Translate this page into:
Diagnostic accuracy of real-time PCR assay ‘Quantiplus® MTB FAST’ for detection of adult pulmonary tuberculosis (PTB): A multi-centric study
For correspondence: Dr Nivedita Gupta, Division of Communicable Diseases, Indian Council of Medical Research, New Delhi 110 029, India e-mail: drguptanivedita@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
The global target set by the United Nations (UN) high-level meeting on Tuberculosis (TB) for coverage of rapid molecular tests is 100 per cent by 2027. Rapid, affordable molecular tests for early detection of TB are the need of the hour. This study aimed to evaluate the diagnostic accuracy of an open real-time PCR (RT-PCR) assay, Quantiplus®, with reference to Mycobacteria Growth Indicator Tube (MGIT) liquid culture.
Methods
We conducted a prospective multi-centric diagnostic accuracy study of Quantiplus® assay (version 2.0) at three sites in India for the detection of pulmonary TB in sputum with culture as the reference standard, compared with Xpert® MTB/RIF. A total of 657 adults (>18 yr) with presumptive TB were enrolled consecutively. The Quantiplus® assay uses an extraction-free, quick-lysis protocol and three gene targets for RT-PCR.
Results
Of the 644 samples analysed, 37 per cent were culture-positive and 32 per cent were smear-positive. The sensitivity and specificity of Quantiplus® assay with reference to MGIT culture were 86 per cent [95% confidence interval (CI): 81-90] and 96 per cent (95% CI: 94-98), respectively, at Ct ≤ 38. The positive and negative predictive values (PPV/NPV) were 93 per cent (95% CI: 89-96%) and 92 per cent (95% CI: 89-94%), respectively. Among the 73 smear-negative culture-positive specimens, the sensitivity and specificity were 61.6 per cent (95% CI: 50-73) and 97 per cent (95% CI: 92-98.6), respectively. The performance of Quantiplus® assay(v2.0) was comparable to Xpert MTB/RIF® (κ=0.83, SE=0.02) at Ct ≤38.
Interpretation & conclusions
The flexibility of the open RT-PCR assay to be used in any RT-PCR machine makes it a very low-cost (<2 US$) alternative to the expensive cartridge-based tests. This is the first report of validation of an open system RT-PCR assay for the detection of pulmonary TB.
Keywords
Diagnosis
low-cost
molecular test
open RT-PCR
presumptive TB
pulmonary tuberculosis
Tuberculosis (TB) continues to be the world’s leading cause of death due to a single infectious agent in 20231. India accounts for 26 per cent of the total global TB disease. New global targets have been set for 2027 during the United Nations (UN) high-level meeting on TB held in 2023, including 100 per cent coverage of rapid molecular testing for newly diagnosed TB1. According to the national TB report of India, 358 per lakh population in 2023 were offered a molecular test, which was only 21 per cent of total presumptive TB testing2. Reliable, rapid, and affordable molecular tests for TB have been the priority in high-burden countries.
The nucleic acid amplification tests (NAAT) Xpert® MTB/RIF and Truenat™ MTB have been approved and introduced by India’s National Tuberculosis Elimination Programme (NTEP)2. Currently, the national programme depends on these closed system machines for testing the sputum of presumptive TB patients. However, this approach becomes limiting, especially in low- and middle-income countries (LMICs) due to the high cost of cartridges, the requirement for sophisticated infrastructure, and the inflexibility of the cartridges to be used in other platforms. Therefore, there is an urgent need for newer and more flexible tools that can be positioned at different levels of healthcare to improve access and ease of testing.
The Quantiplus® MTB FAST detection assay, developed by Huwel Lifesciences, is an open system Real-Time PCR (RT-PCR) based assay for detecting MTB Complex (MTBC) DNA. The original version of the kit was validated earlier and was found to have a sensitivity of 83 per cent [95% confidence interval (CI) 76 to 88%] and specificity of 91 per cent (95% CI: 88 to 93%). Based on expert suggestions, the manufacturer developed an improved version of the kit with three gene targets, viz, IS1081, MPT64, and IS6110. The present study was carried out to evaluate the diagnostic accuracy of an improved version of ‘Quantiplus® MTB FAST Detection Kit’ using the reference standard, liquid culture [Mycobacterium Growth Indicator Tube (MGIT)], and in comparison to Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA). This is the first report of an open system RT-PCR assay for the detection of pulmonary tuberculosis.
Materials & Methods
Study design and participants
This is a prospective multicentric diagnostic accuracy study for the detection of pulmonary TB with MGIT liquid culture as the reference standard in individuals showing symptoms of presumptive TB. The study was conducted at three sites, Christian Medical College (CMC), Vellore, King George’s Medical University (KGMU), Lucknow, and ICMR-National Institute of Research in Tuberculosis (NIRT), Chennai, from January 2024 to December 2024. Although the study site, PD Hinduja Hospital, Mumbai, was involved in validating version 1.0 of the kit, the site could not perform validation of the improved version 2.0 and hence was not included in this report.
The Institutional Ethics Committee (IEC) of these participating centres approved the study. The study was carried out in accordance with the National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, Indian Council of Medical Research (ICMR)3, and guidance on ethical requirements for laboratory validation testing, 20244. Participants were recruited by the study sites from the district TB centres (DTC), the directly observed treatment short-course (DOTS) centre, the chest clinic, or the hospital outpatient department (OPD), as given in the Box.
Definition of presumptive TB
As per the NTEP guideline, the following definition was used for presumptive TB: ‘Patients with any of the following symptoms, regardless of duration, will be considered to have ‘presumptive TB’: cough for two weeks or more, fever for two weeks or more, night sweats, unintentional weight loss, haemoptysis, chest pain or loss of appetite or any abnormality in chest radiograph’5.
Enrolment of participants
Adults >18 yr attending the DTC, DOTS centres, chest clinic, or OPD with symptoms of presumptive pulmonary TB, who are representative of the population among whom the test (if found satisfactory) will be used, were recruited consecutively by the study sites. Individuals who were on anti-TB treatment (ATT) for more than 96 h and those who could not produce two sputum specimens of >3 ml were excluded from the study. Written informed consent was obtained from the individuals consenting to participate in the study. A total of 685 participants from three sites were screened for the study. After excluding 28 participants, 657 eligible participants were enrolled in the study from January 2024 till August 2024 for validation of the original version (version 1.0) of the kit (Fig. 1). Both reference standard and index test being assessed were applied to each patient independently. The improved version of the kit (version 2.0) was evaluated from September to December 2024 by three sites using the same leftover sputum samples stored at -20°C.

- STARD Flow diagram of study participants. ATT, anti-tuberculosis treatment; MTB, Mycobacterium tuberculosis.
Sample size
The sample size was calculated with the expected sensitivity of 90 per cent with five per cent precision and the expected specificity of 99 per cent with 1 per cent precision as reported earlier5. The required sample size was to be 150 culture positives. To achieve this, approximately 610 samples need to be screened, assuming a 24 per cent prevalence6 of culture-positive patients among presumptive PTB patients in India and a five per cent loss due to indeterminate samples.
Index test and reference test
The index test ‘Quantiplus® MTB FAST Detection assay’ is a Real-Time PCR(RT PCR)-based in vitro diagnostic test for detecting MTB Complex (MTBC). The kit consists of an advanced formulated RT-PCR mix with Uracil DNA Glycosylase (UDG/UNG) and Far-Red Neutralizer (FRN), primers and probes mix specific to MTBC and internal control, positive control, and molecular biology grade water. The assay targets MTB-specific genes: IS1081, MPT64, and IS6110. The manufacturer added the IS6110 gene as an additional target in version 2.0 to increase the sensitivity. Additionally, the salt concentration of the enzyme mix was optimised to reduce background noise and improve specificity. The test comes with an endogenous internal control, beta globulin, and a positive control.
Quantiplus® MTB FAST Detection Kit uses an extraction-free quick lysis protocol using Quick Lysis Buffer (Supplementary Figure A). A sterile swab was carefully rotated after dipping into the sputum sample in the container for 10 to 15 sec. The sampled swab was then inserted into the vial containing 0.6 ml of quick lysis buffer. The absorbent tip of the swab was completely immersed in the buffer. The swab head was then twisted against the bottom and sides of the tube 10-12 times. The swab head was then pinched to the wall of the vial, and the swab stick was broken at the break point. The buffer with swab head was then placed in a dry bath at 95°C for 10 min. The lysate was given a short spin, and 5 μl of the supernatant was used as the template for RT-PCR assay.
The RT-PCR was set up with 15 μl of MTB Ready Mix and 5 μl template (Supplementary Figure B). The RT-PCR assay was carried out with an initial denaturation of 95°C for 15 min, followed by 42 cycles with 95°C for 10 sec and 60°C for 10 sec. The time required for DNA extraction was 12-15 min, while the time required for RT-PCR was 120 min. Hence, the overall turnaround time was 2.5 h.
Since the index test “Quantiplus” is a NAAT-based molecular test, it was necessary to compare its performance with another standard NAAT test. Xpert® MTB/RIF assay is the existing molecular NAAT test approved by the NTEP and the World Health Organization (WHO) and is currently used in the national programme. Hence, Xpert® MTB/RIF was used as the comparator. The liquid culture method of the BD BACTEC MGIT test was used as the reference standard.
Blinding
All study sites performed the laboratory tests at their respective sites using a common protocol5. The reference standard and the index test being assessed were applied to each patient blindly. Two different technicians were assigned to perform the reference standard test and the index test at each site. The site investigator compiled the results from each site, and the overall results were compiled by the coordinating site at the ICMR headquarters in New Delhi.
Test methods
Two sputum samples, each of a minimum of 3 ml, were collected (one spot and one morning specimen) and sent to the laboratory. Direct and deposit smears of all samples were subjected to Ziehl-Neelsen acid-fast stain microscopy. Approximately 1 ml of pooled sample was used for the Xpert MTB/RIF and Quantiplus® assay. The Quantiplus® assay was used in this study for the detection of MTB complex only and not for drug resistance. The remaining sputum samples were decontaminated by the N-acetyl-L-Cysteine (NACL)-NaOH method, and the concentrated material was inoculated into MGIT liquid culture as per standard laboratory protocol as described in the algorithm (Fig. 2).
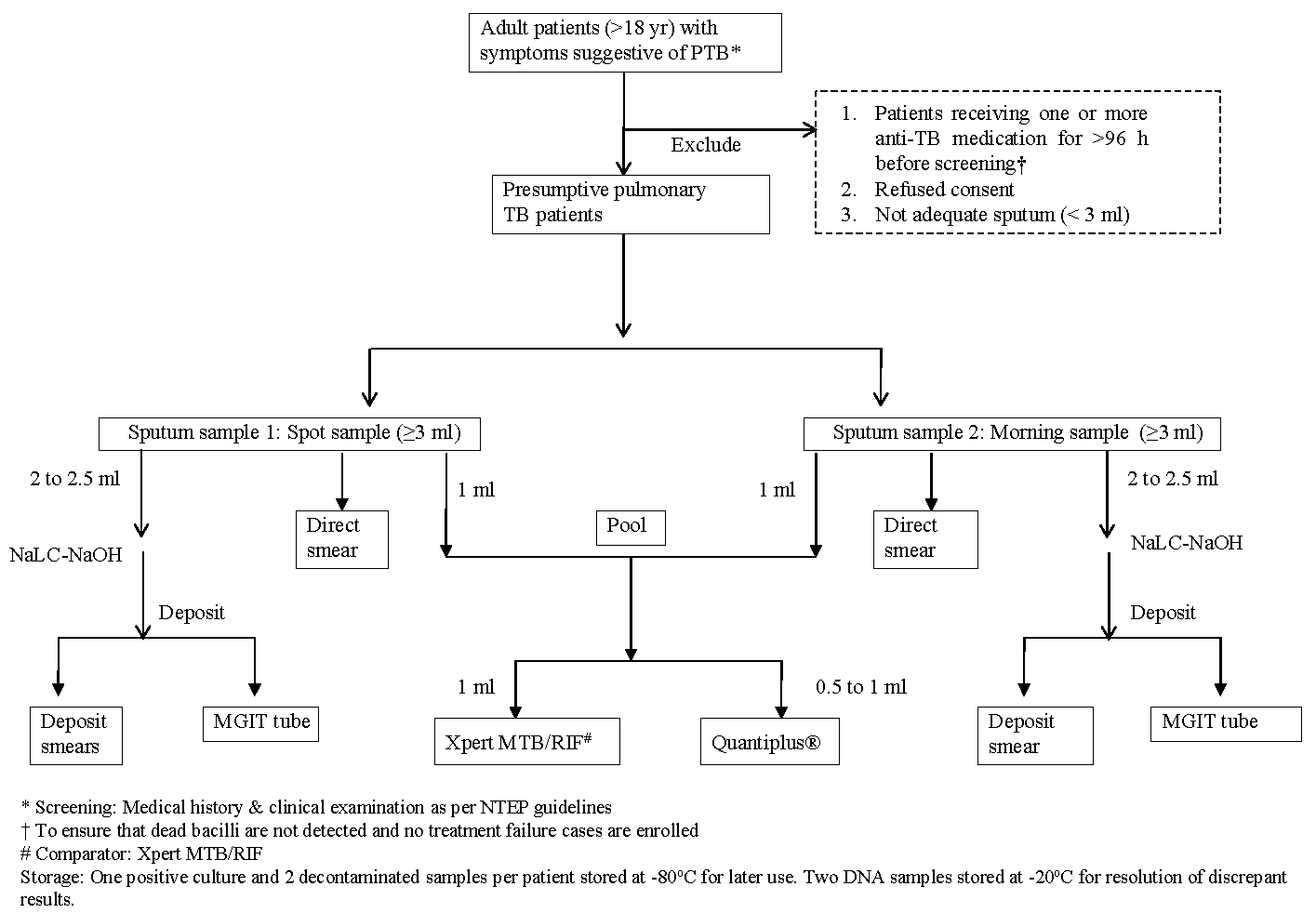
- Workflow of enrolment and laboratory testing for evaluating Quantiplus® assay.
The Mycobacterium tuberculosis complex (MTBC) was identified by an immuno-chromatographic test kit (SD MPT64TB Ag kit). One positive culture and two decontaminated deposits were stored at -80°C for potential later use, if needed. Two extracted DNA samples from each sample were stored at -20°C till the end of the study for the resolution of discrepant results. The laboratory procedures for smear microscopy, mycobacterial culture, and Xpert® MTB Rif assay were performed according to NTEP laboratory guidelines7, standard operating procedures (SOP) of ICMR-NIRT8, and WHO manual9. All participating laboratories were accredited by NTEP and the National Accreditation Board for Testing and Calibration Laboratories (NABL) for conventional test procedures. All the sites participated in internal quality control (IQC) and external quality assurance (EQA).
Result interpretation and data analysis
The interpretation of the index test was done by the site investigator using the cut-off for the cycle threshold value (Ct) of ≤ 38 as suggested by the manufacturer. All the study sites followed the same cut-off value, which was also verified by the coordinating site using Receiver Operating Characteristic (ROC) curve analysis of Ct values of all samples. The analysis of combined results from three sites was carried out by the coordinating centre at ICMR headquarters, New Delhi.
The STATA statistical software version 12.1 (StataCorp LP, College Station, TX, USA) was used for data analysis. The sensitivity, specificity, negative predictive value, positive predictive value, accuracy, and their 95 per cent confidence interval (CI) were computed. The strength of agreement between Quantiplus® and Xpert MTB/RIF tests was determined by calculating the Kappa statistic (k) for agreement corrected for chance.
Results & Discussion
A total of 657 eligible participants were enrolled in the study. Thirteen samples were excluded from analysis either because of contamination of culture (n=7) or invalid results for Quantiplus® assay (n=6). Hence, 644 samples were included for analysis. Among the enrolled participants, the majority (69 %) were male, while 31 per cent were female (Table I). The mean age (±standard deviation) of the participants was 48.2±15.4 yr; 51 of them had a history of TB (Table I).
| KGMU, Lucknow | CMC, Vellore | NIRT, Chennai | Frequency (%) | |
|---|---|---|---|---|
| Total number of eligible participants | 200 | 199 | 245 | 644 |
| Male | 120 | 137 | 189 | 446 (69.3) |
| Female | 80 | 62 | 56 | 198 (30.7) |
| Mean age±SD | 46.4±17.9 | 48.6±14.2 | 49.4±13.9 | 48.2±15.4 |
| Median age (IQR) | 47.5 [30-59.5] | 49 [38-59] | 52 [38.5-60] | 50 [37-60] |
| History of past TB | 26 | 25 | 0 | 51 |
KGMU, King George’s Medical University; CMC, Christian Medical College; NIRT, National Institute of Research in Tuberculosis; SD, standard deviation; TB, tuberculosis; IQR, inter-quartile range
Of the analysed samples, 37 per cent were culture positive (241/644), while 32 per cent (204/644) were smear positive (Table II). The Quantiplus® assay yielded minimum and maximum cycle threshold (Ct) values of 20 and 45, respectively.
| Assay | Total No. | No. of positives (%) | No. of negatives (%) | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Accuracy % (95% CI) | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Smear test | 644 | 204 (31.7) | 440 (68.3) | - | - | - | - | - | - | - |
| MGIT culture | 644 | 241 (37.4) | 403 (62.6) | - | - | - | - | - | - | - |
| Quantiplus® (version 2) at cut off Ct ≤38 | 644 | 222 (34.5) | 422 (65.5) | 85.9 (81-90) | 96.3 (94-98) | 93.2 (89-96) | 91.9 (89-94) | 92.4 (90-94) | 23.1 (14- 38) | 0.15 (0.11-0.2) |
| Quantiplus® In smear-negative | 440 | 57 | 383 | 61.6 (50-73) | 96.7 (94-98) | 78.9 (68-87) | 92.3 (91-94) | 90.9 (88- 93) | - | - |
| Quantiplus® In smear-positive | 204 | 165 | 39 | 96.4 (92- 99) | 91.7 (78-98) | 98.2 (95-99) | 84.6 (71-92) | 95.6 (92- 98) | - | - |
| Xpert MTB/RIF® | 644 | 252 (39.1) | 392 (60.8) | 98.3 (96-99) | 96.3 (94-98) | 94.1 (91-96) | 98.9 (97-99) | 97.1 (95-98) | 26.4 (16- 43) | 0.02 (0.01-0.05) |
Ct, cycle threshold; CI, confidence interval; RIF, rifampicin; MGIT, Mycobacteria growth indicator tube; PPV, positive predictive value; NPV, negative predictive value
The overall sensitivity and specificity of Quantiplus® for detection of MTB with reference to MGIT culture were 85.9 per cent (95% CI: 81 to 90%) and 96.3 per cent (95% CI: 94 to 98%), respectively, when the cut-off value was taken as Ct≤ 38 (Table II). The positive predictive value (PPV) was 93.2 per cent (95% CI: 89 to 96%) and the negative predictive value (NPV) was 91.9 per cent (95 % CI: 89 to 94%). The positive and negative likelihood ratios of Quantiplus® for detection of MTB were 23 (95% CI: 14 to 38%) and 0.15 (95% CI: 0.1 to 0.2%), respectively, indicating that a positive test is 23 times more likely among patients with PTB than among those without PTB.
The sensitivity and specificity of Xpert MTB/RIF® for detection of MTB with reference to MGIT culture were found to be 98.3 per cent (95% CI: 96 to 99.6%) and 96.3 per cent (95% CI: 94 to 97.9%), respectively, in our study (Table II). The diagnostic performance of Quantiplus® MTB assay was comparable to Xpert MTB/RIF® with a kappa value of 0.83 (95% CI: 0.79 to 0.88) at Ct ≤ 38.
Out of 644 samples tested by smear microscopy in our study, 204 were smear-positive and 440 were smear-negative. Among the 440 smear-negatives, 73 were smear-negative and culture-positive. The sensitivity of Quantiplus® for the detection of MTB in smear-negative culture-positive samples was 61.6 per cent (45/73), while the specificity in smear-negative culture-negative samples was 96.7 per cent (355/367; Table II).
The diagnostic accuracy of the NAAT test currently used in the national TB programme in the country, Truenat MTB, has been reported by several studies. The meta-analysis of diagnostic performance of Truenat MTB assay for pulmonary TB with MGIT culture as reference standard reported a pooled sensitivity and specificity of 87.6 per cent (95% CI: 82 to 92%) and 86.1 per cent (95% CI: 70 to 94%), respectively10. In a multi-country study, Penn-Nicholsan et al6 reported a sensitivity of 79.5 per cent (95% CI: 75 to 83%) and specificity of 96.3 per cent (95% CI: 95 to 97%) for Truenat MTB in a reference laboratory setting. In the same study, the sensitivity of smear-negative culture-positive specimens (n=110) for Truenat MTB and Truenat MTB Plus assays was reported to be 44.5 per cent (95% CI: 36 to 54%) and 57.3 per cent (95% CI: 48 to 66%), respectively, when tested at a reference laboratory.
A meta-analysis of 25 studies of Xpert MTB/RIF assay on sputum with reference to culture showed a pooled sensitivity and specificity of 95 per cent (95% CI: 91 to 98%) and 96 per cent (95% CI: 93 to 98%) respectively11, which corroborate with our results for Xpert MTB/RIF assay. In another meta-analysis of 74 studies with pulmonary TB samples (sputum and broncho-alveolar lavage), the pooled sensitivity was 87 per cent (95% CI: 83 to 90%) with reference to culture12. In patients with negative smears, the pooled sensitivity was 70 per cent (95% CI: 64 to 75%)12.
The recent target product profiles (TPP) for diagnostic tests to detect pulmonary TB indicate potential for new solutions with more realistic targets13. The modelled estimate of minimum acceptable sensitivity for a sputum-based low-complexity test for India is 82 per cent13. The sensitivity of Quantiplus® MTB is higher than the minimum acceptability criteria for India, but lower than the global recommendation for a low-complexity assay. However, it is worth mentioning that the WHO TPP refers to the closed system NAAT tests as low-complexity assays. The TPP specifically designed for open PCR systems are not available for MTB.
The limitation of the assay is that it requires a clean environment to avoid cross-contamination, and it is recommended to take adequate precautions to avoid any cross-contamination. The validation study of Quantiplus® MTB assay was carried out in a high-burden setting with a prevalence of 25-35 per cent for MTB by culture, and hence, the results may not be generalisable to low-prevalence settings.
Quantiplus® MTB assay has the flexibility of utilising the real-time PCR machines commonly used in laboratories. The test is highly affordable with one-fifth the price of the currently available tests (<2 US $), which uses a closed NAAT system and hence will reduce the cost of TB testing tremendously. The laboratory infrastructure in India was exponentially enhanced during the COVID-19 pandemic, with more than 3300 RT-PCR machines available across the country, which can be utilised for TB testing. The assay uses a simple and rapid DNA extraction method and can be used to test 96 samples in a single run. Hence, this test has the potential to expand TB testing.
Overall, the Quantiplus® assay demonstrated sensitivity and specificity of 86 per cent and 96 per cent, respectively, for the detection of pulmonary MTB in sputum samples with reference to liquid culture and showed significant agreement with the Xpert MTB/RIF assay. The diagnostic performance of Quantiplus® assay is comparable to that of the Truenat MTB assay reported earlier. The limitation of the assay is that it requires a clean environment to avoid cross-contamination.
Financial support & sponsorship
The study was funded by the Indian Council of Medical Research, Department of Health Research, Ministry of Health & Family Welfare, Government of India (Grant Number: NHRP06508).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Global tuberculosis report 2024. Available from: https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports/global-tuberculosis-report-2024, accessed on April 28, 2025.
- National TB Elimination Program. India TB report 2024. Available from: https://tbcindia.mohfw.gov.in/wp-content/uploads/2024/10/TB-Report_for-Web_08_10-2024-1.pdf, accessed on April 28, 2025.
- National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. Available from: https://ethics.ncdirindia.org/asset/pdf/ICMR_National_Ethical_Guidelines.pdf, accessed on April 28, 2025.
- Guidance on ethical requirements for laboratory validation testing. Available from: https://www.icmr.gov.in/icmrobject/uploads/Documents/1724842064_guidance_on_ethical_requirements_for_laboratory_validation_testing.pdf, accessed on April 28, 2025.
- Evaluation of molecular diagnostic test for detection of adult pulmonary tuberculosis: A generic protocol. Indian J Med Res. 2024;159:246-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective multicentre diagnostic accuracy study for the Truenat tuberculosis assays. Eur Respir J. 2021;58:2100526.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Revised National TB control Programme. Manual of standard operating procedures (SOPs). Culture of Mycobacterium tuberculosis and drug susceptibility testing on solid medium. Available from: https://tbcindia.mohfw.gov.in/wp-content/uploads/2023/05/7293794058standard-operating-procedures-for-CDST-labs-1.pdf, accessed on April 28, 2025.
- Standard operating procedures for Mycobacteriology laboratory. Available from: https://nirt.res.in/pdf/bact/SOP.pdf, accessed on April 28, 2025.
- Practical manual on tuberculosis laboratory strengthening, 2022 update. Geneva: WHO; 2022.
- Truenat MTB assays for pulmonary tuberculosis and rifampicin resistance in adults and adolescents. Cochrane Database Syst Rev. 2025;3:CD015543.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of Xpert in tuberculosis diagnosis based on various specimens: A systematic review and meta-analysis. Front Cell Infect Microbiol. 2023;13:1149741.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: A systematic review and meta-analysis. PLoS One. 2017;12:e0180725.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Target product profiles for tuberculosis diagnosis and detection of drug resistance. Geneva: WHO; 2024.






