Translate this page into:
Differential invasiveness & expression of antimicrobial peptides in Shigella serotypes
For correspondence: Dr Neelam Taneja, Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: drneelampgi@yahoo.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The study of Shigella pathogenesis at present is severely hampered by the lack of a relevant animal model that replicates human bacillary dysentery. Different Shigella serogroups cause varying severity of clinical illness. Ex vivo colonization of Shigella flexneri, S. dysenteriae and S. sonnei were characterized in human paediatric colonic pinch biopsies in the in vitro organ culture (IVOC) model to study the invasiveness of Shigella by gentamicin protection assay (GPA). Furthermore, the expression of antimicrobial peptides (AMPs) in response to different serotypes of Shigella was also studied in IVOC model.
Methods:
IVOC explants were inoculated with 109 colony forming units of different serotypes of Shigella and recovery of bacteria studied. Histopathological analysis was carried out to study inflammatory immune responses. GPA was done to elucidate the invasiveness of different serotypes of Shigella. Secretions of AMPs were measured by enzyme-linked immunosorbent assay (ELISA). Western blotting was performed to check the expression of AMPs and nuclear factor kappa B in IVOC explants.
Results:
After 24 h post-infection, the colon biopsies showed intense inflammatory reaction. In both IVOC and GPA, S. dysenteriae 1 was the most invasive as compared to S. flexneri and S. sonnei. S. sonnei was the least invasive. ELISA demonstrated that S. sonnei dampened the HBD (human β-defensin)-2 responses whereas there was augmentation by S. dysenteriae and there was a modest but non-significant increase by S. flexneri. A modest increase in HBD-3 by S. sonnei and S. flexneri was observed but was not found to be significant. However, western blotting data showed upregulation of all AMPs by all serotypes. Western blotting is more sensitive than ELISA.
Interpretation & conclusions:
In the present study, differences in invasiveness and AMP production induced by different serotypes of Shigella were found. Human intestinal IVOC represents a model system to investigate early interaction between pathogenic bacteria and the human gut.
Keywords
Antimicrobial peptides
cathelicidins
Shigella
α-defensins
β-defensins
Shigella are causative agents of shigellosis which is characterized by acute intestinal enteritis with symptoms ranging from watery diarrhoea to severe abdominal cramping, high fever and blood or mucous in the stool1. Shigellosis is regarded as one of the major public health burdens, especially in resource-poor countries where the incidence rate of endemic shigellosis still remains high2. Shigellae are contagious enteric pathogens that invade and elicit intense inflammation and tissue damage of the colorectal epithelium3-5. By colonizing intestinal epithelial cells, shigellae avoid extracellular components of the host immune system such as antibodies or complement factors. Antimicrobial peptides (AMPs) against Shigella are produced by Paneth cells that maintain intestinal homoeostasis6-8. The efficiency of antimicrobial host defence in the human intestinal tract depends on the capacity of the mucosal immune system to identify and neutralize pathogens. In this context of innate immunity, recognition of the bacteria occurs mainly through the engagement of host cell Toll-like receptors and other pattern recognition molecules, such as the nucleotide oligomerization domain proteins, through pathogen-associated molecular patterns9-11. After the pathogen detection step, the subsequent antimicrobial response is accomplished by recruitment of immune cells and synthesis of antimicrobial factors by the injured mucosa, including the production of molecules such as defensins, cathelicidins, lysozyme, phospholipases and proteases that exhibit a broad range of actions against a variety of pathogens12,13. Production of the best-known AMPs such as defensins, HBD-1-4 and cathelicidins is either constitutive or inducible in response to a pro-inflammatory situation. AMPs have been linked to the bridging of innate and acquired immune responses. Cathelicidins and various defensins show chemotactic activity for immune cells such as monocytes, T-cells or immature dendritic cells and can induce cytokine production by monocytes and epithelial cells14,15.
The severity of shigellosis varies with the serotype involved with Shigella dysenteriae producing the most severe infections and complications and S. sonnei being at the other end of spectrum usually causing mild self-limiting diarrhoea. The study of Shigella pathogenesis is severely hampered at present due to lack of a relevant animal model that can replicate human bacillary dysentery. Therefore, at present, there is no successful animal model, which mimics shigellosis in humans because Shigella are highly host-restricted pathogens. Hence, the goal of this study was first to characterize ex vivo colonization of S. flexneri 2a, S. dysenteriae serotype 1 and S. sonnei in human paediatric colonic pinch biopsies in the co-culture system and look for their invasiveness. Furthermore, to study the functional relevance of cathelicidins and defensins, (two major families of AMPs in mammals), in the context of experimental infections of Shigella in in vitro organ cultures (IVOCs) of human biopsies. The differences in induction of these AMPs generated in response to three serotypes of Shigella were also studied.
Material & Methods
This in vitro study of three years (from September 2013 to August 2016) was undertaken at the department of Medical Microbiology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India. All experiments were undertaken after clearance from the Institute Ethics Committee.
Bacterial strains and culture conditions: S. dysenteriae 1 (CIP 58.1), S. flexneri (CIP 56.19) and S. sonnei (CIP 82.49) were a kind gift from Pasteur Institute, Paris. The strains were grown in BHI broth (Difco Laboratories, MA, USA) at 37°C for 18-24 h. The culture broths were centrifuged at 1200 g for 10 min, and the supernatants were discarded. Cell pellets were suspended in phosphate-buffered saline (PBS), and optical density (OD) was measured at 600 nm using spectrophotometer, LMSPUV 1200 (Labman, Chennai, India). An inoculum of 109 colony forming units (CFU) was used to infect the explant.
In vitro organ culture (IVOC): Standard IVOC was performed as described previously16,17. Briefly, colonic pinch biopsies were collected from healthy portions of intestines from children undergoing intestinal biopsies for other indications. The mucosa and submucosa were gently teased away from the muscularis propria and cut into pieces of 3-4 mm3 in size. The pieces were placed, luminal side up, into 35 mm2 wells of a 6 well culture plate (Corning, Corning, NY, USA). IVOC medium was prepared by adding 10 per cent heat-inactivated foetal calf serum (F7524, Sigma Aldrich Chemical Pvt. Ltd., Bengaluru, India) in RPMI-1640 medium supplemented with glutamine and NaHCO3 (Sigma Aldrich).
The explants were inoculated with 109 CFU of S. flexneri 2a, S. dysenteriae serotype 1 and S. sonnei. The IVOC medium was replaced after four hours, to maintain pH and nutrient levels and the tissue re-inoculated with bacteria as before and after 24 h post infection culture media were collected and centrifuged at 13000 g, 4°C for 15 min. Culture supernatants were taken in a fresh tube and cultured for Shigella quantitively and aliquots were made and preserved at –20°C. These were used to detect antimicrobial secretion in response to Shigella infection.
Histopathological examination of mucosa and submucosa by haematoxylin and eosin (H & E) stain: Tissue biopsies were fixed overnight in neutral formalin followed by washing. Dehydration was done in a graded series of ethanol concentrations to remove the fixative and water from tissue and replacing it with dehydrating fluid. Tissue samples were embedded in the paraffin to provide sufficient external support during sectioning. 5 µm thick sections were cut at the median transverse level. Sections were de-paraffinized using xylene. Hydration of tissue sections in graded series of ethanol was carried out. Sections were mounted on glass slides and stained and counterstained with H & E, respectively. The histology of mucosa and submucosa was examined under the light microscope by an experienced pathologist.
Gentamicin protection assay (GPA): A total of 2×106 Caco-2 human colon epithelial cells were cultured in T25 flasks in Dulbecco’s Modified Eagle Medium (DMEM) with 10 per cent heat-inactivated foetal bovine serum and 1× penicillin-streptomycin and glutamine at 37°C in a CO2 incubator by adding 3 ml DMEM per flask and incubated for 72 h or till ≥80 per cent (confluent) growth18. Media were changed every 24 h. Flasks were set up one each for control, S. dysenteriae, S. flexneri and S. sonnei and incubated.
Overnight cultures of S. dysenteriae, S. flexneri and S. sonnei in 5 ml of brain heart infusion (BHI) broth (LQ003, Himedia, Maharastra, India) were incubated at 37°C with shaking at 7.5 g. The multiplicity of infection (MOI = number of bacteria per Caco-2 cell) was determined. For MOI of 100, 1011 CFU of Shigella was added to each flask containing 109 Caco-2 cells. Pelleted bacterial cells were added to twice washed Caco-2 monolayers and co-culture was incubated at 37°C, five per cent CO2 for 3 h. All experiments were carried out in triplicates. The supernatant was removed and washed with PBS. One millilitre of DMEM with gentamicin (150 µg/ml) was added per flask. Plates were incubated at 37°C, five per cent CO2 for 30 min. After 30 min, the supernatants were removed and cells were lysed with 1 ml of filter sterilized 0.1 per cent Triton X in H2O per well by incubating for 10 min at room temperature (RT) followed by vigorous pipetting. The extent of lysis was determined using a standard inverted microscope. Serial dilutions were made and poured on Mueller Hinton agar (MHA) plate after overnight incubation to count the bacterial colonies.
ELISA for human β-defensins and LL-37: Colonic biopsies were infected with different serotypes of Shigella at 109 CFU in 2 ml complete Roswell Park Memorial Institute (RPMI) media (R8755, Sigma Aldrich Chemical Pvt. Ltd., Bengaluru, India) and culture media were changed every 4 h to maintain pH and nutrients. After 24 h post infection, culture media were collected and centrifuged at 13000 g for 15 min at 4°C. Culture supernatants were taken in fresh tubes and aliquots were made and preserved at –20°C. These were used to detect antimicrobial peptides (AMPs) secretion in response to Shigella infection.
Sample standard cathelicidin (PEP0633), β-defensin-2 (HBD-2, SC-4835) and β-defensin-3 (HBD-3, SC-4836) were purchased from Thermo Scientific, Pierce, MA and Santa Cruz, Dallas, TX, USA, respectively. Different concentrations of the standard (0-1 ng/ml) were made by serial dilutions. The culture supernatant of uninfected biopsy was treated as control.
The primary antibody for cathelicidin (sc-166770), β-defensin-2 (sc-134314) and β-defensin-3 (sc-59495) (Santa Cruz, Dallas, TX, USA) was diluted as per the manufacturer’s instructions in coating buffer and 50 µl was poured into each well followed by incubation overnight at 4°C. The plate was washed thrice with PBS-tween 20 (PBST) for 5 min each on a shaking platform. A 100 µl of blocking buffer was added to each well and plates were incubated for 1 h at room temperature (RT). For preparing the samples and standards, 100 µl of sample or standard was added per well. The plate was incubated on rocking platform for 2 h at RT. The plate was washed with washing buffer thrice on a shaking platform. 100 µl of the diluted biotinylated detection antibody was added to each well of the plate, which was covered and incubated for 1 h at RT. The plate was washed with washing buffer thrice on a shaking platform. Further, 50 µl diluted streptavidin was added and incubated for 1 h at RT. The plate was washed six times on a shaking platform. Finally, 100 µl of substrate solution was added to the plate which was incubated for 30 min in dark at RT. The reaction was stopped by adding 100 µl of the stop solution. Absorbance was taken at 450 nm in an ELISA plate reader.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting for antimicrobial peptides (AMPs): Proteins were isolated from explants (biopsies) using lysis buffer containing 50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.5 per cent Triton X-100, 5 mM Ethylenediaminetetraacetic acid (EDTA) (pH 8) and protease inhibitor cocktail. 50 μg/well of protein was loaded on each lane of 15 per cent tricine SDS-PAGE for antimicrobial peptides β-defensin-2, β-defensin-3 and cathelicidin (LL-37) and 12 per cent SDS-PAGE for nuclear factor kappa B (NF-kB). Separated proteins were transferred to the polyvinylidene difluoride (PVDF) membranes at 20V/4°C overnight. The membranes were blocked with five per cent non-fat dry milk in TBST (1× TBS, 0.2% Tween 20) buffer at RT for 1 h. The membrane was washed thrice with TBST and incubated separately in the TBST buffer containing either primary antibody β-defensin-2, β-defensin-3, cathelicidin (LL-37) NF-kB (Thermo Fisher Scientific, Waltham, MA, USA) and GAPDH separately, each having a dilution of 1:1000. Horseradish peroxidase-conjugated species-specific secondary antibody with a dilution of 1:5000 at RT for 1 h incubation. After washing thrice with TBST chemiluminescence substrate was poured and chemiluminescence emission was detected with the Kodak X-ray film.
Statistical analysis: Data presented as mean±standard error of mean from three independently conducted assays. Data was analysed using Graphpad Prism version 8 (Graphpad Prism, Boston, MA, USA), and P value was calculated by Bonferroni’s one-way ANOVA multiple comparison test. P<0.05 was considered as significant.
Results
Colonic biopsies were collected from 26 children out of which 16 were infected with Shigella serotypes at 109 CFU and incubated for 24 h at 37°C under five per cent CO2 (Supplementary Table). After 24 h post infection, the colon biopsies showed intense inflammatory reaction (Fig. 1A) and intact mucosa and submucosa (Fig. 1B). Table and Figure 2 show the average number of intracellular bacteria that could be retrieved. S. dysenteriae 1 was more invasive as compared to S. flexneri and S. sonnei. S. sonnei was the least invasive. All experiments were conducted in triplicates. Both the differences between S. dysenteriae and S. flexneri and S. dysenteriae and S. sonnei were significant (P<0.05, P<0.01, repectively).
| S.No | Date | Age (yr) | Sex |
|---|---|---|---|
| E1 | 26.11.14 | 2 | male |
| E 2 | 19.01.15 | 1 | male |
| E 3 | 21.01.15 | 9 month | female |
| E 4 | 21.01.15 | 2 | male |
| E 5 | 22.01.15 | 12 | male |
| E 6 | 28.01.15 | 1 | male |
| E 7 | 28.01.15 | 12 | female |
| E 8 | 12.02.15 | 1 | male |
| E 9 | 10.04.15 | 10 month | male |
| E 10 | 10.04.15 | 1 | male |
| E 11 | 16.04.15 | 2 | male |
| E 12 | 15.05.15 | 1 | male |
| E 13 | 25.06.15 | 1 | male |
| E 14 | 24.07.15 | 1 | female |
| E 15 | 31.07.15 | 1 | male |
| E 16 | 13.08.15 | 4 month | male |
| E 17 | 13.08.15 | 5 month | male |
| E 18 | 18.11.15 | 6 month | male |
| E 19 | 27.11.15 | 3 | female |
| E 20 | 01.12.15 | 3 days | female |
| E 21 | 13.01.16 | 1 | female |
| E 22 | 13.01.16 | 6 | male |
| E 23 | 15.01.16 | 2 | male |
| E 24 | 20.01.16 | 10 month | female |
| E 25 | 21.01.16 | 1 | male |
| E 26 | 27.01.16 | 2 | male |

- (A) Histopathological analysis of normal human colon tissue section after 24 h post infection stained with H & E, shows strong inflammatory reaction in mucosa and submucosa. (B) Histopathological analysis of normal human colon tissue section without Shigella infection stained with H & E shows mucosa, submucosa and muscularis. H & E, haematoxylin and eosin.
| Serotypes | Average value of bacterial cell retrieval |
|---|---|
| S. dysenteriae | ~5.91×108±7.17×107 |
| S. flexneri | ~2.7×108±9.7×107 |
| S. sonnei | ~1.91×108±4.66×107 |
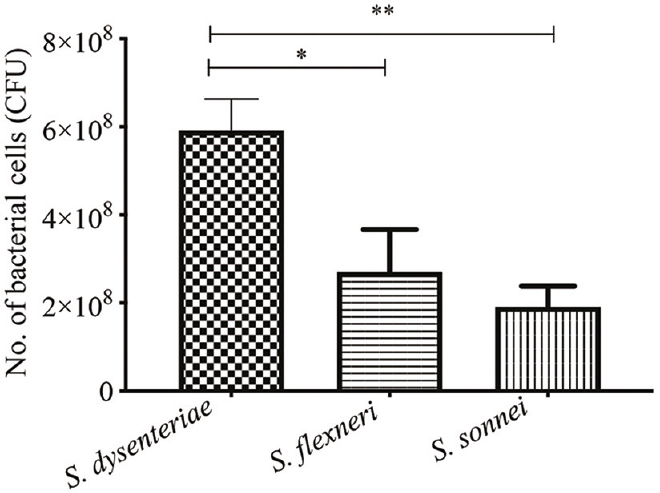
- Bacterial cell retrieval after 24 h post infection. All experiments were conducted in triplicates. P *<0.05, **<0.01. S. dysenteriae, Shigella dysenteriae; S. flexneri, Shigella flexneri, S. sonnei, Shigella sonnei
Histopathological analysis of normal human colon tissue sections without Shigella infection stained with H & E showed normal crypts in a perfectly oriented pattern parallel to one another and extending from the surface to the muscularis mucosa (Fig. 1B) Human colon tissue section after 24 h post infection and stained with H & E showed disruption of the mucosa and intense inflammatory reactions in mucosa and submucosa (Fig. 1A).
Similar results were obtained in the GPA. S. dysenteriae serotype 1 was clearly more invasive followed by S. flexneri and S. sonnei. The differences of invasion between S. flexneri and S. sonnei and S. dysenteriae and S. sonnei were found to be significant (P<0.05, P<0.001, respectively; Fig. 3).
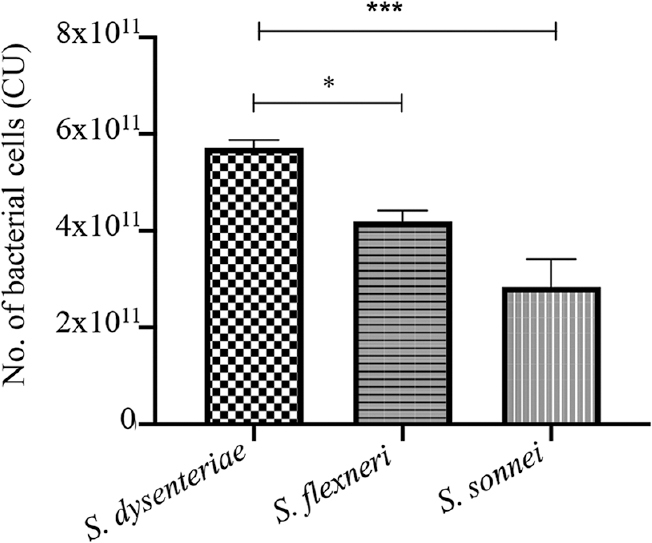
- Shigella invaiveness by gentamicin protection assay (GPA). P *<0.05, ***<0.001
The secretion of human β-defensins and LL-37 in response to infection with different serotypes of Shigella showed highest level of induction of β-defensin-2 in S. dysenteriae followed by S. sonnei. The difference between S. dysenteriae vs. S. sonnei and S. flexneri vs. S. sonnei was also significant (P<0.05 by Bonferroni’s multiple comparison test; Fig. 4). The β-defensins-3 production by S. dysenteriae was similar to control. S. flexneri and S. sonnei produced modest levels of LL-7. None of the serotypes could induce a significant β-defensin-3 level (Fig. 5). Similarly, none of the serotypes could induce cathelicidin (LL-37) production (Fig. 6).
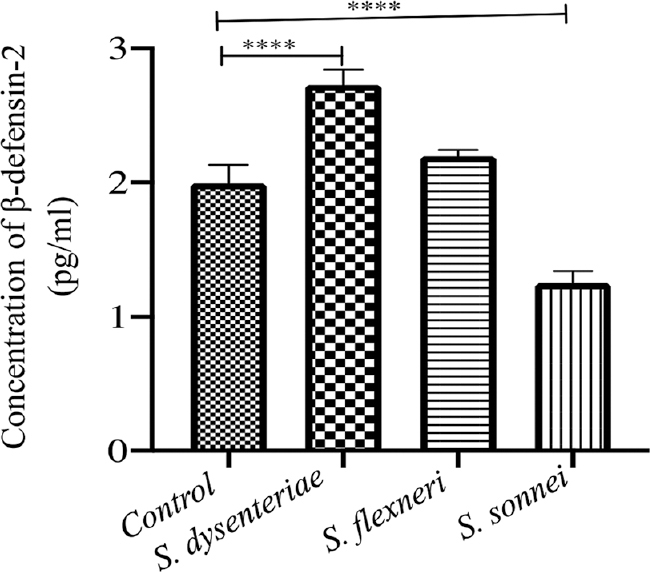
- Levels of β-defensin-2 induced by different serotypes by ELISA: Significant induction was seen by S. dysenteriae and S. sonnei (P**<0.01, ***<.001). ELISA, enzyme-linked immunosorbent assay
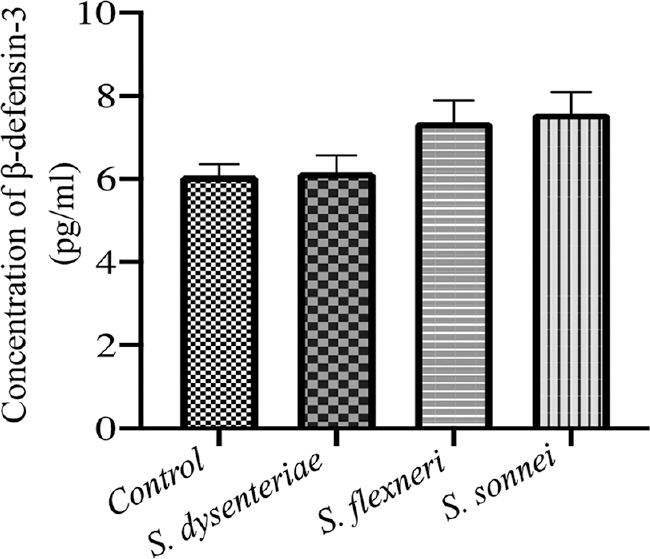
- Levels of β-defensin-3 induced by different serotypes by ELISA
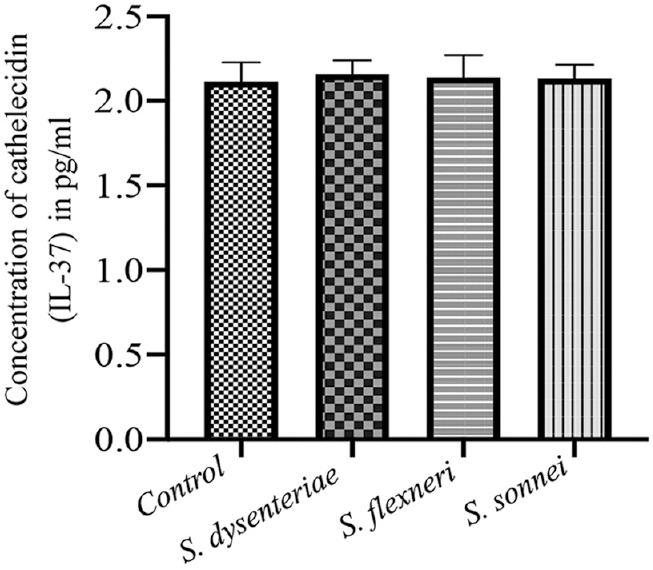
- Induction of cathelicidin (LL-37) production by various serotypes
The expression of antimicrobial peptides β-defensin-2, β-defensin-3, cathelicidin (LL-37) and NF-κB at translational level of Shigella infected and control biopsies after 24 h (Fig. 7) were checked by western blotting. Immunoblots revealed the expression level of β-defensin-2, β-defensin-3 and cathelicidin (LL-37) and NF-κB in Shigella-infected biopsies increased by 3.5, 4.1, 5.2 and 20.5 folds, respectively, in S. dysenteriae infected; 1.6, 1.6, 2 and 26.25 folds respectively in S. flexneri infected and 1.1, 3.2, 9.9 and 21.25 folds, respectively, in S. sonnei infected in comparison to non-infected biopsies.
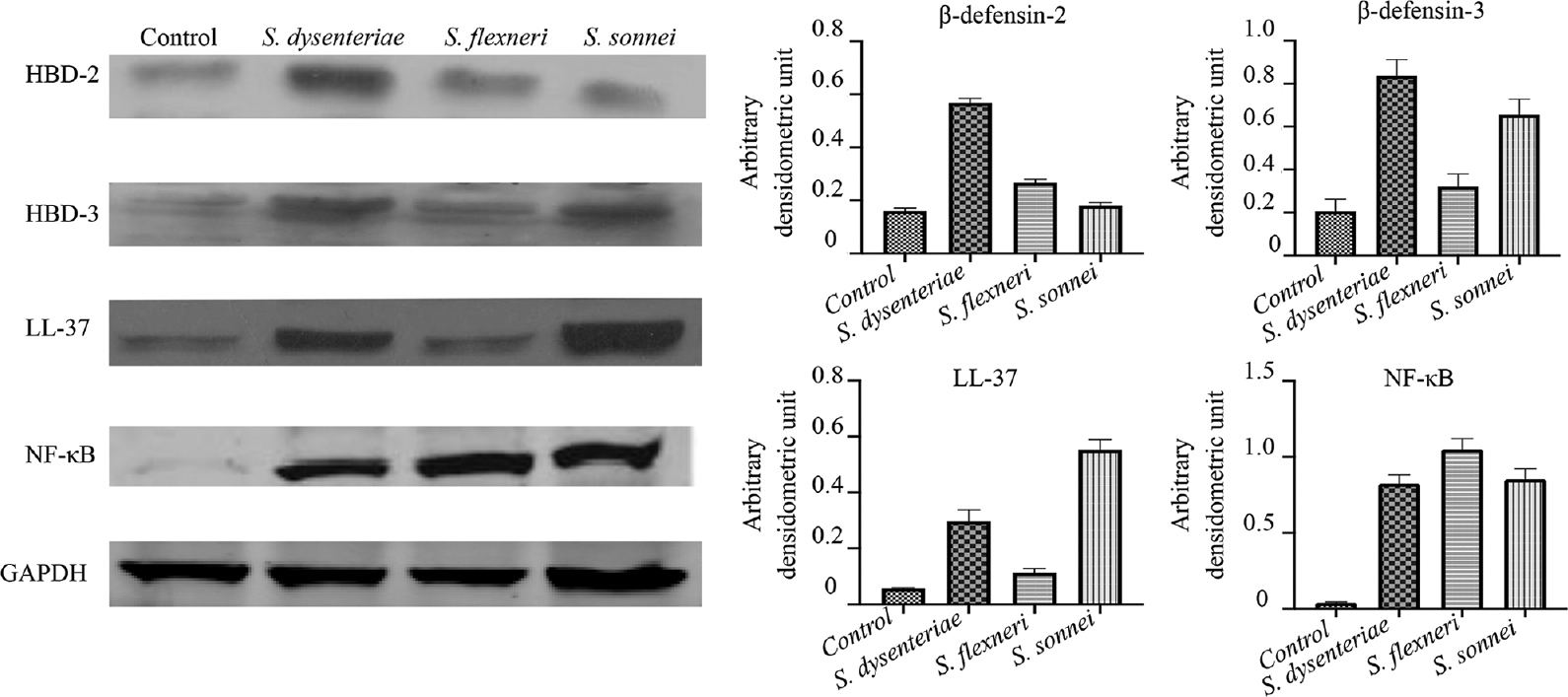
- Western blot analysis of antimicrobial peptides β-defensin-2, β-defensin-3 and cathelicidin (LL-37) in biopsies after infection with different serotypes of Shigella. HBD, human β-defensin; NF-κB, nuclear factor kappa B; GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Discussion
Human intestinal IVOC provides an apical exposure to simulate an in vivo infection route. Human intestinal IVOC has been extensively applied to studies of various enteric infections ranging from chronic Helicobacter pylori infection to acute bacterial infections including diarrhoeagenic E. coli, Salmonella, Campylobacter jejuni and Shigella as well as bacterial toxin-related enteropathy19. These have been used to provide a better understanding of bacterial virulence factors, human host responses and intestinal pathophysiology.
The ability to invade colonic epithelium is a hallmark of Shigella pathogenesis. This invasion triggers the inflammatory cascade. In the results of both IVOC and GPA in the present study, it was clear that S. dysenteriae was more invasive as compared to S. flexneri and S. sonnei. It also caused maximum necrotic damage (data not shown). Although the invasion plasmids were the same in the three serotypes, we presumed that differential invasiveness could be due to differences in the uptake of bacteria by M-cells. Alternatively could attract more neutrophils which may loosen the intracellular junctions by juxtaposing between two epithelial cells which may further enhance the entry of more Shigella through basolateral route. We believe that comparative genomics, transcriptomics and proteomics will be required to fully explain this finding.
In the course of the invasion of the human intestinal epithelial lining, Shigella are confronted by the surface antimicrobial innate defence systems. Recent studies reported that S. flexneri may interfere with this host barrier defence system by downregulating expression of genes from the innate immune response such as LL-3720. In the study by Sperandio et al21, it was clearly demonstrated that S. flexneri actively dampens host innate immune responses by suppressing transcription of specific AMP genes. In our study, by ELISA, we demonstrated that S. sonnei dampened the HBD-2 responses whereas there was augmentation by S. dysenteriae and there was a modest but non-significant increase by S. flexneri, as shown in Figure 4. A modest increase in HBD-3 by S. sonnei and S. flexneri was observed but was not significant, as shown in Figure 5. There was no increase or decrease in LL-37, as shown in Figure 6. However, western blotting data showed upregulation of all AMPs. Western blotting is more sensitive than ELISA. ELISA measures secreted proteins whereas western blotting measures even cell-bound proteins. Conflicting data are available in the literature about the up- or downregulation of HBD-2 and HBD-3 in mucosal gastrointestinal infections. For example, in a study by Zilbauer et al22, upregulation of β-defensin gene and peptide expression during C. jejuni infection was observed and recombinant β-defensins were shown to have a direct bactericidal effect against C. jejuni through disruption of cell wall integrity. On the other hand, early Shigella spp. infection or watery diarrhoea downregulated HBD-1 transcripts and LL-37 in gut biopsies21. Likewise, virulent S. flexneri suppressed transcription of HBD-3, which is especially active in vitro against S. flexneri, in HT-29 cells and human intestinal xenografts. This downregulation of HBD-3 by S. flexneri was also observed in IL-1β-stimulated colonic cells21. In this study, we found a modest augmentation of HBD-2 and HBD-3 in the IVOC by ELISA and upregulated expression of HBD-2, HBD-3 and LL-37 at translational level, as shown in Figure 7. The observed differences from other studies may be explained by the time points at which assays were done/use of different assays or experimental systems. Sperandio et al21 also showed that by blocking antimicrobial factors expression, S. flexneri seems to progress deeply and massively towards intestinal crypts at the early time point of infection21. It has been postulated that the downregulation of β-defensin depends on Shigella plasmid DNA and MxiE transcriptional activator that interfere with NF-κB and mitogen-activated protein kinase signalling pathways for LL-37 and HBD-3 expression. It was also observed that Shigella causes NF-κB, activation and increased expression of transcription factor NF-κB at translational level. Earlier reports revealed that NF-κB like binding motif is present in HBD-2 gene promoter region, which suggests transcriptional control of AMPs such as HBD-2, HBD-3 and LL-37 by NF-κB23,24.
Besides antimicrobial activity, several AMPs participate in immunomodulatory activities such as chemotaxis, activation and alteration of cytokine/chemokine responses of both innate and adaptive immune cells25,26. AMPs thus form a bridge between innate and adaptive immune systems. Additional functions attributed to these peptides are neutralization of lipopolysaccharides (LPS)27,28, regulation of the normal flora29, wound repair and maintenance of epithelial barrier integrity30,31. Because, of their multifaceted activities in host defence, AMPs are often termed as host defence peptides (HDPs) and for their immunomodulatory properties32, they are also designated as promising therapeutic agents33.
This study demonstrates differential invasiveness and AMP induction by different serotypes of Shigella. Different serotypes of Shigella cause variable severity of illness with S. dysenteriae causing the severest infections and S. sonnei being at the other end of spectrum usually causing mild self limiting diarrhoea. The reasons for this differential severity are not known as studies are hampered by unavailability of a suitable animal model. The human IVOC overcomes this challenge. AMPs are expressed and secreted by epithelial cells at the intestinal mucosal surface where these play a crucial role in the host intestinal homoeostasis. Boosting of secretion/expression of human β-defensins and LL-3 may present a promising therapeutic strategy to treat infections and dysbiosis-driven diseases in humans. This study has some limitations such as IVOC is a difficult and expensive technique. Tissue putrefaction after 24 h, each experiment requires a fresh biopsy sample, so reproducibility for expression of AMPs is low and high variation.
Overall, differences were found in invasiveness and AMP production induced by different serotypes of Shigella. Human intestinal IVOC represents a valuable addition to traditional model systems to investigate early interaction between Shigella and the human gut.
Financial support and sponsorship
This study received funding from the Indian Council of Medical Research (grant no. 5/8-1(39)/D/2012-13/ECD-II), New Delhi, India.
Conflicts of interest
None.
References
- Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis. 2010;16:1718-23.
- [Google Scholar]
- The inside story of Shigella invasion of intestinal epithelial cells. Cold Spring Harb Perspect Med. 2013;3:a016717.
- [Google Scholar]
- Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506.
- [Google Scholar]
- Shigella's ways of manipulating the host intestinal innate and adaptive immune system:A toolbox for survival? Immunol Cell Biol. 2007;85:119-29.
- [Google Scholar]
- Mucosal immunology (3rd ed). New York: Academic Press; 2004. p. :95-110.
- Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356-68.
- [Google Scholar]
- Nodlike proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250-7.
- [Google Scholar]
- Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551-7.
- [Google Scholar]
- Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181-215.
- [Google Scholar]
- Beta-defensins:Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525-8.
- [Google Scholar]
- The ex vivo response of human intestinal mucosa to enteropathogenic Escherichia coli infection. Cell Microbiol. 2009;11:521-30.
- [Google Scholar]
- Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and E. coli. J Med Microbiol. 1992;37:319-25.
- [Google Scholar]
- Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405-20.
- [Google Scholar]
- Human intestinal in vitro organ culture as a model for investigation of bacteria-host interactions. J Exp Med. 2013;5:43-50.
- [Google Scholar]
- Downregulation of bactericidal peptides in enteric infections:A novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180-5.
- [Google Scholar]
- Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J Exp Med. 2008;205:1121-32.
- [Google Scholar]
- Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun. 2005;73:7281-9.
- [Google Scholar]
- Induction of endogenous antimicrobial peptides to prevent or treat oral infection and inflammation. Antibiotics. 2023;12:361.
- [Google Scholar]
- Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387-97.
- [Google Scholar]
- The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 20145621;5
- [Google Scholar]
- β-Defensins:Multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337-48.
- [Google Scholar]
- Inhibition of lipopolysaccharide- and lipoprotein-induced inflammation by antitoxin peptide pep19-2.5. Front Immunol. 20181704;9
- [Google Scholar]
- Antimicrobial peptides mechanism of action, activity and clinical potential. Military Med Res. 2021;8:48.
- [Google Scholar]
- Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during Candida albicans infections enhancing the intestinal barrier integrity:In vitro study. Front Cell Infect Microniol. 2021;11:666900.
- [Google Scholar]
- Regulation of the intestinal barrier function by host defense peptides. Front Vet Sci. 2015;2:57.
- [Google Scholar]
- Antimicrobial peptides (AMPs) as drug candidates:A patent review (2003-2015) Expert Opin Ther Pat. 2016;26:689-702.
- [Google Scholar]






