Translate this page into:
Detection of Chlamydia trachomatis infections by polymerase chain reaction in asymptomatic pregnant women with special reference to the utility of the pooling of urine specimens
Reprint requests: Dr. Sunil Sethi, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh 160 012, India e-mail: sunilsethi10@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Genital Chlamydia trachomatis (CT) infections are one of the most prevalent sexually transmitted infections across the world. In pregnant women, if not detected and treated early, these may result in poor pregnancy outcomes and complications. The present study was aimed to screen CT infections from first void urine (FVU) samples of asymptomatic pregnant women using molecular methods. The secondary objective was to evaluate cost-effectiveness in pooling FVU samples for their diagnostic application.
Methods:
FVU samples were collected from 1000 asymptomatic pregnant women over a period of three years. Pooling was done by including five specimens in one pool in the amount of 10 µl and subjected to polymerase chain reaction (PCR) and further confirmed by direct fluorescent antibody assay (DFA).
Results:
The age of study participants ranged from 18 to 43 yr with the median±standard deviation of 26±3.84 yr. Majority of positive participants were younger than 25 years. A total of 200 pools were prepared and 20 of these were PCR positive. When individual specimen in 20 positive pools was tested, 20 PCR-positive specimens were identified from 19 pools, of which 16 were positive by DFA. Thus, CT was detected in 1.6 per cent asymptomatic pregnant women in India and pooling strategy resulted in 70 per cent reduction in a number of tests performed.
Interpretation & conclusions:
Our study detected C. trachomatis infection in 1.6 per cent asymptomatic pregnant women, and pooling of FVU specimens for PCR testing was found to be a cost-saving strategy in comparison to testing individual samples. Further evaluation and studies on the bigger sample size are warranted to validate these results.
Keywords
Asymptomatic pregnant
Chlamydia trachomatis infection
direct fluorescent antibody assay
first void urine
North India
polymerase chain reaction
pooling
prevalence
Genital Chlamydia trachomatis (CT) infections are one of the most prevalent sexually transmitted infections across the world with an annual incidence of 100 million cases1. Infections caused by this organism have been associated with several complications mainly in sexually active young individuals. Eighty five per cent of CT-infected women including pregnant ones present with clinically vague or no signs and symptoms related to the disease attributing to increased number of cases in the community who are not seeking treatment1. In pregnant women, if not detected and treated early, these cases further present with poor pregnancy outcomes such as abortion, chorioamnionitis, premature rupture of membrane, preterm labour, stillbirth and low birth weight and adverse neonatal sequelae. Moreover, they may transfer infection to their regular or new sexual partner increasing disease burden23.
In India, there is no recommendation for routine antenatal screening of pregnant women for CT infections. Scarce data are available on the prevalence in the group of asymptomatic pregnant women in our country and ranges from 0.1 per cent to as high as 20 per cent in some healthcare centres depending on the geographic region, settings and laboratory methods used to detect CT infections45. Other studies from developing countries have shown the prevalence ranging from 7 to 45 per cent6.
Although it is possible to isolate the organism, but it is technically difficult and the need of endocervical swabs to obtain the live organism is another limitation of the culture. Most of the commercially available serological diagnostic kits are costly and have poor sensitivity and specificity5. Thus, we aimed in the present study to estimate the presence of CT infections in asymptomatic pregnant women in India with the use of sensitive molecular techniques. We also evaluated the utility of pooled urine samples to perform polymerase chain reaction (PCR) to decrease the costs of testing.
Material & Methods
In the present study, a total of 1000 asymptomatic pregnant women having gestational age <24 wk were recruited during an antenatal outpatient visit in the Gynaecology department of Postgraduate Institute of Medical Education and Research, Chandigarh, India over the period of three years from July 2009 to June 2012. The patients were selected according to the inclusion criteria which was representative of the study group when attributed to the general population also. Symptomatic patients and those on treatment were excluded from the study. Informed written consent was obtained for the collection of urine samples. All women were provided sterile urine container to collect 30-40 ml of first void urine (FVU). The samples were transported to the laboratory as soon as possible, preferably within two hours of collection. Before the processing, FVU specimen was kept at 4°C and then aliquoted in two parts. One part was stored at -20°C for the future use. All urine samples were processed within seven days of collection.
The study protocol was approved by the institutional ethics committee and written informed consent was obtained from all participants.
DNA extraction for PCR: DNA extraction was done according to manufacturers’ instructions given in the PCR Kit [Roche Amplicor CT/NG test for CT (Roche Molecular Systems, Inc., Branchburg, USA)]. Briefly, 500 µl aliquot of FVU at room temperature was centrifuged at 12,500 g for five minutes with the equal amount of wash buffer. Then, supernatant was discarded, and pellet resuspended in 250 µl of lysis buffer and 250 µl specimen diluents and vortexed and centrifuged at 12,500 g for 10 min. These processed specimens were used for amplification.
Pooling of urine samples: A pool of urines was made by placing 10 µl of each processed specimen into an amplification vial to create a 5x pool for a total volume of 50 µl. If pools of specimens demonstrated positive result, individual urine was tested for CT.
PCR assay: PCR was performed using primer consisting of 207 nucleotide sequences within the cryptic plasmid of CT. Internal control (IC) primers, provided by manufacturer, were also used in each run. IC primers were DNA plasmid with primer binding regions identical to those of CT target sequence. Pooled and individual processed urine specimens were tested on the same run according to manufacturer instructions (Roche Diagnostics, USA). The PCR process included hold programme for two minutes at 50°C and five minutes at 95°C followed by 35 cycle run at 91°C, 62°C and 72°C for 10, 50 and 30 sec, respectively and then hold programme for five minutes at 95°C. Following amplification, end products detection process was based on biotin-avidin interactions. Specimens were labelled positive if showed OD value >2. The samples from positive pools were retested by PCR to find out the individual positive samples amongst them.
Direct fluorescent antibody assay (DFA): All PCR- positive samples were further confirmed by direct fluorescent antibody assay. DFA utilizes a fluorescein-tagged monoclonal antibody for direct visualization of elementary bodies (EBs) of CT in the collected specimens. Tests were carried out according to manufacturer’s instructions (Cell Labs, Australia). Smears in which >10 EB were seen, labelled as positive. Less than ten EBs in smear were taken as doubtful. In negative smear, there was no EB.
The statistical analysis was done using the SPSS software version 17 (SPSS Inc., Chicago, IL, USA) and calculation of statistical parameters according to standard formula.
Results
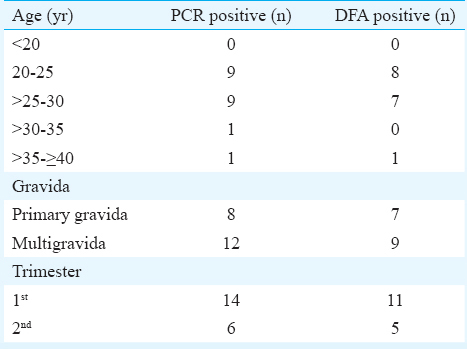
A total of 1000 asymptomatic pregnant women were included in the present study. The characteristics of all 1000 women screened for CT are listed in Table I.

The age of study participants ranged from 18 to 43 yr with the median±standard deviation of 26±3.84 yr. Majority of positive participants were younger than 25 yr. Of the 16 CT-positive women, 11 (68.75%) were in the first trimester of pregnancy whereas rest of the CT positive women were in the second trimester (31.25%). Seven of the 16 (43.75%) asymptomatic pregnant women were primigravida followed by nine multigravida (56.25%) (Table II).
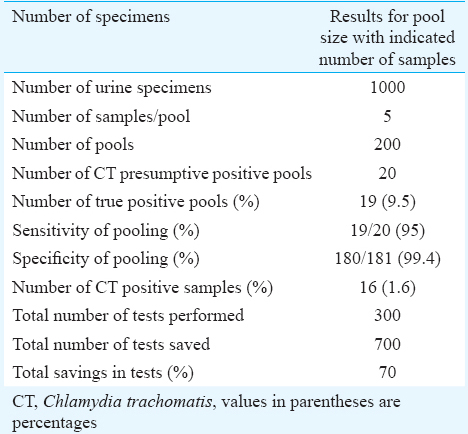
There were 1000 urine samples available for pooling. A total of 200 pools were made including five specimens in each pool. Twenty of these 200 pools which showed OD value more than 0.2 tested by PCR were labelled positive. When individual specimen in 20 positive pools was tested, 20 PCR-positive specimens were identified from 19 pools. One pool was false positive. All PCR-positive urine samples were further confirmed by DFA. Of the 20 PCR-positive urine samples, 16 (16/1000=1.6%) were positive by DFA. Table III demonstrates data for the urine specimens collected, prepared pools, number of positive pools, sensitivity and specificity of pooling, individual positive specimen and cost saving based on the reduction in the number of tests required.
Discussion
Genital CT infections and its consequences are serious health-related issues in the developing countries like India. Screening of symptomatic and asymptomatic population has become mandatory to reduce the morbidity and mortality due to infections caused by this organism in these countries. Widespread screening is economical if the prevalence is >3 per cent7. In low prevalence resource-poor settings, screening of high-risk population is more common. Several studies have shown decrease in prevalence and incidence of CT infections whenever screening initiatives are undertaken. Hillis et al8 from the USA have reported 40 per cent decrease in the incidence of new CT infection between 1987 and 1991 when screening, preventive and intervention measures were adopted in asymptomatic young women. Centers for Disease Control and Prevention have also approved antenatal screening of CT infections9.
In the present study, genital CT infection was observed more in women under 25 yr of age (50%) which was consistent with other studies1011. This could be due to sexually more active behaviour of the younger individuals which predispose them to genital CT or other sexually transmitted diseases.
To date, FDA has approved only four nucleic acid ampliflication techniques (NAATs) for the detection of CT12. The DNA sequence target cryptic plasmid is present in more copy numbers (7-10/cell) contributing to better sensitivity of Cobas Amplicor assay than other commercially available assays which target major outer membrane protein. Since urine is non-invasive specimen, easier to collect from patients, requires non-specific shipping necessities and stable on storage at 2-8°C, we preferred collecting urine samples over endocervical swabs. Several investigators have demonstrated urine PCRs sensitivity is equal to endocervical swab PCR or higher in some cases if infection is present in urethra only, not in cervix1314. There is evidence of comparable sensitivity (100%) of pooled and individual testing of urine samples1516, thus pooling of urine samples was done by adding five samples in each pool to reduce the costs of CT screening. Some studies have suggested lower sensitivity on addition of eight or more samples in a pool; therefore, we did not increase the number more than five in a single pool1718. Furthermore, amplification of internal DNA control (provided by manufacturer) was observed in every PCR run, supported no inhibitory effect of pooling on assay performance. In contrast, after pooling, it was easier to separate supernatant from pellet following centrifugation and probably it had diluted inhibitory factors of PCR, thus false-negative results were none. In the present study, pooling strategy resulted in 70 per cent of cost saving based on the reduction in the number of tests performed and potential saving of kits. Kacena et al17 and Peeling et al19 had carried out studies in fairly large samples and similarly published the benefits of the pooling of urine specimens to reduce the costs of PCR assays for the detection of CT.
Cobas Amplicor yielded 20 positive test results out of 1000 urine specimens which were further confirmed by DFA. From these 20 PCR-positive specimens, DFA confirmed 16 positive tests out of 1000 urine samples. In four urine specimens, discrepancy between the results of PCR and DFA was noted. Contrary to the finding of our study, a study conducted in north India (New Delhi) in 2003 has reported 18.8 per cent prevalence (95% CI: 14.76-22.96%) in endocervical swabs collected from 350 pregnant women using technique similar to our study5. There are other studies from India also available which reported the prevalence of genital CT in higher range 4, 6 and 14 per cent, respectively20-23. These studies have used different specimens and techniques, enzyme immune assay or other laboratory methods, with lower sensitivity and specificity as compared to PCR and DFA which could be the possible reason of overestimation of the problem. Pooling strategy in clinical samples, especially in low prevalence setting, has proven to be a cost-effective tool for epidemiological and screening programmes2425.
In conclusion, screening of FVU samples of asymptomatic pregnant women yielded 1.6 per cent positivity for CT infection. Pooling strategy was quick and cost saving; however, further evaluation and studies on the bigger sample size are warranted to validate these findings.
Acknowledgement
Authors are thankful to the Indian Council of Medical Research for funding the study.
Conflicts of Interest: None.
References
- 2008. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. Available from http://www.who.int/
- Sexually transmitted infections in pregnancy: Prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect. 2005;81:294-302.
- [Google Scholar]
- Characteristics of Chlamydia trachomatis infection in hospitalized infants with lower respiratory tract infection. J Microbiol Immunol Infect. 2007;40:255-9.
- [Google Scholar]
- Low prevalence of Chlamydia trachomatis infection in non-urban pregnant women in Vellore, S. India. PLoS One. 2012;7:e34794.
- [Google Scholar]
- Effect of treatment for Chlamydia trachomatis during pregnancy. Int J Gynaecol Obstet. 2003;80:129-37.
- [Google Scholar]
- Sexually transmitted diseases: a challenge to reproductive health. In: Khanna J, Van Look PFA, Griffin PD, eds. Challenges in reproductive health research: Biennial report 1992-1993. Geneva: WHO; 1994. p. :83-97.
- [Google Scholar]
- Cost effectiveness of screening for Chlamydia trachomatis: A review of published studies. Sex Transm Infect. 2002;78:406-12.
- [Google Scholar]
- The impact of a comprehensive Chlamydia prevention program in Wisconsin. Fam Plann Perspect. 1995;27:108-11.
- [Google Scholar]
- Guidelines for Treatment of Sexually Transmitted Diseases. Atlanta, GA: Centers for Disease Control and Prevention; 1998.
- Use of ligase chain reaction with urine versus cervical culture for detection of Chlamydia trachomatis in an asymptomatic military population of pregnant and nonpregnant females attending Papanicolaou smear clinics. J Clin Microbiol. 1998;36:1300-4.
- [Google Scholar]
- Prevalence of Chlamydia trachomatis and genital mycoplasmas in asymptomatic women. Can Med Assoc J. 1985;133:34-5.
- [Google Scholar]
- Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J Clin Microbiol. 2014;52:2305-10.
- [Google Scholar]
- Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet. 1995;345:213-6.
- [Google Scholar]
- Noninvasive tests for diagnosis of Chlamydia trachomatis infection: Application of ligase chain reaction to first-catch urine specimens of women. J Infect Dis. 1995;172:1411-4.
- [Google Scholar]
- Pooling of clinical specimens prior to testing for Chlamydia trachomatis by PCR is accurate and cost saving. J Clin Microbiol. 2004;42:4866-7.
- [Google Scholar]
- Major improvements in cost effectiveness of screening women for Chlamydia trachomatis using pooled urine specimens and high performance testing. Sex Transm Infect. 2002;78:74-5.
- [Google Scholar]
- Pooling urine samples for ligase chain reaction screening for genital Chlamydia trachomatis infection in asymptomatic women. J Clin Microbiol. 1998;36:481-5.
- [Google Scholar]
- The impact on accuracy and cost of ligase chain reaction testing by pooling urine specimens for the diagnosis of Chlamydia trachomatis infections. Sex Transm Dis. 1999;26:504-7.
- [Google Scholar]
- Pooling of urine specimens for PCR testing: A cost saving strategy for Chlamydia trachomatis control programmes. Sex Transm Infect. 1998;74:66-70.
- [Google Scholar]
- Low prevalence of chlamydial endocervical infection in antenatal South Indian women. Genitourin Med. 1993;69:240-1.
- [Google Scholar]
- Seroprevalence of Chlamydia trachomatis infection in women with bad obstetric history and infertility. J Commun Dis. 2011;43:243-7.
- [Google Scholar]
- Chlamydia trachomatis antigen detection in pregnancy and its verification by antibody blocking assay. Indian J Med Microbiol. 2006;24:97-100.
- [Google Scholar]
- Prevalence of genital Chlamydia trachomatis by first void urine polymerase chain reaction test in women attending out patient clinic. J Obstet Gynecol India. 2006;56:511-3.
- [Google Scholar]
- Pooling of urine specimens for detection of asymptomatic Chlamydia trachomatis infections by PCR in a low-prevalence population: Cost-saving strategy for epidemiological studies and screening programs. J Clin Microbiol. 2000;38:1679-80.
- [Google Scholar]
- Utility of pooled urine specimens for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in men attending public sexually transmitted infection clinics in Mumbai, India, by PCR. J Clin Microbiol. 2005;43:1674-7.
- [Google Scholar]






