Translate this page into:
Development of a cost-effective ex vivo lung perfusion system for lung transplantation in India
For correspondence: Dr Goverdhan Dutt Puri, Department of Anaesthesia & Intensive Care, C Block, 4th Floor, Advanced Cardiac Centre, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: gdpuri007@hotmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Standard donor lung preservation with cold flush and storage allows up to six hours between retrieval of lungs from the donor and transplantation in the recipient. Ex vivo lung perfusion (EVLP) systems mimic physiological ventilation and perfusion in the donor lungs with potential for prolonged lung preservation and donor lung reconditioning. In this study, it was aimed to perform EVLP on discarded donor lungs using a locally developed EVLP system.
Methods:
Equipment that are routinely used for cardiac surgeries were collected and a functional EVLP system was assembled. This system was used on five pairs of lungs retrieved from brain-dead organ donors. The lungs were ventilated and pulmonary circulation was continuously perfused with a solution containing oxygen and nutrients for four hours. The system was tested without red blood cells (RBCs) added to the solution (acellular group; n=3; A1, A2 and A3) and also with RBCs added to the solution (cellular group; n=2; C1 and C2).
Results:
The EVLP system was successfully used in four (A1, A2, A3 and C2) of the five lung pairs. Mechanical and gas exchange functions of the lungs were preserved in these lung pairs. One lung pair (C1) worsened and developed pulmonary oedema. Histopathological examination of all five lung pairs was satisfactory at the end of the procedure. Major challenges faced were leakage of solution from the system and obstruction to drainage of RBCs containing solution from the lungs.
Interpretation & conclusions:
The results of the present study suggest that, it is possible to maintain the lungs retrieved for transplantation in a physiological condition using a locally prepared EVLP system and a solution without RBCs.
Keywords
Erythrocytes
lung transplantation
organ preservation
organ preservation solutions
perfusate
Standard donor lung preservation technique involves cold flush and storage where the pulmonary vasculature is flushed with cold preservative solution and the lungs are placed on melting ice1. While this technique typically reduces ischaemia to the lungs till transplant, the optimal use of this technique is only for six hours beyond which the outcome of transplant worsens2. In addition, the lungs from brain dead donors may first need resuscitation from neurogenic pulmonary oedema before they can become suitable for transplant3. Owing to these factors, out of every five lungs being donated in developed countries, only one lung is being transplanted (21% utilization rate)4. The number is even lower for developing countries because of cost constraint.
To overcome these shortcomings, ex vivo lung ventilation and perfusion (EVLP) was developed by researchers worldwide56. Here, the lungs are first ventilated with a low tidal volume and the pulmonary circulation is continuously perfused with a hyperoncotic, hyperosmolar, oxygen and nutrient containing solution at normothermia. This system can potentially increase the time allowed between recovery of lungs and their subsequent implantation7. It can also reverse neurogenic pulmonary oedema by drawing fluid away from the interstitium8. Universal availability of such an EVLP system and the experience to operate it can increase the lung utilization rate.
In this study, the aim was to assemble a functional EVLP system with the components available locally at the cardiovascular surgical centre at the host institute.
Material & Methods
The present study was carried out in the Department of Anaesthesia & Intensive Care, Postgraduate Institute of Medical Education & Research, Chandigarh, India, after procuring approval from the Institutes Ethics Committee between April 2015 and July 2016. A separate written informed consent was obtained from the relative of the brain dead donor for the use of donor lungs for the study. Inclusion criterion for human DBD lungs was the absence of a prospective recipient for the lungs of a multi-organ donor, as notified by the National Transplant Regulatory Authority. Exclusion criteria for the lungs were the presence of pneumonia, positive donor serology (hepatitis B, C and human immunodeficiency virus) or severe mechanical lung injury. Based on the inclusion/exclusion criteria, five pairs of lungs from brain-dead donors were included in the study.
Perfusate: 12 l of lung perfusate was prepared for each pair of donor lungs. Each litre of this low potassium dextran solution contained dextran (40.5 %), sodium (148 mmol), potassium (5 mmol), magnesium (0.8 mmol), chloride (140 mmol), sulphate (0.8 mmol) and phosphate (0.8 mmol) and glucose (5 mmol). The perfusate was used without adding RBCs for perfusing the first three pairs of lungs (acellular group; Group A; A1, A2 and A3). Type-matched packed RBCs were added to the perfusate to a target haematocrit of 12-14 per cent in the latter two lung pairs (cellular group; Group C; C1 and C2).
Lung recovery: Donor demographics, drug use, smoking history, chest X-ray, bronchoscopy and the best ratio of partial pressure of oxygen in arterial blood to the fraction of inspired oxygen concentration (PO2:FIO2) of the donor were recorded. These six criteria were used to calculate lung donor score, which has been shown to predict donor lung quality and recipient survival after transplant in a previous study9. A high donor lung score indicates poor lung quality and vice versa.
Multi-organ recovery started with the liver, renal and cardiothoracic organ transplant teams performing the respective organ dissections. On completion of dissection, lung biopsy was taken from each lung, and 4 mg/kg systemic heparin was administered to the donor. Prostaglandin E1 500 µg was injected into the main pulmonary artery, the aorta was cross-clamped and the main pulmonary artery was perfused with 4 l of cold perfusate through a 14 Fr DLP™ paediatric one-piece arterial cannula (77014, Medtronic, MN, USA). Pressure bags were used for perfusion. The DLP™ cannula was sutured in the pulmonary artery so that it can be connected to the EVLP circuit at a later stage. An additional 1 l of perfusate was perfused through the pulmonary veins. Individual pulmonary veins were perfused through a 14 Fr self-inflating retrograde cardioplegia catheter (RC014, Edwards Lifesciences Corporation, Irvine, California, USA). The endotracheal tube was pulled up in the trachea and the trachea was stapled with the lungs in an inflated state. A double-layered plastic bag was used for transporting the lungs, with the outer bag containing ice and the inner bag containing the donor lungs immersed in 3 l of perfusate with a temperature target of 4°C10. The lungs were then transported to the site of EVLP, which was 15 min away from the site of recovery.
Hardware: The EVLP system (Figs 1 and 2) consisted of an EVLP circuit, a ventilator - Servo I (Maquet, Solna, Sweden), a flexible fibre-optic bronchoscope, a suction apparatus, two fluid-filled pressure transducers with high-pressure tubing to monitor the pressure in the pulmonary artery and left atrium; a sterile tray for placing the lungs, and a weighing scale.
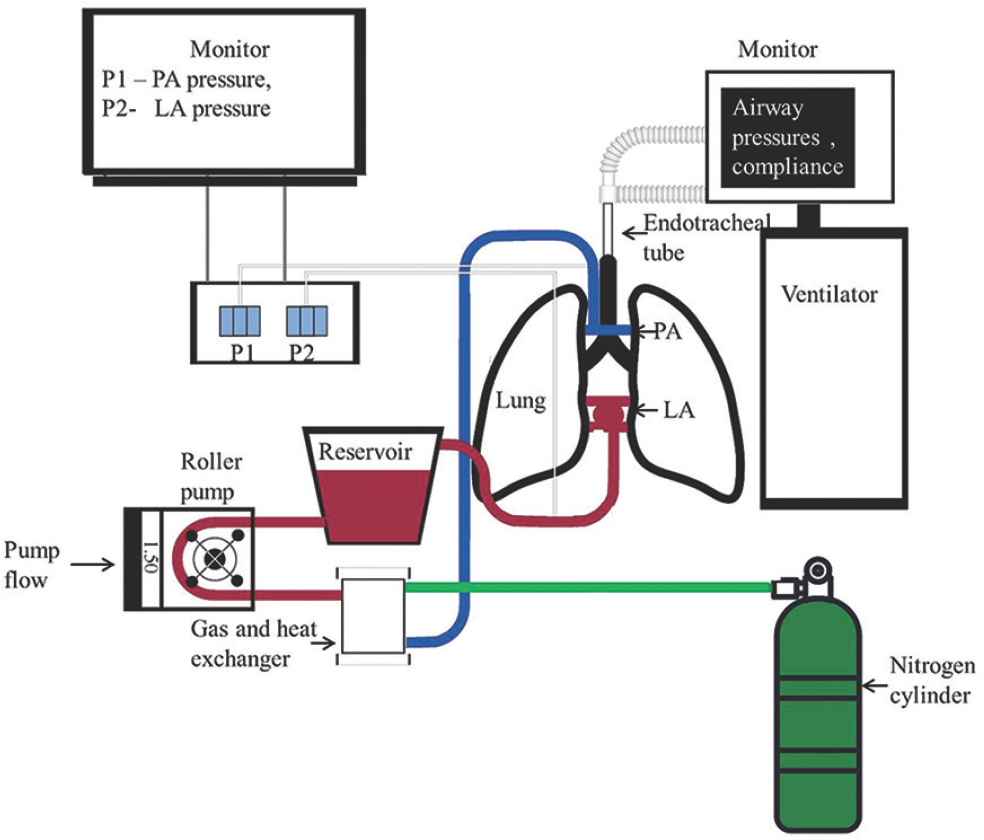
- Schematic of an ex vivo lung perfusion setup; LA, left atrium; PA, pulmonary artery; P1, P2, pressure transducers.
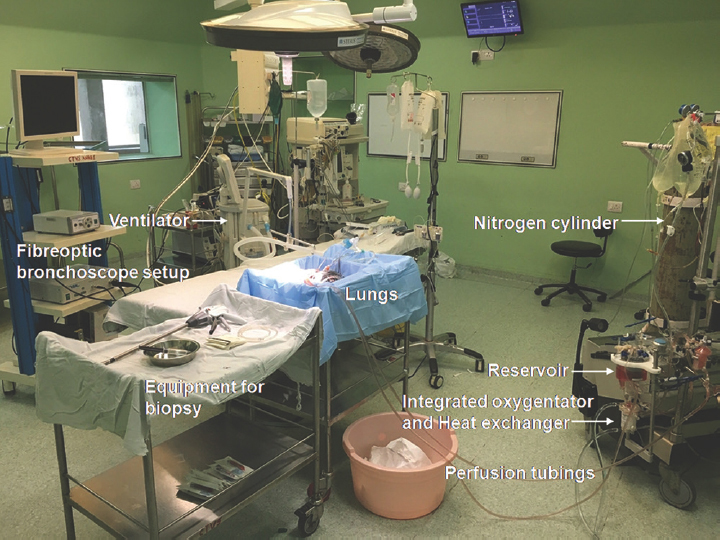
-
Ex vivo lung perfusion setup in the cardiac surgical operating room.
The components of the EVLP circuit included, (i) disposable circuit tubings to carry the perfusate between the various components of the system; (ii) hard shell reservoir to hold the perfusate; (iii) positive displacement pump – roller pump to circulate the perfusate through the components; (iv) oxygenator with an integrated heat exchanger to perform gas exchange with the perfusate; (v) wall oxygen and air supply connected to oxygenator; (vi) 100 per cent nitrogen cylinder with Y connection to oxygenator; and (vii) heater–cooler system connected to the heat exchanger.
EVLP initiation: At the site of the EVLP, the lungs were removed from the plastic cover and placed on a sterile platform. A 7.5 mm I.D. endotracheal tube (Rusch, Rusch Inc., GA, USA) was sutured to the trachea with silk ties, and the left atrial cuff was trimmed and sewn to a 22 Fr DLP™ single-stage venous cannula (66122, Medtronic, MN, USA) with polypropylene running sutures. A 14G cannula (BD Venflon™) was placed in the main pulmonary artery and left atrial pouch for pressure measurement and sampling (Fig. 3).
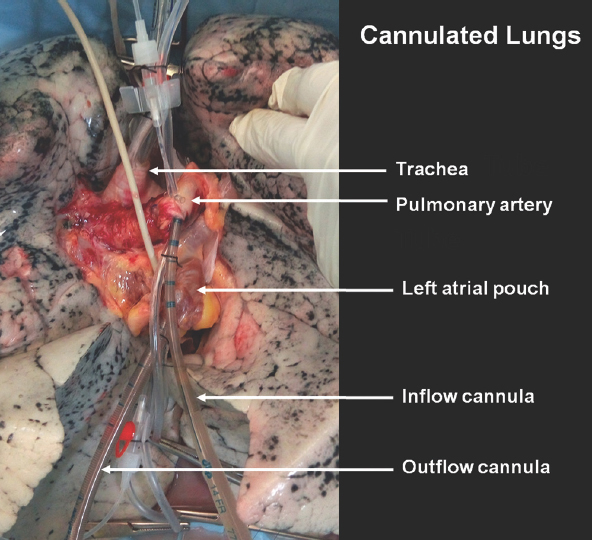
- Donor lung at the start of ex vivo lung perfusion with the endotracheal tube and cannula in place.
The method described by the Toronto EVLP group was followed for initiation of perfusion, target flows, ventilation strategy and recruitment manoeuvre8. Briefly, the lungs were initially perfused through the left atrial cannula to remove debris. This was followed by gradual ante-grade perfusion to avoid shear stress. The perfusate flow was gradually increased, ensuring that the pulmonary artery pressure remained below 15 mmHg. Since the circuit was a closed system, the left atrial pressure could be controlled by adjusting the height of the venous reservoir as long as there was no resistance in the venous cannula. The mean left atrial pressure was targeted at 2-3 cm of H2O by adjusting the height of the reservoir. The temperature control machine was set at 27°C to warm the perfusate to room temperature. Simultaneously, a flexible fibre-optic bronchoscopy was performed to remove secretions from the bronchi.
The temperature and flow were increased every 10 min till a temperature of 37°C and a flow rate of 40 per cent of donor predicted cardiac output were reached, except in the cellular perfusate group (C1 and C2), where only 30 per cent of donor predicted cardiac output could be achieved. The left atrial pressure rose exponentially beyond this flow rate in C1 and C2. This indicated that the outflow cannula from the lungs was causing obstruction to the drainage of RBC containing fluid.
Ventilation was initiated with 7 ml/kg donor’s calculated ideal body weight, respiratory rate of 7/min, FIO2 of 0.21 and positive end-expiratory pressure of 5 cm of H2O in pressure-regulated volume control mode of the ventilator once the perfusate temperature reached 32°C. Air was used through the oxygenator in the initiation and maintenance phases.
Fluid leak from the left atrial cuff and pulmonary artery was significant in lung pairs A1 and C1, which necessitated the use of suction to return the fluid to the circuit. In the remaining three cases, the leaking fluid was drained and replenished with new fluid. Lung recruitment maneuver was performed once every hour for four hours by maintaining the airway pressure at 20 cm H2O for 30 sec.
Assessment: An hourly assessment of the lungs was done by changing the gas flow to the oxygenator to 100 per cent nitrogen and ventilating with a tidal volume of 10 ml/kg donor ideal body weight at a frequency of 10 breaths/min and FIO2 of 1.0 for five minutes.
Sample from the left atrium, aspirated through the pressure transducer cannula, was used for measuring the PO2 in the perfusate leaving the lungs. PO2:FIO2 was used for evaluating the gas exchange function. Peak inspiratory pressure, mean airway pressure and dynamic compliance were noted, as displayed on the ventilator, for measuring the lung mechanics.
Lung tissue samples were also collected from both the lungs every hour. The site was chosen adjacent to the previous biopsy site. The histopathological team fixed the sample in buffered formalin 10 per cent for 24 h and embedded in paraffin. Five-mm thick sections were analyzed for extravascular fluid in the lungs in the form of interstitial and intra-alveolar oedema, signs of inflammation by the presence of alveolar macrophages, peri-bronchiolar inflammation, vasculitis and necrosis to calculate the lung injury score as has been described in a previous study11. Of note, the components of this score include chronic lung changes in addition to acute changes. The ex vivo lung perfusion was considered as successful if the PO2:FIO2 at the end of EVLP was more than 350 mmHg and if the deterioration of pulmonary vascular resistance, dynamic compliance and peak inspiratory pressure was <15 per cent from baseline in line with the Toronto protocol3.
Results
The donor demographics and the perfusate used are described in Table I. Of the five donors, donors of lung pairs A1 and A3 had the best PO2:FIO2 ratio of less than 300 mmHg, which was marginal. The donor of lung pair C2 had a history of smoking that resulted in a higher donor score.
| Lung pair number | A1 | A2 | A3 | C1 | C2 |
|---|---|---|---|---|---|
| Perfusate | Acellular | Acellular | Acellular | Cellular | Cellular |
| Donor age (yr)/sex | 47/male | 35/female | 35/female | 23/male | 55/male |
| Mechanism of injury | Vascular (embolism) | Trauma | Vascular (SAH) | Trauma | Trauma |
| Best PO2:FIO2 (mmHg) | 165 | 310 | 280 | 330 | 360 |
| Lung donor score | 8 | 7 | 8 | 7 | 8 |
| Cold ischaemia duration (min) | 105 | 155 | 160 | 90 | 170 |
PO2:FIO2, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen concentration in donor; SAH, sub-arachnoid haemorrhage
There was the collapse of dependent portions of all five pairs of lungs at the beginning of the EVLP, and these had expanded at the completion of the procedure. Maximum expansion was subjectively noted to be in the first hour after bronchoscopic clearance of secretions. The lung pairs in the acellular group (A1, A2 and A3) developed reddening of the lobe tips. However, there was no histological feature of lung damage in these regions. There was gross worsening in the appearance of the lung pair C1 in the form of pulmonary oedema.
Lung oxygenation, as measured by PO2:FIO2, showed improvement in all pairs of lungs during EVLP (Fig. 4A). This was observed even in the lung pair C1. Lung mechanics, as indicated by peak inspiratory pressure and mean airway pressure, showed stable or improving trend in lung pairs A1, A2, A3 and C2 during EVLP (Fig. 4B and C). In the acellular group (A1, A2 and A3), dynamic compliance at the end of the procedure was higher than that at the start of EVLP (Fig. 4D). The compliance in lung pair C1 had reduced by 56 per cent and in lung pair C2 by 14 per cent at the end of EVLP, which was acceptable.
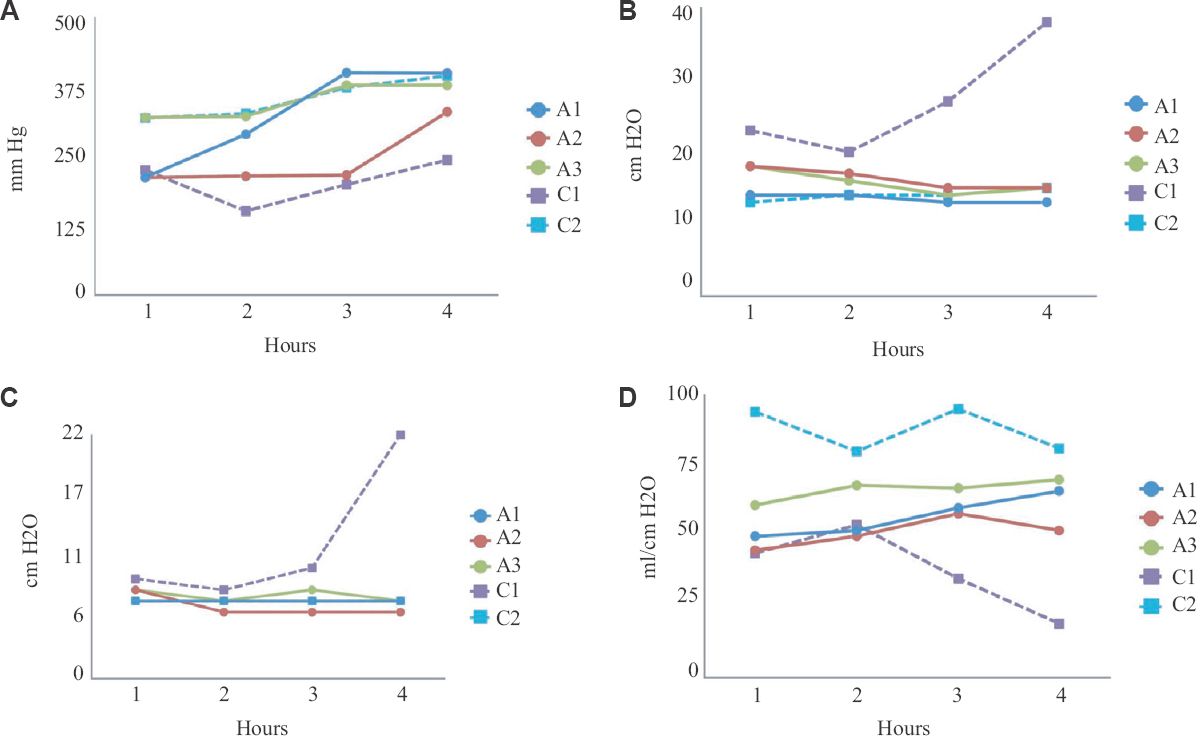
- Parameters measured in the donor lungs during ex vivo lung perfusion; (A) ratio of partial pressure of oxygen in perfusate sampled from left atrium to the fraction of oxygen in the gas given through the ventilator (PO2:FiO2; in mm Hg); (B) Peak inspiratory pressure (in cmH2O); (C) Mean airway pressure (in cmH2O); (D) Dynamic compliance (in ml/cmH2O). EVLP, ex vivo lung perfusion; A1, A2, A3, lung pairs in the acellular group, C1, C2, lung pairs in the cellular group.
Histopathological examination did not reveal worsening in any of the lung pairs (Table II). Areas of intra-alveolar and septal haemorrhage were noted in lung pair C1 (Fig. 5). However, there was no evidence of tissue ischaemia or thrombosis in any of the lung pairs. Lung pair C2 had higher scores from the beginning because of emphysematous changes.
| Time (hour) | Lung pair | Median (range) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||
| Side | |||||||||||
| L | R | L | R | L | R | L | R | L | R | ||
| Baseline | 2 | 2 | 0 | 2 | 2 | 1 | 3 | 1 | 2 | 4 | 2 (0-4) |
| Zero | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 5 | 3 | 2 (1-5) |
| One | 1 | 1 | 3 | 2 | 2 | 2 | 1 | 1 | 4 | 5 | 2 (1-5) |
| Two | 1 | 3 | 2 | 2 | 4 | 3 | 4 | 2 | 3 | 4 | 3 (1-4) |
| Three | 0 | 1 | 1 | 2 | 3 | 4 | 2 | 3 | 4 | 3 | 2.5 (0-4) |
| Four | 0 | 1 | 2 | 1 | 3 | 3 | 1 | 3 | 4 | 3 | 2.5 (0-4) |
L, left lung; R, right lung
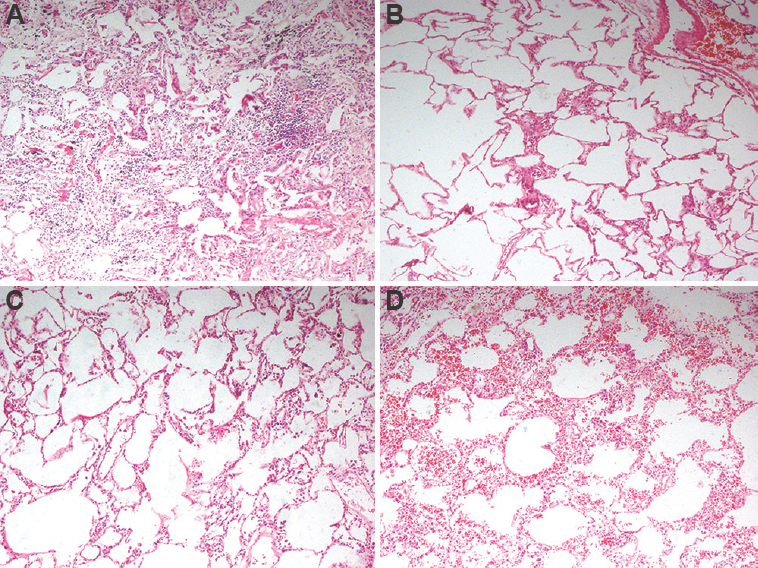
- Representative histopathology images from two lung pairs which underwent EVLP; (A) Lung pair A2 at lung recovery from the donor; (B): Lung pair A2 after four hours of EVLP demonstrating scant infiltration of lymphocytes; (C) Lung pair C1 at recovery demonstrating expanded alveoli and scant intra-alveolar macrophages; (D) Lung pair C1 after four hours of EVLP demonstrating mild intra-alveolar and septal haemorrhage. No evidence of ischaemia or thrombosis noted in any of the images. EVLP, ex vivo lung perfusion; A2, lung pair in the acellular group, C1, lung pair in the cellular group.
Discussion
Ex vivo lung perfusion is an emerging strategy for lung preservation and reconditioning. Perfusing the lungs at normal temperature with a hyperoncotic perfusate removes debris from the pulmonary circulation and provides nutrients. This strategy may prolong the time interval allowed between retrieval and transplantation, recondition donor lungs having neurogenic pulmonary oedema, and can almost double donor lung availability for transplant12. Setting up an ex vivo lung perfusion system locally is a crucial step in the establishment of a successful EVLP programme13. All five pairs of lungs in this study were recovered and used for research in the absence of a prospective recipient. Apprehension from the patients’ relatives was regarding the cosmetic effect of organ recovery. After this was addressed, consent was more forthcoming.
Standard preservation of donor lungs till transplant is usually done with cold flush and storage where the pulmonary vasculature of the donor is flushed with a cold preservative solution at 4°C and the lungs are placed in an ice box till transplant1. The time interval between organ retrieval and transplant is the graft ischaemia time and hypothermia mitigates the ischaemia in the graft during this time by causing a reversible reduction in cellular metabolism. Nevertheless, the protection is not complete and the risk of death in the recipient increases with increase in the graft ischaemia time2.
To overcome these limitations, lung preservation by simulating the internal milieu was developed. At the turn of this century, Steen et al5 designed the Lund protocol to resuscitate marginal donor lungs. This was followed by further developments in lung preservation, resuscitation and evaluation. At present, there are three common systems of EVLP, Lund protocol, Organ Care System protocol and Toronto protocol, which have been studied in large multicentre trials (Table III)14151617.
| Study | Develop-UK trial13 | Novel lung trial16 | Inspire trial14 | Expand trial15 |
|---|---|---|---|---|
| Objective | Evaluate reconditioning | Evaluate reconditioning | Compare donor lung preservation with cold static storage | Evaluate reconditioning |
| Location | Multicentre-UK | Multicentre-US | Multinational | Multinational |
| EVLP arm | 53 EVLP of which 18 were selected for transplant | 76 EVLP of which 42 were transplanted | 141 lungs (randomized) | 93 lungs on EVLP, 81 suitable for transplant, 79 transplanted |
| Standard arm | 184 standard lung recipients | 42 controls | 165 lungs (randomized) | None |
| EVLP strategy | 22 - Hybrid protocol - Toronto and Lund 31 - Lund protocol |
XPS™ system, Steen solution™ | Organ Care System lung device | Organ Care System lung device |
| Conclusion | Increased PGD, ECMO in EVLP arm | Equivalent result at one year | No short-term survival benefit | Missed the pre-specified composite primary outcome |
EVLP, ex vivo lung perfusion; PGD, primary graft dysfunction; ECMO, extracorporeal membrane oxygenation; XPS, XVIVO perfusion system
Gas exchange in the EVLP occurs at two points, first, at the level of oxygenator. The term oxygenator is a misnomer as the apparatus only performs gas exchange between the gas passing through it and the fluid passing through it. Normally, an oxygenator is used in cardiopulmonary bypass to oxygenate the blood. However, in EVLP, it is used to deoxygenate the perfusate. When 100 per cent nitrogen is passed through the oxygenator, the oxygen dissolved in the perfusate is depleted. This perfusate, depleted of oxygen, subsequently passes through the lungs and then the gas exchange function of the lungs begins, oxygenating the perfusate. The PO2 in the perfusate leaving the lungs is an indicator of gas exchange function of the lungs.
A hyperoncotic perfusate is common to all EVLP protocols. This helps prevent pulmonary oedema and assists in drawing fluid away from the interstitium. The oncotic pressure in our perfusate was provided by five per cent dextran, a relatively cheap and freely available colloid. Dextran containing solution is also used in the Organ Care System protocol, where it has been successfully used in lung preservation and resuscitation1516. The Lund protocol contains albumin in addition to dextran. Apart from contributing to the oncotic pressure, the antioxidant effect of albumin is said to be beneficial18.
The perfusate in EVLP may or may not contain RBCs. Initially, the Lund protocol was successfully performed with RBC containing perfusate. Subsequently, EVLP has been demonstrated with acellular perfusate as well8. Animal studies have also been performed to evaluate the physiological benefits of RBCs given their buffering action, rheology and oxygen-carrying capacity. However, these have yielded conflicting results19202122. In the present study the procedure was attempted both with and without RBCs. Autologous blood was avoided as tissue damage is a possibility due to the chemical mediators in autologous blood of brain dead donors21.
A controlled positive left atrial pressure in EVLP is important to preserve the geometry of the alveolar spaces and reduce lung injury23. Two left atrial strategies have been described earlier. In the open left atrial strategy, there is no cannula in the left atrium and the perfusate drains passively into a biocompatible EVLP platform. In the closed left atrial strategy, which was used in the present study, a cannula was sewn to the left atrium and the venous pressure was controlled by altering the height of the reservoir. If the closed left atrial strategy is chosen, the fluid leak can be avoided with a commercially available funnel-shaped left atrial cannula22. However, when cellular perfusate is used, the viscosity of the RBC containing solution will mandate a large outflow cannula. The product catalogue of 22 Fr DLP™ single-stage venous cannula displays a pressure drop of less than 10 mmHg for flows of up to around 1.5 l/min of water24. However, the addition of RBCs may significantly alter the flow characteristics and result in an increased pressure drop25. Use of a 32 Fr or bigger venous cannula and/or increasing the height of the platform can ameliorate this problem.
Success rates of EVLP in marginal lungs can be as high as 86-88 per cent in high-volume centres, and the extension of graft preservation time with EVLP to more than 12 h may not negatively affect the outcome2627. However, our EVLP system will need to be further evaluated for safety and efficacy as we did not clinically evaluate the lungs. Furthermore, we could not comment on the pulmonary vascular resistance as the perfusate carbon dioxide level could not be controlled. Nevertheless, serial histopathological examination of the lungs did not demonstrate any evidence of ischaemia during or after EVLP.
EVLP is a complex system that requires teamwork and trained workforce, and time is of essence. The components of our EVLP setup can also be assembled on a portable platform, which can be used to facilitate the transfer of lungs from lung retrieval site to the site of transplant. When used in such a manner, it can avoid ischaemia–reperfusion injury as well as any potential deleterious effect of cold flush and storage1.
To conclude, ex vivo lung perfusion can be performed using locally available components. The left atrial pressure strategy in the form of closed or open left atrium may depend on the type of the perfusate and is a crucial part in the circuit design, especially with the use of RBCs.
Acknowledgment:
Authors acknowledge Dr T. Balamurugan, PGIMER, Chandigarh, for providing relevant histopathological images; Dr Aveek Jayant, Amrita Institute of Medical Sciences, Kochi, India, for critically reviewing the proposal; Dr Subraashis Guha Niyogi, PGIMER, Chandigarh, for the given illustrations in the manuscript; Servshri Sunil Kumar, Naseeb Minhas, Amit Kumar, Navdeep Bansal, Ms Ramandeep Kaur and Ms Swati Bansal who are the participating investigators of the original proposal.
Financial support & sponsorship: This work was supported by Institute Research Grant (2017), Postgraduate Institute of Medical Education & Research, Chandigarh, India.
Conflicts of Interest: None.
References
- Thoracic organs:Current preservation technology and future prospects;part 1:Lung. Curr Opin Organ Transplant. 2010;15:150-5.
- [Google Scholar]
- Graft ischemic time and outcome of lung transplantation:A multicenter analysis. Am J Respir Crit Care Med. 2005;171:786-91.
- [Google Scholar]
- Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation:A pilot study of 12 patients. Lancet. 2012;380:1851-8.
- [Google Scholar]
- Successful transplantation of extended criteria lungs after prolonged ex vivo lung perfusion performed on a portable device. Transpl Int. 2015;28:248-50.
- [Google Scholar]
- Normothermic ex vivo lung perfusion in clinical lung transplantation. Curr Transplant Rep. 2015;2:324-8.
- [Google Scholar]
- Defining an extended criteria donor lung:An empirical approach based on the Eurotransplant experience. Transpl Int. 2011;24:393-400.
- [Google Scholar]
- Single-lung and double-lung transplantation:technique and tips. J Thorac Dis (10):2508-18.
- [Google Scholar]
- Cold ischemia or topical-ECMO for lung preservation:A randomized experimental study. Sao Paulo Med J. 2014;132:28-35.
- [Google Scholar]
- Transplantation of initially rejected donor lungs after ex-vivo lung perfusion. J Heart Lung Transplant. 2013;32:S153.
- [Google Scholar]
- How to establish a successful ex vivo lung perfusion program. Ann Transl Med. 2017;5:S12.
- [Google Scholar]
- An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation:DEVELOP-UK. Health Technol Assess. 2016;20:1-276.
- [Google Scholar]
- Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE):A randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6:357-67.
- [Google Scholar]
- Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND):A single-arm, pivotal trial. Lancet Respir Med. 2019;7:975-84.
- [Google Scholar]
- New insights into the Steen solution properties:Breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev. 2014;2014:242180.
- [Google Scholar]
- Evaluating acellular versus cellular perfusate composition during prolonged ex vivo lung perfusion after initial cold ischaemia for 24 hours. Transpl Int. 2016;29:88-97.
- [Google Scholar]
- Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant. 2015;34:978-87.
- [Google Scholar]
- Prolonged EVLP using OCS lung:Cellular and acellular perfusates. Transplantation. 2017;101:2303-11.
- [Google Scholar]
- Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319-25.
- [Google Scholar]
- Importance of left atrial pressure during ex vivo lung perfusion. J Heart Lung Transplant. 2016;35:808-14.
- [Google Scholar]
- Pressure and flow properties of cannulae for extracorporeal membrane oxygenation II:Drainage (venous) cannulae. Perfusion. 2019;34:65-73.
- [Google Scholar]
- Transplantation after ex vivo lung perfusion:A midterm follow-up. J Heart Lung Transplant. 2016;35:1303-10.
- [Google Scholar]
- Outcomes after transplantation of lungs preserved for more than 12 h:A retrospective study. Lancet Respir Med. 2017;5:119-24.
- [Google Scholar]






