periodic clinical and diagnostic monitoring were undertaken. Similar results have been reported by Ahern et al8.
As far as common DIDs caused by most common suspected drugs and class were concerned, varied results were noticed on comparison with various studies. Atiqi et al9 recorded cardiac disease, hypertension and gastrointestinal conditions as most common DIDs resulting due to anticoagulant treatment and use of non-steroidal anti-inflammatory drugs (NSAIDs). Gastrointestinal bleeding due to NSAID, acetylsalicylic acid and warfarin were the most common DIDs reported by Brvar et al11. Unlike our study, phlebitis at the injection site has been reported as most frequently occurring iatrogenic event in another study12.
Thiessard et al27 recorded skin and subcutaneous tissue disorders (29%), followed by nervous system (19%), gastrointestinal (12%), blood and lymphatic system (12%) and vascular disorders (12%) as most common DIDs in their study. Peripheral neuropathy, anaemia, hepatitis and gastritis were the most prevalent DIDs with use of highly active anti-retroviral therapy (HAART) treatment in the study of Anwikar et al28. Rather et al29 reported anaemia, hepatic toxicity, itching, skin rash, elevated triglycerides and peripheral neuropathy to be the most common DIDs in their study due to ART. Common cardiovascular adverse drug events reported were drug-induced arrhythmias, blood pressure abnormalities and heart failure22. The specific drug-induced events included bradycardia, tachycardia, corrected QT interval prolongation, hypertension, hypotension and heart failure exacerbation. In the present study hypotension, hypertension, bradycardia, arrythimias and irregular pulse were recorded as common cardiovascular DIDs.
Drug-induced immune haemolytic anaemia has been commonly reported in few studies1718 with cefotetan, ceftriaxone, and piperacillin. However, ART, tirofiban and methotrexate were most commonly offending agents to cause anaemia in our study.
Enoxaparin, tirofiban, paclitaxel, trimethoprim/sulphamethoxazole, injection vancomycin and quinine were responsible for thrombocytopenia, as also reported by Arnold et al19. They recorded quinine, quinidine, trimethoprim/sulphamethoxazole and vancomycin as the most common culprit for drug induced thrombocytopenia.
Anti-tuberculosis treatment (ATT) induced hepatic and renal dysfunction were common DIDs in our study which were in accordance to Tariq et al20. However, our results were in variance to the results of another study21 where antidepressants were shown to be associated with causing hepatotoxicity. Paroxetine, fluoxetine, fluvoxamine, citalopram, mirtazapine and venlafaxine were associated with reversible liver injury. This was because their field of research was exclusive with anti-depressants unlike ours which was a cross-sectional study.
The major limitation of the current study is that it does not represent the true prevalence of the problem due to voluntary/spontaneous nature of reporting. Risk factor correlation was not done in the current study. The present data were generated by spontaneous reporting system as proposed by PvPI. Thus, there might be many other confounding factors which could have affected the final outcome of the study. There exist a lot of variations in the trends of DIDs reported worldwide. Such studies carried out across the country in future shall go long way to provide clinicians and policy regulators valuable information about DIDs which can be largely prevented in the interest of patient safety.
Acknowledgment
The authors acknowledge the Indian Pharmacopeia Commission, for all support provided under Pharmacovigilance Programme of India (PvPI) and WHO for this important Initiative on Patient Safety
References
- Hospital admission associated with adverse drug reaction: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42:1017-25.
- [Google Scholar]
- Adverse drug reactions and prescription errors: morbi-mortality. Medicina (B Aires). 2013;73:111-8.
- [Google Scholar]
- Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clin Belg. 2013;68:15-21.
- [Google Scholar]
- Adverse drug reactions which provoke hospital admission. Farm Hosp. 2011;35:236-43.
- [Google Scholar]
- Incidence and costs of adverse drug reactions in a tertiary care pediatric intensive care unit. J Clin Pharmacol. 2013;53:567-73.
- [Google Scholar]
- Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf. 2008;31:789-98.
- [Google Scholar]
- Iatrogenic disease in the elderly: risk factors, consequences, and prevention. Clin Interv Aging. 2011;6:77-82.
- [Google Scholar]
- Determining the frequency and preventability of adverse drug reaction-related admissions to an Irish University Hospital: a cross-sectional study. Emerg Med J. 2014;31:24-9.
- [Google Scholar]
- Prevalence of iatrogenic admissions to the Departments of Medicine/Cardiology/Pulmonology in a 1,250 bed general hospital. Int J Clin Pharmacol Ther. 2010;48:517-24.
- [Google Scholar]
- Iatrogenic medication: estimation of its prevalence in French public hospitals. Regional Centers of Pharmacovigilance. Therapie. 1999;54:21-7.
- [Google Scholar]
- The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol. 2009;9:8.
- [Google Scholar]
- Iatrogenic illness in a department of general internal medicine: a prospective study. Mt Sinai J Med. 1989;56:267-71.
- [Google Scholar]
- [Adverse drug effects observed at French admissions departments and emergency services (Prospective study of the National Educational Association for Teaching Therapeutics and proposals for preventive measures)] Bull Acad Natl Med. 2003;187:647-66. discussion 666-70
- [Google Scholar]
- Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29:35-9.
- [Google Scholar]
- Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing. 1997;26:375-82.
- [Google Scholar]
- Drug-induced immune hemolytic anemia. Hematol Am Soc Hematol Educ Program. 2009;1:73-9.
- [Google Scholar]
- Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev. 2013;27:137-45.
- [Google Scholar]
- Frequency of anti-tuberculous therapy-induced hepatotoxicity in patients and their outcome. J Ayub Med Coll Abbottabad. 2009;21:50-2.
- [Google Scholar]
- Drug-induced cardiovascular adverse events in the intensive care unit. Crit Care Nurs Q. 2013;36:323-34.
- [Google Scholar]
- Pharmacovigilance programme of India (PvPI) National Co-ordination centre Indian Pharmacopeia Commission, Ghaziabad. Available from: http://www.pic.gov.in/PvPI/pv_amcs.htmli
- [Google Scholar]
- Adverse drug reactions: Definitions, diagnosis and management. Lancet. 2000;356:1255-9.
- [Google Scholar]
- Drug induced disease. In: Tisdale JE, Miller DA, eds. Prevention, detection, and management (2nd ed). Bethesda: American Society of Health-System Pharmacists; 2005. p. :870-7. Nova South eastern University, College of Pharmacy - West
- [Google Scholar]
- Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87-97.
- [Google Scholar]
- Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986-2001) Drug Saf. 2005;28:731-40.
- [Google Scholar]
- HAART induced adverse drug reactions: a retrospective analysis at a tertiary referral health care center in India. Int J Risk Saf Med. 2011;23:163-9.
- [Google Scholar]
- Evaluation of the adverse reactions of antiretroviral drug regimens in a tertiary care hospital. Indian J Pharmacol. 2013;45:145-8.
- [Google Scholar]






