Translate this page into:
Distribution trends & antibiogram pattern of Salmonella enterica serovar Newport in India
Reprint requests: Dr Yashwant Kumar, National Salmonella and Escherichia Centre, Central Research Institute, Kasauli 173 204, Himachal Pradesh, India e-mail: yasht26@yahoo.co.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Salmonellosis is a major public health concern worldwide. Besides typhoidal salmonellae, infections due to non-typhoidal serovars of Salmonella are also associated with high morbidity and mortality leading to huge economic losses. Among non-typhoidal serovars, Salmonella Newport has been reported as a major cause of foodborne infections resulting in outbreaks due to consumption of contaminated food items. Little data related to this serovar are available from India leading to the scarcity of information on the distribution trends of this important serovar in the country. Therefore, an effort was made in the present study to generate data on distribution trends and antibiogram of S. Newport in the country.
Methods:
S. Newport isolates received at the National Salmonella and Escherichia Centre at Kasauli, India, during January 2010 to December 2013 were analysed for their distribution trends and antibiogram data were also generated using standard methods.
Results:
In the present study, S. Newport isolates were received from eight States and one union territory of the country and highest proportion of S. Newport isolates were found to be from humans (53.61%) followed by animals (27.84%) and food (18.56%). S. Newport isolates exhibited resistance to all drugs used in the present study except chloramphenicol, ciprofloxacin and cefuroxime.
Interpretation & conclusions:
Considering distribution of this important serovar of Salmonalla and its wide range of reservoirs, steps towards formulation and execution of efficient surveillance programmes should be taken.
Keywords
Antibiogram
distribution
foodborne
outbreaks
Salmonella Newport
Genus Salmonella is comprised of two species and 2,579 serovars1. Salmonella enterica subsp. enterica comprises 1,531 serovars which are largely associated with human infections2, commonly acquired by consumption of contaminated food. Although typhoidal Salmonellae remains to be the important cause of morbidity and mortality, non-typhoidal Salmonellae are a leading cause of food poisoning and enteric infections and emerged as an important public health problem worldwide3. It is estimated that non-typhoidal serovars of Salmonella cause 93.8 million human infections and 1,55,000 deaths annually4. These serovars account for 38 per cent of foodborne illnesses5, and are responsible for 35 per cent of hospitalizations and 28 per cent of deaths due to foodborne illnesses6. Moreover, non-typhoidal Salmonellae have a wide range of reservoirs and are widely distributed7.
Salmonella Newport is one of the most important serovars and it is ranked in top three Salmonella serovars associated with foodborne outbreaks in the United States5. S. Newport has been reported to be responsible for several major outbreaks associated with tomatoes, ground beef, alfalfa sprouts, mung beans, cantaloupe and many other food products8. S. Newport has been reported to cause a wide spectrum of clinical diseases in humans, such as diarrhoea, ileocecal lymphadenitis, chest wall abscess, pyosalpinx, osteomyelitis, endocarditis, meningitis, splenic abscess, septicaemia9 and bacteraemia10.
Although S. Newport has emerged as an important human pathogen in the last few decades and initiation of enhanced surveillance of this serovar is advocated in several countries, there is scarcity of data about the prevalence and distribution of S. Newport in India. Therefore, an attempt was made in the present study to generate data on the distribution trends of S. Newport throughout the country.
Material & Methods
Ninety seven S. Newport isolates, received at the National Salmonella and Escherichia Centre, Central Research Institute, Kasauli, India, from various medical, veterinary and research institutes throughout the country, during January 2010 to December 2013 constituted the material for the study. Bacterial isolates were identified on the basis of culture characteristics, Gram staining and conventional biochemical tests11. Isolates identified as salmonellae were further subjected to serotyping1 using an array of various Salmonella antisera (Statens Serum Institute, Copenhagen, Denmark; Denka Seiken Co. Ltd., Tokyo Japan).
Antimicrobial susceptibility testing: All serologically confirmed S. Newport isolates were tested for antimicrobial susceptibility by disc diffusion method12 using the following 12 antimicrobials (Hi Media Laboratories, Pvt. Ltd., Mumbai, India): ampicillin (10 μg), ceftazidime (30 μg), cefotaxime (30 μg), cefuroxime (30 μg), chloramphenicol (30 μg), trimethoprim-sulphamethoxazole (co-trimoxazole) (1.25/23.75 μg), kanamycin (30 μg), norfloxacin (10 μg), ciprofloxacin (5 μg), nalidixic acid (30 μg), tetracycline (30 μg), and nitrofurantoin (300 μg), according to the Clinical and Laboratory Standards Institute (CLSI) guidelines and interpretative criteria12. Escherichia coli ATCC 25922 was used as standard strain.
Results
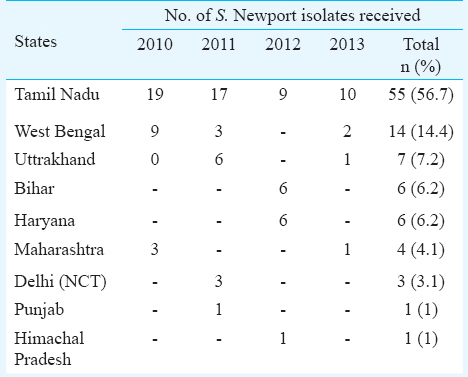
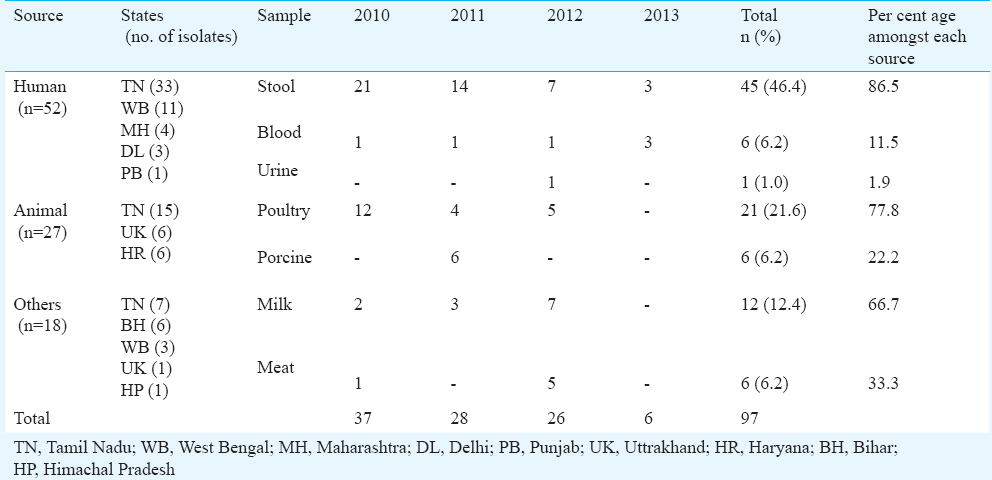
A total of 3,924 suspected Salmonella isolates were received during the study period. Of these, 1,850 (47.1%) isolates were identified as Salmonella. Among serotyped isolates, 97 (5.24%) were serologically identified as S. Newport which were received from eight States and one union territory viz. Tamil Nadu (56.7%), West Bengal (14.4%), Uttrakhand (7.2%) Bihar (6.2%), Haryana (6.2%), Maharashtra (4.1%) Delhi (NCT) (3.1%), Himachal Pradesh (1%) and Punjab (1%) (Table I). Among isolates from humans, highest proportion was found from stool (86.5%) followed by blood (11.5%) and urine (1.9%) (Table II). S. Newport isolates from animals were found to be of poultry origin (77.7%) and porcine origin (22.2%) whereas milk (66.6%) and meat (33.3%) were found to be the main sources of S. Newport amongst food items. S. Newport isolates were found to be resistant to ampicillin (87.62%), cefotaxime (30.9%), ceftazidime (30.9%), kanamycin (30.9%), co-trimoxazole (25.7%), tetracycline (24.7%), nalidixic acid (23.7%), nitrofurantoin (23.7%) and norfloxacin (22.7%) whereas, all the isolates were found to be susceptible to chloramphenicol, ciprofloxacin and cefuroxime.
Discussion
Of the 97 S. Newport isolates analysed in the present study, maximum number of isolates was found to be from humans followed by animals and food indicating its importance as human pathogen. Among human isolates, maximum number of S. Newport isolates was from stool. Inappropriate treatment and disposal of sewage continues to be the major hurdle in attaining good hygiene and sanitary conditions and therefore, may contribute to dissemination of S. Newport into the environment. Presence of S. Newport in human faeces may result in possible transmission of S. Newport through contaminated water bodies and vegetables irrigated using contaminated water sources apart from the other animal reservoirs. S. Newport outbreaks due to the consumption of contaminated mung beans8, cantaloupe13, ready to eat salad vegetables14, watermelon15 and mango16 have been reported earlier from various countries. Isolation of S. Newport from blood and urine shows the potential of this serovar to cause invasive infections as has been reported earlier9.
S. Newport isolates obtained from animals and food products of animal origin have caused septicaemic illness in both animals and humans17. Amongst S. Newport isolates from animals, 77.8 and 22.2 per cent were found to be of poultry and swine origin, respectively. Both of these reservoirs may act as an important source of S. Newport infections in humans due to consumption of undercooked poultry18, eggs19 and pork20. Live poultry may also act as an important source of S. Newport infection21. Swine can be asymptomatic reservoirs of salmonellae22 and may become colonized by ingesting contaminated faeces or through snout to snout contact23. Salmonellae can be found commonly in the environment of pig farms which helps in the maintenance of the bacteria in the herds24.
Both vegetarian and non-vegetarian populations are susceptible to S. Newport infections due to the consumption of contaminated meat and milk25. Therefore, high standards of hygiene are required to be maintained in dairy farms and food processing industries. Administration of S. Newport vaccine in cattle may further help in controlling infections in dairy herds. It will also check environmental dissemination of S. Newport by controlling faecal shedding into the environment26. No specific trends were observed in the number of the S. Newport isolates from various parts of the country.
Highest resistance was observed against ampicillin and third generation cephalosporins followed by co-trimoxazole, tetracycline, and nalidixic acid. However, all isolates were found to be susceptible to chloramphenicol and ciprofloxacin. Third-generation cephalosporins are the drugs of choice for treatment of persons with non-typhoidal Salmonella infections that require chemotherapy or when fluoroquinolones are contraindicated.
Although an attempt has been made in the present study to generate data on the distribution of S. Newport in India, the scenario may actually be much complicated and, therefore, necessitate formulation and execution of efficient surveillance programmes which will result in generation of enough epidemiological data, further facilitating public health authorities to understand the epidemiological trends of S. Newport in the country. Moreover, shifts in the prevalence of specific strain types and serovars in human and animal populations is a consequence of its introduction through international travel, human migration, food, animal feed and livestock trade. Therefore, national level surveillance programmes should be collaborated with global monitoring programmes.
Acknowledgment
The authors thank the heads of all the laboratories that referred Salmonella isolates to this Centre. The technical assistance of Shri Gian Chand is also acknowledged. Thanks are due to Shriyut Jiwa Ram and Devanand for supplying media and biochemicals for biotyping.
Conflicts of Interest: None.
References
- Antigenic formulae of the Salmonella serovars (9th ed). WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, France; 2007.
- Salmonellosis and the gastrointestinal tract: more than just peanut butter. Curr Gastroenterol Rep. 2008;10:424-31.
- [Google Scholar]
- Antimicrobial resistance among invasive nontyphoidal Salmonella enteric isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother. 2011;55:1148-54.
- [Google Scholar]
- International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882-9.
- [Google Scholar]
- Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006-2013. MMWR Morb Mortal Wkly Rep. 2013;62:283-7.
- [Google Scholar]
- Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011;17:7-15.
- [Google Scholar]
- Attributing the human disease burden of foodborne infections to specific sources. Foodborne Pathog Dis. 2009;6:417-24.
- [Google Scholar]
- An outbreak of Salmonella Newport associated with mung bean sprouts in Germany and the Netherlands, October to November 2011. Euro Surveill. 2014;19:1-9.
- [Google Scholar]
- Isolation of Salmonella enterica Serotype Newport from a partly ruptured splenic abscess in a traveler returning from Zanzibar. J Clin Microbiol. 2007;45:3115-7.
- [Google Scholar]
- Salmonella Newport bacteremia in a 12-day-old infant. J Am Board Fam Med. 2011;24:214-7.
- [Google Scholar]
- Group 5: Facultatively anaerobic Gram negative rods. Bergey manual of determinative bacteriology. Baltimore, MD: The Williams & Wilkins Co; 1994. p. :175-289.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, CLSI document M7-A7. Wayne, PA: CLSI; 2006.
- Centres for Disease Control and Prevention. Multistate Outbreak of Salmonella Typhimurium and Salmonella Newport Infections Linked to Cantaloupe (Final Update). Available from: http://www.cdc.gov/salmonella/typhimuriumcantaloupe-08-12/index.html?s_cid=cs_852
- Microbiological study of ready-to-eat salad vegetables from retail establishments uncovers a national outbreak of salmonellosis. J Food Prot. 2003;66:403-9.
- [Google Scholar]
- Public Health England. Outbreak of Salmonella Newport. Available from: http://www.hpa.org.uk/NewsCentre/NationalPressReleases/2012PressReleases/120202SalmonellaNewportoutbreak/
- A multistate outbreak of Salmonella enterica Serotype Newport infection linked to mango consumption: impact of water-dip disinfestation technology. Clin Infect Dis. 2003;37:1585-90.
- [Google Scholar]
- Characterization of antimicrobial resistance of Salmonella Newport isolated from animals, the environment, and animal food products in Canada. Can J Vet Res. 2006;70:105-14.
- [Google Scholar]
- Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: comparison between 1993 and 2006. Int J Food Microbiol. 2012;153:281-7.
- [Google Scholar]
- Salmonella Newport: outbreak of food poisoning among college students due to contaminated undercooked eggs. Ethiop Med J. 1994;32:1-6.
- [Google Scholar]
- Laboratory infection caused by Salmonella Newport of porcine origin. Med Trop (Madr). 1965;41:535-7.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Notes from the field: Multistate outbreak of Salmonella infantis, newport, and lille infections linked to live poultry from a single mail-order hatchery in Ohio--March-September, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:213.
- [Google Scholar]
- Preslaughter holding environment in pork plants is highly contaminated with Salmonella enterica. Appl Environ Microbiol. 2003;69:4489-94.
- [Google Scholar]
- Herd-level risk factors for subclinical Salmonella infection in European finishing-pig herds. Prev Vet Med. 2004;62:253-66.
- [Google Scholar]
- Longitudinal study of Salmonella enterica in growing pigs reared in multiple site swine production systems. Vet Microbiol. 2001;83:45-60.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Outbreak of multidrug-resistant Salmonella Newport - United States, January-April 2002. MMWR Morb Mortal Wkly Rep. 2002;51:545-8.
- [Google Scholar]
- Effects of a commercially available vaccine against Salmonella enterica serotype Newport on milk production, somatic cell count, and shedding of Salmonella organisms in female dairy cattle with no clinical signs of salmonellosis. Am J Vet Res. 2008;69:1229-34.
- [Google Scholar]






