Translate this page into:
Dried blood spot HIV-1 RNA quantification: A useful tool for viral load monitoring among HIV-infected individuals in India
Reprint requests: Dr Anita Shet, Department of Pediatrics, St. John's Medical College & Hospital, St. John's National Academy of Health Sciences, Sarjapur Road, Bangalore 560 034, India e-mail: anitashet@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Monitoring of HIV-infected individuals on antiretroviral treatment (ART) ideally requires periodic viral load measurements to ascertain adequate response to treatment. While plasma viral load monitoring is widely available in high-income settings, it is rarely used in resource-limited regions because of high cost and need for sophisticated sample transport. Dried blood spot (DBS) as source specimens for viral load measurement has shown promise as an alternative to plasma specimens and is likely to be a useful tool for Indian settings. The present study was undertaken to investigate the performance of DBS in HIV-1 RNA quantification against the standard plasma viral load assay.
Methods:
Between April-June 2011, 130 samples were collected from HIV-1-infected (n=125) and non-infected (n=5) individuals in two district clinics in southern India. HIV-1 RNA quantification was performed from DBS and plasma using Abbott m2000rt system after manual RNA extraction. Statistical analysis included correlation, regression and Bland-Altman analysis.
Results:
The sensitivity of DBS viral load was 97 per cent with viral loads >3.0 log10 copies/ml. Measurable viral load (>3.0 log 10 copies/ml) results obtained for the 74 paired plasma-DBS samples showed positive correlation between both the assays (r=0.96). For clinically acceptable viral load threshold values of >5,000 copies/ml, Bland-Altman plots showed acceptable limits of agreement (−0.21 to +0.8 log10 copies/ml). The mean difference was 0.29 log10 copies/ml. The cost of DBS was $2.67 lower compared to conventional plasma viral load measurement in the setting
Interpretation & conclusions:
The significant positive correlation with standard plasma-based assay and lower cost of DBS viral load monitoring suggest that DBS sampling can be a feasible and economical means of viral load monitoring in HIV-infected individual in India and in other resource-limited settings globally.
Keywords
Dried blood spot
HIV
India
public health
resource-limited settings
viral load
A rapid scale up of the national antiretroviral treatment programme in India initiated in April 2004, has ensured that as of March 2011, over 420,000 people living with HIV infection are currently on first-line antiretroviral treatment (ART) free of cost in the public sector1. The WHO has recommended using clinical monitoring and CD4 measurement as an alternative in resource-limited settings, however, several studies have reported poor sensitivity of these methods in the detection of virological failure23. Thus, patients in these settings run the risk of being treated with failing regimens and accumulating drug resistant mutant viruses, thus restricting treatment options further and reducing the chance of complete viral suppression even when switched to second-line treatment in the future. Given this situation, it becomes important to explore a feasible viral load measurement procedure that works in low income settings. An alternative strategy of viral load measurement using dried blood spot (DBS) has shown promise as a reliable method of viral load measurement, which can significantly reduce transport difficulties as well as cost4–8. In addition, DBS requires a lesser volume of blood with reduced infectious risk making it safer to handle and also can be stored and transported at room temperature910. Recent reports on the evaluation of DBS in resource-limited settings from Sub-Saharan Africa show promising trends61112. DBS has not been utilized or evaluated systematically in India. The present study was, therefore, conducted to compare the feasibility and cost of DBS HIV-1 RNA quantification with standard plasma viral load measurement for HIV-infected individuals in India receiving antiretroviral therapy.
Material & Methods
Study settings and patients: The patients included in the study were aged between 18-60 years, had documented HIV-1 infection and were part of the larger HIVIND cohort13. The HIVIND is a randomized control trial (Trial registration: ISRCTN79261738). The study protocol and patient inclusion and exclusion criteria were mentioned elsewhere13. The specific sites included the Infectious Disease Clinic of St. John's Medical College Hospital, Bangalore (Site 1) and ART Centre, K.R. Hospital, Mysore (peripheral centre) (Site 2; 200 km away from site 1). Both sites were situated within tertiary care hospitals located in two cities in Karnataka State in southern India. The main laboratory was situated in site 1. Ethical approval of the study protocol was obtaining from the ethics committees at both sites and patients were recruited after written obtained informed consent. During summer season, between April and June 2011, a total of 125 whole blood samples (2 ml) from HIV-1 positive samples were collected in EDTA vacutainers from patients. Among the 125 samples, 73 were collected from site 1 and 52 from site 2 from therapy-naïve and experienced participants. Samples were selected based on the plasma viral load in three strata; viral load (VL) 150 to 1000 copies/ml (n=30), VL 1000 to 5000 copies/ml (n=20) and VL>5000 copies/ml (n=56). Samples with VL not detected (n=19) were also included in the analysis. In addition, samples were collected from 5 HIV-1 uninfected individuals to serve as true negatives in every run to check for cross-contamination.
Preparation of DBS: Dried blood spot strips were prepared by spotting 50 ΅l of whole blood onto a Whatman 903 filter paper (3 spots per card). In site 1, filter papers were air dried overnight at room temperature and stored at 4°C in plastic sealed bag with a silica desiccant until they were processed. In site 2, collection and spotting of the DBS occurred in a similar fashion; however, after air drying overnight the DBS strips were stored at room temperature (Temperature: 30 to 35°C and humidity 71 to 82%) until transport. The DBS strips were transported at regular intervals (every 15 to 20 days) in plastic sealed bags at room temperature, followed by storage at 4°C until assay performance.
Preparation of plasma: Whole blood was centrifuged at 800 × g for 15 min, and plasma was extracted, aliquoted and stored at −20°C. Plasma samples separated in the peripheral centre were stored at −20°C and were transported to the primary laboratory in dry ice and stored at −80°C until analysis.
Plasma viral load: Plasma viral load was determined by Abbott Real Time PCR (RT-PCR) m2000rt system (Abbott Molecular, Germany), using manual RNA extraction procedure as per manufacturer's instructions. In short, plasma was treated with mLysis buffer (provided with Abbott RNA sample preparation system) to lyse the viral coat and release the nucleic acid. The armoured internal control was added to the lysis buffer before the treatment of the plasma. The lysed viral and internal control RNA was captured on magnetic beads followed by several washing steps to remove contaminants and inhibitors of RNA. Pure RNA was then eluted and detected using quantitative real-time RT-PCR. The internal control ensured correct extraction and non-inhibition of the RT-PCR reaction. The assay detects a dynamic range of viral load ranging from 40 to 10,000,000 copies/ml. Quality control of the assay was maintained by certification from Quality Control for Molecular diagnostics (www.qcmd.org, Glasgow, Scotland).
DBS viral load: Dried blood spot viral load was measured as described previously with modifications4. Briefly, two blood spots from the same patient were punched out using a sterile puncher, and placed into 1.7 ml of mLysis buffer provided with the Abbott sample preparation system (m2000sp) in 50 ml sealed conical tubes. The tubes were incubated at room temperature for 2 h, with intermittent mixing. RNA was extracted manually from the lysate according to the standard HIV-1 RNA 1.0 ml extraction protocol using Abbott RNA sample preparation system. The viral load was measured from the extracted RNA using “m2000 DBS HIV-1 RNA ‘open-mode’ protocol” (Abbott Molecular, Germany). Laboratory researchers analysing the DBS were blinded about the true plasma viral load values.
Statistical analysis: Viral load was stratified into three levels: (i) VL 2.17 to 3 log10 copies/ml (corresponding to 1000 copies/ml), (ii) VL >3 to 3.7 log10 copies/ml, (1000- about 5000 copies/ml), and (iii) VL >3.7 log10 copies/ml (corresponding to approximately 5000 copies/ml). Sensitivity and specificity of DBS viral load using plasma assay as the gold standard was assessed at all three viral load strata. Pearson corelation analysis was performed, as well as Bland-Altman analysis14 to examine the level of agreement between the two tests. Bland-Altman analysis was performed using MedCalc version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium). With the given sample size that was used for Bland Altman analysis, the 95% CI for the limits of agreement were +/− 0.11 log10 copies/ml. This narrow range in the precision of the limits of agreement was deemed to be clinically acceptable. In addition, the costs incurred for viral load estimation from the DBS and plasma were assessed and compared. For both the assays, costs considered included sample collection (filter paper and vials), envelopes, transport by courier (with cold chain maintenance for plasma and regular surface mail for DBS), laboratory consumables, external quality control, power consumption and real-time PCR assay including instrument cost, reagent cost and labour cost as per the prevailing retail price in India in July 2011 and for an assumed testing volume of approximately 1000 samples/year.
Results
Plasma viral load was obtained from a total of 125 individuals, and included those who were ART naive (n=54); individuals recently initiated on ART, (n=71; 50 at 4 wk and 21 at wk 24 after ART initiation) and 5 HIV-uninfected individuals. Among HIV-infected individuals, 106 (84.8%) had detectable plasma viraemia ranging from 150 to 6,613,818 copies/ml and 19 (15.2%) had undetectable viraemia (viral load <150 copies/ml).
Sensitivity and specificity analysis: Among samples where plasma viral load was >3.7 log10 copies/ml (n=56) the DBS assay had a sensitivity of 100 per cent and a specificity of 100 per cent. DBS sensitivity was 90 per cent for samples with plasma viral load ranging from >3 to 3.7 log10 copies/ml (n=20). Among samples with plasma viral load levels of 2.17 to 3 log10 copies/ml (n=30), sensitivity of DBS was low at 50 per cent although specificity remained 100 per cent.
Correlation and Bland-Altman analysis: Of the 125 samples, 74 plasma-DBS pairs with detectable viral load in both the assays with >3 log10 copies/ml viral load were included in the analysis. There was a positive correlation between the two assays with a Pearson correlation coefficient of r= 0.96; P<0.05 (Fig. 1). A good correlation was observed between the DBS and plasma viral load values from samples obtained in both the sites (site 1: r=0.96; P<0.05 and site 2: r=0.97; P<0.05). Bland-Altman plots for samples with the clinically relevant viral load threshold values of >3.7 log10 copies/ml (~5,000 copies/ml) (n=56) showed good level of agreement with a mean difference (bias) of 0.29 log10 copies/ml) with acceptable limits of agreement (-0.21 and +0.8 log10 copies/ml) (Fig. 2). Overall, 80.4 per cent (45/56) of the samples had DBS values which differed less than 0.5 log10 units15 compared to the corresponding plasma viral load value. This difference is clinically acceptable15. Among the remaining 11 samples, the highest difference was 0.75 log10 copies/ml, which still remains within the clinically acceptable range.

- Correlation between HIV-1 viral loads measured with the Abbott Real-Time m2000rt assay in dried blood spot (DBS viral load; y-axis) and liquid plasma (Plasma viral load, x-axis) samples. Each data point represents one of the 74 individual study samples with viral load >3 log10 copies/ml. The Pearson correlation coefficient was 0.96 (P<0.05).

- Bland-Altman plot with 95% CI of limits of agreement between HIV-1 viral loads measured with the Abbott Real-Time m2000rt assay in dried blood spot and liquid plasma samples >3.7 log10 copies/ml (n=56). Results indicated a good limit of agreement with -0.21 and +0.80 log10 copies/ml (mean ± 2 SD). The mean difference or bias was 0.29 log10 copies/ml.
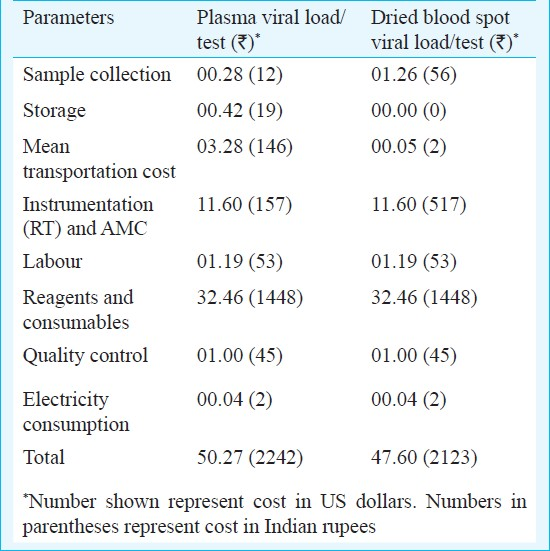
Cost description: The total cost for DBS viral load and plasma viral load in the study setting at prevailing rates was calculated as $ 47.60 (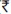 2,123) and $ 50.27 (
2,123) and $ 50.27 (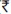 2,242) respectively Table. Costs of plasma transport varied within States and between States, and ranged between $ 2.94-3.08 (
2,242) respectively Table. Costs of plasma transport varied within States and between States, and ranged between $ 2.94-3.08 (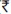 131-137) per sample. The overall cost incurred for testing of the DBS was $ 2.67 (
131-137) per sample. The overall cost incurred for testing of the DBS was $ 2.67 (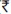 119) less per test compared to direct plasma viral load testing. Cost savings included those incurred for cold chain transportation and other logistics such as electricity and containers for transport required for plasma viral load.
119) less per test compared to direct plasma viral load testing. Cost savings included those incurred for cold chain transportation and other logistics such as electricity and containers for transport required for plasma viral load.
Discussion
Our results showed a significant correlation between plasma and DBS viral load measurement. Moreover, for viral load values >3.7 log10 copies/ml (>5000 copies/ml); 80.4 per cent of samples had less than 0.5 log10 difference, and none exceeded a difference of 0.75 log10 copies/ml, suggesting that DBS is a reliable method for detecting clinically significant virological failure.
The use of dried blood spot specimens as a source for diagnostic tests has become increasingly popular in recent years. DBS has been used to identify genetic and metabolic disorders in neonates16, detection of HIV-1 antibody17 and HIV-1 DNA for infant diagnosis of HIV infection18. The WHO has recommended the use of DBS for HIV drug resistance (HIVDR) surveillance for monitoring transmitted drug resistance in resource-limited settings19. The reasons for the tremendous popularity of DBS as a method of sample collection storage and analysis can be understood when one considers the numerous advantages with this method. In regions of limited expertise and infrastructure and non-reliable functioning of the cold chain for transport, DBS can be prepared by spotting whole blood onto a filter paper, either from venous blood or directly from a finger prick, and transported at ambient temperature by road or via the postal system to the reference laboratory for analysis. In addition, DBS requires less sample volume and also has reduced biohazard risk that makes this an ideal method of transport.
Recent studies indicate that viral load measurement using plasma and DBS are comparable, although this is dependent on the RNA extraction method used42021. Many of these studies had been conducted in controlled conditions in reference laboratories in high-income settings in Europe and United States with automated RNA extraction processes which may not be feasible in basic laboratories in resource-limited settings. In a study using Abbott m2000rt system and automated RNA extraction procedure conducted by Marconi et al4 in Italy, paired DBS and plasma specimens with viral load levels ranging between 200 to >100,000 copies/ml were used revealing a sensitivity of 97 per cent; and 78.5 per cent pairs differed from each other by less than 0.5 log10 copies/ml. A Spanish study conducted by Garrido et al5 with manual RNA extraction showed a DBS sensitivity of 75.3 per cent, with only 51.9 per cent samples have a difference of < 0.5 log10 viral load. In our study, two thresholds were considered; viral load of 1,000 copies/ml (log10 3.0 copies/ml) which is often used as the cut-off for genotyping22 and 5,000 copies/ml (log10 3.7 copies/ml) which is the WHO threshold for definition of virological failure for individuals on first-line ART and needing to switch to second-line therapy1523. The high sensitivity and specificity of DBS viral load measurement achieved in our study was concordant with previous findings4–812, which precludes the possibility of missing patients with virological failure who are eligible for second-line therapy as per the WHO guideline23. Moreover, the positive correlation of DBS with plasma viral load at these thresholds, with good limits of agreement and minimal bias suggests that DBS is a reliable method for clinical interpretation of virological failure.
Other published studies have also indicated the feasibility of HIV-1 viral load quantification using DBS in varied settings with differing climatic and storage conditions, using an array of different commercial assays in resource-limited settings in Sub Saharan Africa and Asia24–26. Previously, HIV-1 RNA quantitation from DBS has shown good stability under different temperature and storage conditions ranging from ambient to -70°C4712. In our study, site 1 had ideal DBS collection and storage facilities, while site 2 was a setting with very basic laboratory facilities where DBS samples were stored at room temperature and relatively high humidity for a mean duration of 18 days, and then transported to the central laboratory for viral load measurement. Despite the less than ideal conditions at site 2 (DBS stored at room temperature), there was good correlation of plasma and DBS viral load measurements for the samples with detectable viral load. Previous studies from other parts of the world have also supported the robustness of DBS when stored at room temperature81227. India is a tropical country with varied climatic conditions and the summer temperature can be very high. The recommended storage temperature for DBS continues to be 4°C, until further multicentric studies evaluate the efficacy of viral load monitoring by DBS stored at different temperatures in different climatic conditions in India.
The reduction in cost for DBS samples compared to direct plasma viral load testing can be substantial when applied at a national scale. With a cost reduction of $2.67 ( 119) per test, the substitution of DBS testing for annual viral load assessment of approximately 400,000 individuals, the Indian public health system can save over $ 1,000,000 (
119) per test, the substitution of DBS testing for annual viral load assessment of approximately 400,000 individuals, the Indian public health system can save over $ 1,000,000 ( 44,600,000) per year. In India, second-line ART is available at several centres around the country, where patients failing first-line ART are first assessed for virological failure before switching regimens. Even at the current rate of virological testing for potential failures (approximately 10,000 tests per year), the cost savings with substitution of DBS for direct plasma viral load testing would amount to over $ 25,000 (
44,600,000) per year. In India, second-line ART is available at several centres around the country, where patients failing first-line ART are first assessed for virological failure before switching regimens. Even at the current rate of virological testing for potential failures (approximately 10,000 tests per year), the cost savings with substitution of DBS for direct plasma viral load testing would amount to over $ 25,000 ( 1,115,000) a year28.
1,115,000) a year28.
Our study was limited geographically to southern India, a high-prevalence area with a predominance of subtype C virus. The non-inclusion of samples from other areas in India, particularly north-eastern areas where there is a greater prevalence of HIV-1 circulating recombinant forms, as well as hepatitis C co-infection may decrease the generalizability of our results. The relatively small sample size included in this study may also limit the accuracy of the results, however, significant correlation and limits of agreement of the two assay methods found in the study reinforce the usefulness and feasibility of utilizing DBS as a method for clinical viral load monitoring of patients on ART in India. Although the performance of DBS decreased at the lower viral load values (150 to 1,000 copies/ml), the correlation at the higher viral load values underscores the value of DBS as a feasible clinical monitoring tool. One limitation of the DBS method noted previously by investigators suggest that the proviral DNA present in peripheral blood mononuclear cells can contribute towards a falsely high DBS viral load value8. However, our study has not revealed this, and higher DBS values have been noted in samples with viral load levels <3.7 log10 copies/ml, but not in the >3.7 log10 copies/ml group. Further, the dedicated “m2000 DBS HIV-1 RNA open-mode protocol” for DBS viral load in Abbott m2000rt system uses a constant correction factor which is highly reproducible4 and can overcome the interference of proviral DNA in viral load measurement.
In conclusion, our results suggest that the usefulness, feasibility and cost advantage of measuring viral load from DBS samples in tropical climatic conditions in India make DBS as an alternative sampling method for viral load monitoring in resource-limited settings specifically from the rural and remote parts of the country. Further studies of operational research to apply these findings within a clinical setting on a large scale will be useful in translating these findings into policy that will benefit India and the global community.
Acknowledgment
The study has been partially funded by a grant from European Union Framework Program 7.
References
- People alive and on ART, National AIDS Control Organization May 2011. Available from: http://www.nacoonline.org/upload/HIV%20data/Patients%20%20alive%20on%20ART%20_May%202011.pdf
- [Google Scholar]
- Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51-62.
- [Google Scholar]
- Evaluation of the Abbott real-time HIV-1 quantitative assay with dried blood spot specimens. Clin Microbiol Infect. 2008;15:93-7.
- [Google Scholar]
- Correlation between human immunodeficiency virus type 1 (HIV-1) RNA measurements obtained with dried blood spots and those obtained with plasma by use of Nuclisens EasyQ HIV-1 and Abbott RealTime HIV load tests. J Clin Microbiol. 2009;47:1031-6.
- [Google Scholar]
- Measure of viral load by using the Abbott Real-Time HIV-1 assay on dried blood and plasma spot specimens collected in 2 rural dispensaries in Cameroon. J Acquir Immune Defic Syndr. 2009;52:9-16.
- [Google Scholar]
- Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision and RNA stability. J Clin Microbiol. 2003;41:1888-93.
- [Google Scholar]
- Dried blood spots perform well in viral load monitoring of patients who received antiretroviral treatment in rural Tanzania. Clin Infect Dis. 2009;49:976-81.
- [Google Scholar]
- Early detection of human immunodeficiency virus on dried blood spot specimens: sensitivity across serial specimens. Women and Infants Transmission Study Group. J Pediatr. 1996;129:111-8.
- [Google Scholar]
- World Health organization/HIVResNet Drug Resistance Laboratory Strategy. Antiviral Ther. 2008;13(Suppl 2):49-57.
- [Google Scholar]
- Evaluation of filter paper transfer of whole blood and plasma samples for quantifying HIV RNA in subjects on antiretroviral therapy in Uganda. J Acquire Immune Defic Syndr. 2007;46:590-3.
- [Google Scholar]
- Quantitation of HIV-1 RNA in dried blood spots by the real-time NucliSENS EasyQ HIV-1 assay in Senegal. J Virol Methods. 2008;148:291-5.
- [Google Scholar]
- Design of a randomized trial to evaluate the influence of mobile phone reminders on adherence to first line antiretroviral treatment in South India - the HIVIND study protocol. BMC Med Res Methodol. 2010;10:25.
- [Google Scholar]
- Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;307:307-10.
- [Google Scholar]
- Technical Brief on HIV Viral Load Technologies (June 2010) Available from: http://www.who.int/hiv/topics/treatment/tech_brief_20100601_en.pdf,
- [Google Scholar]
- DNA microextraction from dried blood spots on filter paper blotters: potential applications to newborn screening. Hum Genet. 1987;75:213-6.
- [Google Scholar]
- Seroprevalence of human immunodeficiency virus among childbearing women. Estimation by testing samples of blood from newborns. N Engl J Med. 1988;318:525-30.
- [Google Scholar]
- Diagnosis of vertical HIV-1 transmission using the polymerase chain reaction and dried blood spot specimens. J Acquir Immune Defic Syndr. 1992;5:113-9.
- [Google Scholar]
- WHO manual for HIV drug resistance testing using dried blood spot specimen, March 2010. Available from: http://www.who.int/hiv/topics/drugresistance/dbs_protocol.pdf
- [Google Scholar]
- Isolation of HIV-1 RNA from plasma: evaluation of seven different methods for extraction (part two) J Virol Methods. 1998;76:153-7.
- [Google Scholar]
- Comparison of the Abbott m2000 HIV-1 Real-Time and Roche AMPLICOR Monitor v1.5 HIV-1 assays on plasma specimens from Rakai, Uganda. Int J STD & AIDS. 2011;22:373-5.
- [Google Scholar]
- Detection of drug resistance mutations at low plasma HIV-1 RNA load in a European multicentre cohort study. J Antimicrob Chemother. 2011;66:1886-96.
- [Google Scholar]
- Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach.–2010 rev. July 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf
- [Google Scholar]
- Dried blood spots in HIV monitoring: applications in resource-limited settings. Bioanalysis. 2010;2:1893-908.
- [Google Scholar]
- Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review. Antivir Ther. 2009;14:619-29.
- [Google Scholar]
- Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195-208.
- [Google Scholar]
- Quantification of HIV-RNA from dried blood spots using the Siemens VERSANT HIV-1 RNA (kPCR) assay. J Antimicrob Chemother. 2011;66:2823-6.
- [Google Scholar]
- National AIDS Control Program, Phase III (2006-2011), November 2006. Available from: http://www.nacoonline.org
- [Google Scholar]






