Translate this page into:
Do children with severe acute respiratory infection need cohorting & isolation before screening for COVID-19?
For correspondence: Dr Rakesh Kumar, Department of Paediatrics, Postgraduate Institute of Medical Education & Research, Sector 12, Chandigarh 160 012, India e-mail: drrakesh.pgi@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This retrospective analysis was done to ascertain the SARS-CoV-2-positivity rate in children (0-12 yr) with severe acute respiratory infection (SARI) and compare it to those without SARI to determine the need for running a dedicated SARI isolation facility for paediatric COVID-19 care. The case records of 8780 children (0-12 yr) admitted and/or tested for SARS-CoV-2 between June 2020 and May 2021 at a tertiary care centre in north India were analyzed. The overall SARS-CoV-2 reverse transcription (RT)-PCR positivity rate was 3.0 per cent (262/8780). There were 1155 (13.15%) children with SARI. Fifty of these 1155 (4.3%) children with SARI, as against 212 of the 7625 (2.8%) children without SARI, tested positive for COVID-19. The absolute difference in the positivity rate among SARI and non-SARI groups was only 1.54 per cent which translates to cohorting and isolating 65 children with SARI to pick up one extra SARS-CoV-2-positive child (compared to those without SARI). The positive predictive value of SARI as a screening test was 4.3 per cent. Our findings suggest that isolation of children with SARI as a transmission-prevention strategy for COVID-19 may not be required. This is particularly relevant in resource-limited settings.
Keywords
Children
COVID-19
isolation
RT-PCR
SARS-CoV-2
screening
SARI
The World Health Organization (WHO) defines severe acute respiratory infection (SARI) as cough and fever requiring hospitalization within 10 days of symptom onset1. The case definition of SARI among children proposed by the WHO during pre-COVID times has gained more importance with the current SARS-CoV-2 pandemic. As per the guidelines by various health agencies, children (and adults) hospitalized with SARI should be placed in respiratory isolation until the reverse transcription (RT)-PCR for SARS-CoV-2, or screening test report is available2. There are, however, several drawbacks to such screening and isolation policies, especially for children. First, clinical pneumonia secondary to other viruses, bacteria and atypical organisms constitutes 20-30 per cent of hospital admissions, especially in under-five children34. Second, acute COVID pneumonia globally constitutes more than five per cent of total symptomatic cases in children5. Third, in low middle-income countries, the turnaround time for RT-PCR test results may vary between eight and 48 h, necessitating the holding up of these children in the isolation areas for that period. Running dedicated respiratory isolation with healthcare staff with complete personal protective equipment and precautions for a COVID-19-positive patient requires space and staffing. In our observation at a tertiary referral hospital in north India during the two COVID-19 waves, the SARS-CoV-2-positivity rates among children with SARI (isolated in the dedicated SARI ward) were not impressive. Hence, a retrospective data analysis was done to ascertain the SARS-CoV-2-positivity rate in children (0-12 yr) with SARI and compare it to those without SARI to determine the need for running a dedicated SARI isolation facility for paediatric COVID-19 care.
The case records of consecutive children (0-12 yr) admitted and/or tested for SARS-CoV-2 between June 2020 and May 2021 in the department of Pediatrics, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were retrieved from the medical records department after approval by the Institutional Ethics Committee. Details concerning SARS-CoV-2 RT-PCR test result, hospitalization, comorbidities and hospital outcome were analyzed retrospectively.
Since the start of the pandemic, the hospital admission policy had been revised as per the prevailing COVID-19 management guidelines for the isolation and testing of children for SARS-CoV-26. During the study period, all children requiring hospitalization were screened for the presence or absence of SARI. Those fulfilling the SARI definition were admitted to a dedicated SARI ward with augmented personal protection practice. All other (non-SARI) children were admitted to the routine care area; thus, there were two large groups of hospitalized children, SARI and non-SARI, for comparison. As a hospital policy, all hospitalized children (SARI and non-SARI areas) were tested for SARS-CoV-2 using RT-PCR and/or Gene Xpert. The test was done on combined nasopharyngeal and oropharyngeal swabs immersed and transported in a viral transport medium. RNA was extracted, and RT-PCR was performed as per the standard protocol7.
The SARI and non-SARI groups were compared regarding SARS-CoV-2 RT-PCR test positivity. Further, children positive for SARS-CoV-2 were analyzed for the presence of SARI, any contact with COVID-19 patients and comorbidities and outcomes. Statistical analyses were performed using the SPSS software version 20 (SPSS, Inc., Chicago, IL, USA).
The analysis showed that 8780 children underwent SARS-CoV-2 RT-PCR testing during the study period, of which 3.0 per cent (262/8780) tested positive for SARS–CoV-2 (Table I). Only 19 per cent (50/262) had SARI among those who tested positive. There were 1155 (13.15%) children with SARI and 7625 children without SARI. Fifty (4.3%) of the 1155 children with SARI, as against 212 (2.8%) of the 7625 children without SARI, tested positive for SARS-CoV-2 by RT-PCR. The month-wise distribution of patients and positivity rate is shown in Table I and Figure. In the study interval of one year, the maximum positivity rate for SARS-CoV-2, 23.6 per cent (among SARI) and 13.3 per cent (without SARI), was seen during the second wave in May 2021, followed by August 2020 with 10 and 2.9 per cent positivity rate among children with and without SARI, respectively. The difference in the positivity rate among SARI and non-SARI groups was significant only for these two months. The overall (for all cases tested over one year) sensitivity and positive predictive value (PPV) for SARI as a screening test/tool were 19 and 4.33 per cent, respectively. The sensitivity and PPV for August 2020 were 34.2 and 10 per cent, respectively, and that for May 2021 were 21.3 and 23.6 per cent, respectively. Hence, the maximum sensitivity of SARI as a screening test was 34.2 per cent and the maximum PPV was 23.6 per cent (at the peaks).
| Month-Yr | Children with SARI | Children without SARI | ||
|---|---|---|---|---|
| Total number | SARS-CoV-2 positive (%) | Total number | SARS-CoV-2 positive (%) | |
| June 2020 | 4 | 0 | 25 | 0 |
| July 2020 | 84 | 1 (1.2) | 551 | 3 (0.5) |
| August 2020 | 130 | 13 (10)*** | 861 | 25 (2.9) |
| September 2020 | 121 | 11 (9.1) | 797 | 37 (4.6) |
| October 2020 | 155 | 1 (0.6) | 1027 | 22 (2.1) |
| November 2020 | 135 | 2 (1.5) | 889 | 17 (1.9) |
| Decemer 2020 | 144 | 2 (1.4) | 951 | 18 (1.9) |
| January 2021 | 103 | 1 (0.97) | 681 | 7 (1.0) |
| February 2021 | 87 | 1 (1.1) | 574 | 4 (0.7) |
| March 2021 | 88 | 3 (3.4) | 583 | 11 (1.9) |
| April 2021 | 49 | 2 (4.1) | 324 | 20 (6.1) |
| May 2021 | 55 | 13 (23.6)* | 362 | 48 (13.3) |
| Total | 1155 | 50 (4.3)** | 7625 | 212 (2.8) |
P *<0.05, **<0.01, ***<0.001 compared to non-SARI group. SARI, severe acute respiratory infection
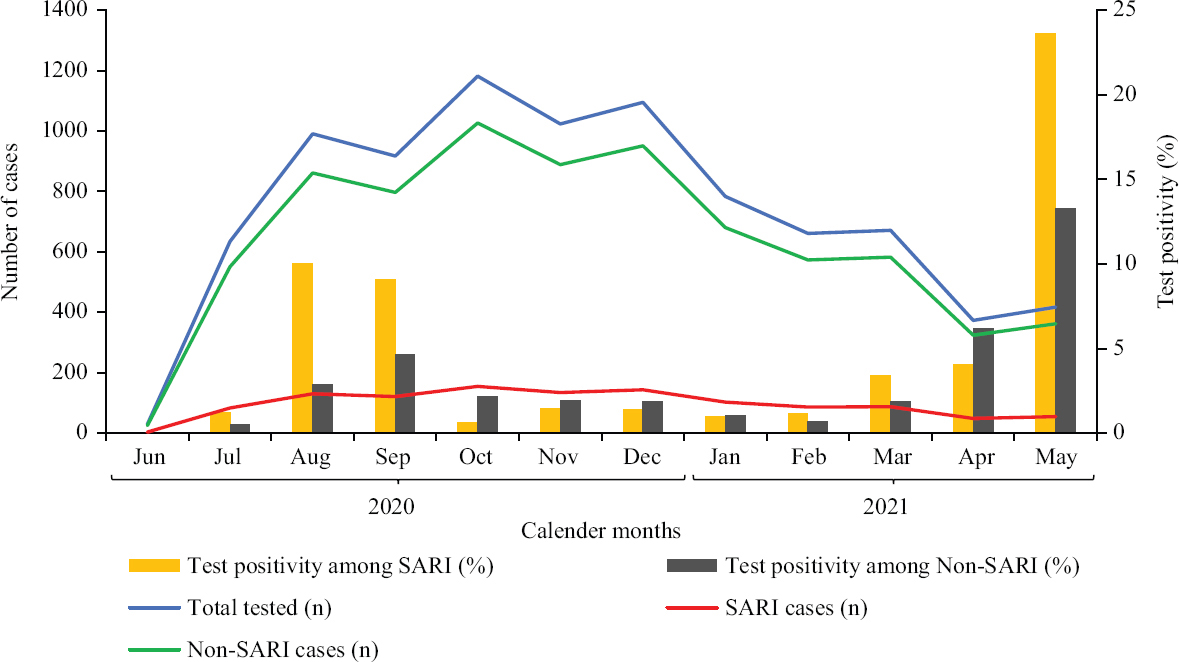
- Month-wise distribution of children with and without SARI tested for SARS-CoV-2 and the positivity rate among the two groups. SARI, severe acute respiratory infection.
Of the 262 children positive for COVID-19, 56 (21.4%) had some form of comorbidities. In-hospital mortality was similar in SARI group (34%) compared to non-SARI group (24.5%). The other details of these SARS-CoV-2-positive children are summarized in Table II.
| Characteristics | All SARS-CoV-2 positive (n=262), n (%) | With SARI (n=50), n (%) | Without SARI (n=212), n (%) |
|---|---|---|---|
| Number of males | 167 | 30 | 137 |
| History of contact with COVID-positive patient | 61 (23.3) | 18 (36)* | 43 (20.3) |
| In-patient/admission | 172 | 50*** | 122 |
| Comorbidities (total) | 56 (21.4) | 13 (26) | 43 (35.2) |
| Malignancy | 35 | 3 | 32 |
| Heart disease | 10 | 5 | 5 |
| Chronic kidney disease | 5 | 3 | 2 |
| Nephrotic syndrome | 3 | 1 | 2 |
| Diabetes | 2 | 0 | 2 |
| Chronic lung disease | 1 | 1 | 0 |
| Hospital outcome (death) | 47 (17.9) | 17 (34) | 30 (24.5) |
P *<0.05,***<0.001 compared to without-SARI group. SARI, severe acute respiratory infection
In our observation, the SARS-CoV-2-positivity rate for children was three per cent. Among children with SARS-CoV-2 RT-PCR positivity, only about one-fifth had SARI while the majority belonged to the non-SARI group (who were not isolated in a SARI Ward). When we compared positivity rates in SARI (4.3%) and non-SARI (2.8%) groups, it was higher (P<0.05) in the former. However, this difference does not appear clinically relevant as the absolute difference in positivity rate was only 1.5 per cent. This translates to cohorting and isolating 65 children with SARI to pick up one extra SARS-CoV-2-positive child (compared to those without SARI). The PPV of SARI as a screening test was 4.3 per cent (95% CI: 3.3-5.5%), which is very low compared to adults possibly related to a lower prevalence of COVID-19 in children.
On month-wise analysis of SARS-CoV-2 positivity among SARI and non-SARI groups, a significant difference was seen only for two of the 12 months of data. These two months (August 2020 and May 2021) were around the first and second peaks of COVID-19 pandemic. However, the sensitivity and PPV of SARI as a screening tool were not enough to warrant the isolation of hospitalized children with SARI awaiting the RT-PCR test report.
Further, there was no significant difference in the presence of comorbidities and in-hospital mortality in the COVID-19 RT-PCR-positive children with or without SARI. Most of the children who died had comorbidities (13 of 17 in the SARI group and 26 out of 30 in the non- SARI group).
The SARS-CoV-2-positivity rate (among SARI patients) reported for adults or adults and children combined was higher than children (below 12 yr). As per the data from the National Institute of Virology, Pune, of the 4939 patients with SARI (>15 yr), 1501 (30.4%) were COVID-19 positive. The same centre reported an overall positivity rate of 17 per cent among all individuals tested (SARI and non-SARI)8. ICMR data from the initial part of the first wave of the pandemic, including 41 centres, showed a 1.8 per cent positivity rate among SARI patients of all ages9. At a similar timeframe, our centre reported a positivity rate of 0.8 per cent among children (below 12 yr)10. Another study from a tertiary centre in New Delhi reported a positivity rate of 39 per cent among adults (median age of 54.5 yr)11.
Hence, it indicates that isolating children with SARI (as against adults) as a transmission-prevention strategy may not be required. This is particularly relevant in resource-limited settings with limited hospital beds, healthcare staff, central oxygen supply, etc. There are many other logistic issues in creating SARI/respiratory isolation rooms, including a separate air conditioning system, negative pressure air circulation, a room with separate entry/exit and donning doffing area. It would require additional physical space, funds and healthcare workers.
Our data over one year (although retrospective), including two pandemic peaks, suggested that the SARS-CoV-2-positivity rate among children with SARI was not high enough to warrant isolation/cohorting of children with SARI. However, there were some limitations to our data. We did not have information on the history of SARS-CoV-2-positive contacts of children presenting with or without SARI to assess the positivity rate among those with or without positive contacts. Further, we did not have information on short-term or long-term outcomes of children with COVID-19.
We propose isolating children with SARI may not be required before screening them for COVID-19. However, it may be prudent to isolate children with SARI during the peak of a pandemic when the positivity rate among children with SARI is likely to be significantly high. This policy decision at the State/national level may be more cost-effective and avoid wasting resources and workforce.
Acknowledgment:
The authors acknowledge the contribution of Dr Jayashree Muralidharan, Professor of Paediatric Critical Care, Department of Paediatrics, PGIMER, Chandigarh, for guiding and improving the scientific content of the manuscript.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Revision of clinical case definitions:Influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018;96:122-8.
- [Google Scholar]
- Revised guidelines on clinical management of COVID – 19. Available from: https://www.mohfw.gov.in/pdf/RevisedNationalClinicalManagementGuidelineforCOVID 1931032020.pdf
- Factors determining the outcome of children hospitalized with severe pneumonia. BMC Pediatr. 2009;9:15.
- [Google Scholar]
- Non-respiratory and non-diarrheal causes of acute febrile illnesses in children requiring hospitalization in a tertiary care hospital in North India:A prospective study. Am J Trop Med Hyg. 2018;99:783-8.
- [Google Scholar]
- Revised strategy of COVID19 testing in India. Available from: https://www.mohfw.gov.in/pdf/ICMRrevisedtestingstrategyforCOVID.pdf
- COVID-19 pneumonia in children:from etiology to management. Front Pediatr. 2020;8:616622.
- [Google Scholar]
- Laboratory surveillance for SARS-CoV-2 in India:Performance of testing &descriptive epidemiology of detected COVID-19, January 22–April 30, 2020. Indian J Med Res. 2020;151:424-37.
- [Google Scholar]
- 30% of SARI patients covid positive:NIV. Available from: https://indianexpress.com/article/cities/pune/30-of-sari-patients-covid-positive-niv-6552380/
- Severe acute respiratory illness surveillance for coronavirus disease 2019, India, 2020. Indian J Med Res. 2020;151:236-40.
- [Google Scholar]
- India's COVID-19 testing strategy:Why pediatric hospitals need to focus more on ILI than SARI? Indian J Pediatr. 2020;87:753.
- [Google Scholar]
- Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Physicians India. 2020;68:19-26.
- [Google Scholar]






