Translate this page into:
Current concepts in the management of small cell lung cancer
Reprint requests: Dr Apar Kishor Ganti, Division of Oncology-Hematology, Department of Internal Medicine, University of Nebraska Medical Center, 987680 Nebraska Medical Center Omaha, NE 68198-7680, USA e-mail: aganti@unmc.edu
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Small cell lung cancer (SCLC) has a clinical course that is distinct from its more common counterpart non-small cell lung cancer. SCLC continues to be a major clinical problem, with an aggressive clinical course and short disease-free duration after initial therapy. Current optimal treatment consists of chemotherapy with platinum-etoposide, given concurrently with thoracic irradiation in patients with limited stage disease and chemotherapy alone in those with extensive stage. Prophylactic cranial irradiation (PCI) is recommended for patients who have responded to initial therapy, as it not only decreases the risk of brain metastases and but also improves overall survival. Newer targeted agents are currently being evaluated for this disease.
Keywords
Cisplatin
etoposide
small cell lung cancer
prophylactic cranial irradiation
thoracic radiation
Introduction
Soon after its first description in 19591, small cell lung cancer (SCLC) was recognized to have a clinical course that was significantly different from its more common counterpart non-small cell lung cancer (NSCLC)2. While the incidence of SCLC is decreasing in the United States from about 25 per cent of all lung cancers in 1993 to about 13 per cent more recently3, it is unclear if the same holds true worldwide. The reasons for this decline in incidence are likely related to the changes in smoking patterns and habits in North America and Europe4. Despite this, SCLC is a significant clinical problem. Approximately two thirds of patients with SCLC are diagnosed with advanced disease, which tends to have an aggressive clinical course, often with paraneoplastic syndromes. This review focuses on the current approaches for the management of SCLC.
Pathology
SCLC was originally thought to be a form of lymphoid neoplasm or unusual sarcoma. In 1926, Barnard first characterized this tumour as an unusual carcinoma and coined the term “oat cell carcinoma”5. The 2004 WHO definition, which is in use currently, classifies SCLC as a “malignant epithelial tumour consisting of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. The cells are round, oval or spindle-shaped. Nuclear molding is prominent. Necrosis is typically extensive and the mitotic count is high”6. This classification includes a variant: combined small cell carcinoma, which has a component of small cell carcinoma admixed with a >10 per cent non-small cell carcinoma component (any histologic subtype).
Despite the name, size alone does not reliably separate SCLC from other pulmonary neoplasms. Hence, in addition to size (2-3 times the diameter of a normal lymphocyte), the neoplastic epithelial cells should have the proper array of characteristic nuclear features, often with nuclear molding, and a high mitotic rate78 to make the diagnosis. Frequently, the tissue available to the pathologist is a small biopsy and/or cytology specimen and the limitations associated with using these small specimens should be acknowledged. The tumour sample often has significant necrosis and “crush” artifact that may further limit the amount of intact tumour that is available for assessment. While “crush” artifact is frequent in SCLC, it is non-specific7. If the tumour does not qualify as SCLC by initial morphologic assessment, additional studies often fail to clarify the diagnosis9.
SCLC is a high grade neuroendocrine carcinoma with a high mitotic activity (>10 mitoses/2 mm2), averaging 60-80 mitoses/2 mm2, and widespread necrosis6. Of the immunoperoxidase antibody panels used to diagnose SCLC, CD45/LCA (leucocyte common antigen) and a keratin cocktail are probably the most useful. In a high grade tumour with the proper morphologic features on initial microscopic evaluation, a positive keratin stain and negative CD45/LCA stain support the diagnosis of SCLC and exclude the possibility of lymphoid tumours10. SCLC show positive keratin stains in virtually all cases and thyroid transcription factor-1 (TTF-1) positivity in the majority (70-90%)79. Neuroendocrine markers such as chromogranin, synaptophysin and CD56/NCAM (neural cell adhesion molecule) are positive in 50-60 per cent of cases, but may be negative in a substantial proportion (10-30%)9. SCLC Ki-67 proliferation rates are high (70-100%) compared to other better differentiated NE tumours (typical carcinoid <5%, atypical carcinoid 5-20%). The p63 staining is generally negative in SCLC, which may be useful to differentiate SCLC from small cell variant of squamous cell carcinoma (positive for p63 in 90% of cases). A reliable diagnosis of SCLC can often be made on routine stains if high quality standard hematoxylin-eosin microscopic sections and well preserved tumour cells are available, often negating the need for additional studies.
Staging system
Since systemic chemotherapy is the main treatment for all patients with SCLC, the main clinical purpose of the staging is to determine whether thoracic radiation should be incorporated in conjunction with chemotherapy for localized disease. Two systems are commonly used to stage SCLC: (i) The tumour-node-metastases (TNM) classification11, which is identical to that used for non-small cell lung cancer, and (ii) The VA Lung Study Group (VALSG) limited disease- extensive stage (LD-ED) system. Limited stage disease (LD) is confined to the ipsilateral hemithorax and all known disease can be encompassed within a single radiation port, while extensive stage disease (ED) includes disease in the contralateral hemithorax and distant metastases. The International Association for the Study of Lung Cancer (IASLC) defines limited stage as absence of distant metastatic disease12.
Although the IASLC system has a higher discriminatory power13, the VALSG system continues to be widely utilized, probably because of its simplicity14. In an analysis of 8088 SCLC patients, survival was found to be directly correlated with both T and N stages15 and hence the TNM system should become the staging system of choice16.
The staging of patients with ipsilateral pleural effusion, supraclavicular nodes and contralateral mediastinal lymph node involvement is debated. Most trials for LD tend to exclude patients with isolated pleural effusions171819, but survival of patients with isolated pleural effusions is similar to other patients with LD-SCLC2021. Supraclavicular lymph node involvement on the other hand, may predict for a slightly inferior survival2223. In a retrospective analysis of 264 patients with LD-SCLC, patients without clinically positive mediastinal lymph nodes had better outcomes compared to those with positive mediastinal or supraclavicular nodes, pleural effusion, or bronchial obstruction24.
Management
Cytotoxic chemotherapy: SCLC is extremely chemosensitive25 and multiple agents have shown activity in this disease, including platinum compounds (cisplatin, carboplatin), camptothecins (topotecan, irinotecan), podophyllotoxins (etoposide, teniposide), anthracyclines (doxorubicin, epirubicin), alkylating agents (cyclophosphamide, ifosfamide), taxanes (paclitaxel, docetaxel) and vincristine.
Trials conducted in the 1970s found that cyclophosphamide, doxorubicin/epirubicin and vincristine [CA(E)V] was effective for SCLC262728. After the introduction of etoposide, randomized trials comparing etoposide-cisplatin (EP) with CA(E)V suggested that EP had superior response rates, and better disease free and overall survival in patients with limited stage disease. Even though response rates were higher with EP in patients with ED, this did not translate into a survival benefit29303132. However, since EP is better tolerated it is currently the regimen of choice for initial treatment of SCLC. While trials using alternating CAV and EP showed slightly better outcomes than either combination alone, this did not represent a major improvement303132.
When carboplatin was used instead of cisplatin in combination with etoposide33, there were no differences in response rates, but carboplatin was associated with significantly less toxicity. A second study evaluated the long-term survival following carboplatin-based chemotherapy with radiotherapy in patients with LD34. Patients had a median survival of 17.4 months, with a 5-yr overall survival of 20 per cent, findings comparable with cisplatin-based regimens.
The Japanese JCOG 9511 trial found higher response rates (84 vs. 68%) and median survival (12.8 vs. 9.4 months) with cisplatin-irinotecan compared to EP in patients with ED-SCLC35. As expected, irinotecan had lower haematologic side effects, but increased diarrhoea compared to etoposide. However, multiple trials outside Japan found similar outcomes with the two regimens36373839. The discordant results may be due to pharmacogenomic differences between the Japanese and Western populations in irinotecan metabolism.
The low percentage of patients with LD-SCLC who experience long-term survival has led to studies examining the role of multi-drug therapy in an effort to increase the proportion of long-term survivors. The role of platinum-based triplet therapy has been investigated following the introduction of additional active agents, but a definite survival advantage has not been demonstrated at this time and cannot be recommended outside of a clinical trial.
Duration of therapy: Two randomized trials have been conducted to evaluate the role of additional cycles of therapy following four cycles of platinum-etoposide in patients with ED-SCLC4041. Both studies showed that although extended therapy improved progression-free survival, overall survival was similar.
A meta-analysis (14 trials; 2550 patients) suggested that maintenance/consolidation chemotherapy was associated with a 33 per cent increased survival at 1 and 2 years42. However, only one trial used what we would consider modern chemotherapy regimens in both induction and consolidation phases. Hence maintenance therapy for SCLC cannot be recommended outside of a clinical trial.
Thoracic radiation
The majority of patients with LD-SCLC treated with chemotherapy alone relapse locally. In contrast, thoracic radiation therapy (TRT) can provide local control, but does not have a major impact on overall disease control43. Multiple randomized trials combined the two modalities to achieve better overall disease control. Two meta-analyses concluded that a combination of TRT and chemotherapy produced a small, but definite improvement in survival4445. This was however, at the expense of a small, but significant increase in the risk of treatment related death. Hence, the addition of thoracic radiation to combination chemotherapy is currently considered the standard of care in patients with LD-SCLC. A Canadian population-based retrospective study found that combined modality therapy improved median, 2- and 5-yr survival as compared to chemotherapy alone (15.1 months, 32 and 12% vs. 14.3 months, 26.9 and 9.9%, respectively)46. However, multiple issues are unresolved, including the total radiation dose, fractionation schedule and timing of radiation vis-à-vis chemotherapy.
The National Cancer Institute of Canada found that patients receiving a 37.5 Gy (Gray) had a better local control than those receiving 25 Gy47. However, this did not translate into better overall survival. A retrospective analysis of patients enrolled in three consecutive chemoradiation trials, treated with 45, 55 and 65 Gy found similar local control and overall survival with the three doses analyzed, suggesting that a dose of at least 45 Gy would be needed to obtain adequate local control48.
Currently, the most commonly utilized fractionation schedules involve single daily treatments of 1.8 to 2.0 Gy, five times per week, over 5 to 6 wk. Hyperfractionated radiotherapy (twice daily radiation with lower individual fractions) improves local control and possibly survival by applying higher doses of radiation given in a shorter time. A randomized phase III trial that randomized patients with LD-SCLC to either 1.8 Gy once-daily over 5 wk (25 treatment days) or accelerated 1.5 Gy twice-daily for 3 wk (15 treatment days) in conjunction with four cycles of EP49, showed that patients receiving the accelerated twice-daily schedule had better median survival (23 vs. 19 months), and 5-year overall survival (26 vs. 16%). Patients randomized to the twice-daily arm had an increased incidence of grade 3 oesophagitis requiring narcotic analgesics or feeding tube placement (27 vs. 11%). A criticism of this study has been that the two radiation doses used were not biologically equivalent and 45 Gy given on an accelerated twice-daily schedule should ideally be compared to 70 Gy given on a once-daily schedule50 to answer this controversy. In addition, twice-daily radiation is not very practical, and, therefore, has never been widely utilized.
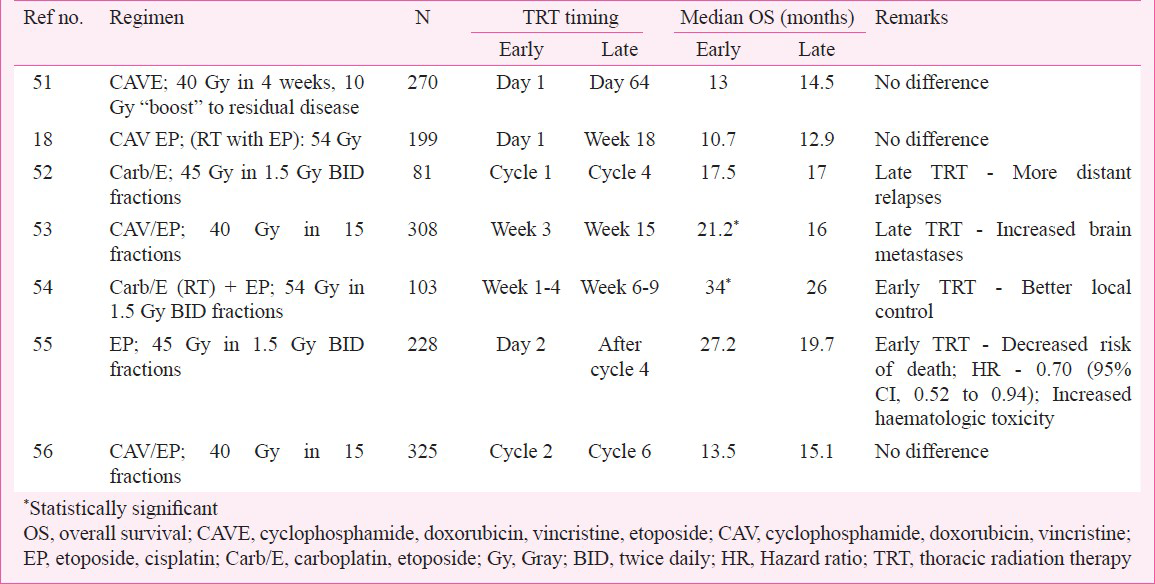
Various trials have compared early versus late TRT in combination with chemotherapy (Table I)18515253545556. Unfortunately the results of these studies were conflicting, with two trials showing a benefit for early radiation5354, while the rest showed that both approaches provided similar results. A meta-analysis57 suggested that while there was no difference between early or late TRT on overall survival, when only trials using platinum-based chemotherapy were considered, there was a significantly improved 5-year overall survival with early TRT.
Surgery
Since SCLC is considered a systemic disease, there has not been a role for surgery in the management of these patients585960. However, recent studies have shown promising results for surgical resection of early stage disease. Approximately 4-12 per cent of lung cancers in patients who present with a solitary lung nodule are SCLC61. Five-year survival rates following surgical resection of SCLC presenting as a solitary pulmonary nodule, ranged from 40-53 per cent61. Other studies have demonstrated 5-year survival rates of 27-42 per cent following resection of stage I or stage II (TNM system) disease6263. It is unclear if the biologic behaviour of SCLC in these patients who present with a solitary nodule is different compared to other forms of LD-SCLC. In these patients, systemic therapy is recommended following resection. Consideration to thoracic radiation must be given in patients who are found to have lymph node involvement in the resection specimen64. These results support continued research on the role of surgical resection in early stage SCLC.
In contrast, there is currently no role for resection in the multimodality treatment of locally advanced SCLC. A trial that randomized 328 patients with LD-SCLC who had achieved at least a partial response following chemotherapy with cyclophosphamide, doxorubicin, and vincristine, to either surgery or observation did not find a survival benefit with surgery65.
Prophylactic cranial irradiation
The central nervous system (CNS) is a common site of metastasis in patients with SCLC. Almost 25 per cent of patients will have brain metastases at presentation66. Furthermore, of the patients who obtain a complete response with initial therapy, approximately 45 per cent will present with brain metastases as the only site of relapse at 2 years6768. CNS relapse is associated with greater deterioration in performance status, and an increased need for hospitalization69. Hence prevention of CNS relapse would be a good option in order to decrease the suffering caused by CNS metastases.
Multiple trials have been conducted to evaluate the role of prophylactic cranial irradiation (PCI) in SCLC70. Unfortunately, these were small and heterogenous and not surprisingly there was a wide variation in their findings. Despite this, all these studies showed a significant decrease in the incidence of brain metastases with PCI. The effects on overall survival however, varied among the trials. Two separate meta-analyses concluded that PCI decreased the incidence of brain metastases by 52-54 per cent and improved survival in patients who had achieved a complete response to therapy, by 16-18 per cent7172. A more recent study randomized patients with ED-SCLC who had not progressed following initial therapy, to either PCI or observation. Here, PCI decreased both the cumulative incidence of brain metastases (14.6 vs. 40.4%), and improved median survival (5.4 vs. 6.7 months)73. The 1-year survival rate was 27.1 per cent in the PCI group and 13.3 per cent in the control group. The similar findings in both meta-analyses in LD-SCLC and the randomized trial in ED-SCLC, have led to an increased use of PCI in patients with SCLC.
The optimal dose and fraction size for PCI are unclear. A randomized trial compared a standard dose of 25 Gy in 10 fractions PCI to high dose (36 Gy in 18 fractions once-daily or 36 Gy in 24 fractions using 1.5 Gy twice-daily PCI) in 720 patients with LD-SCLC who achieved complete response after chemotherapy and thoracic RT, showed no significant reduction of brain metastasis with higher dose PCI, but significant increase in mortality. Therefore, at present time, PCI at 25 Gy in 10 fractions should remain standard of care74.
One of the major concerns with the use of PCI is long-term neurotoxicity. However, the frequency and severity of the long-term toxicities associated with PCI are unclear. Given the survival advantage of PCI, patients who receive PCI tend to live longer and thus are at a greater risk of developing chronic neurotoxicity. In a retrospective analysis of 98 patients, those who received PCI had a significant improvement in the mean Q-TWiST (quality time without symptoms and toxicity) survival75. In another similar analysis that evaluated quality-adjusted life expectancy (QALE), PCI offered a benefit over no PCI (QALE = 4.31 and 3.70 for mild toxicity and 4.09 and 3.70 for substantial toxicity, respectively)76. The results of these analyses would suggest a benefit to utilization of PCI without undue toxicity; however, well designed clinical trials with this specific objective in mind are needed to answer this question conclusively.
Relapsed/refractory SCLC
Although SCLC is very chemosensitive, most patients will relapse at varying intervals following initial treatment. Treatment of these patients is often challenging in the absence of good data to guide therapy in this setting.
In patients with chemosensitive disease, repeating the original regimen may be worthwhile777879, especially if the patient had a sustained response initially (>6 months). Topotecan, a water-soluble, semi-synthetic derivative of camptothecin has demonstrated antitumour activity in relapsed/refractory SCLC. In a randomized phase III study of 210 patients with sensitive relapse, topotecan showed similar response rates, time to progression and median survival with cyclophosphamide, anthracycline and vincristine (CAV)80. More significantly, topotecan had greater symptom control and decreased interference with daily activities. The oral formulation of topotecan has similar response rates (18 vs. 22%), median survival (33 vs. 35 wk) and 1-year survival rates (33 vs. 29%) in patients with a chemosensitive relapse compared to intravenous topotecan81.
Amrubicin, a synthetic 9-amino anthracycline with minimal cardiotoxicity, has shown promising activity in SCLC82. A phase III trial comparing amrubicin to topotecan in 637 patients83 found that although survival was similar in the two groups, amrubicin had a better median survival (6.2 vs. 5.7 months, P=0.047) in patients with refractory disease. Patients treated with amrubicin had significant improvement in their symptoms.
Multiple other agents, including paclitaxel, docetaxel, irinotecan, oral etoposide, vinorelbine and gemcitabine have been studied with modest activity in relapsed/refractory SCLC.
Future directions
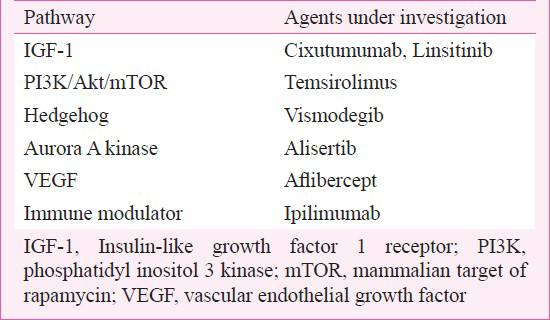
Despite various strategies, the overall survival in patients with SCLC has not improved significantly over the past decade. An increasing knowledge regarding the molecular biology of SCLC has led to the development of targeted agents that could potentially be useful in SCLC. Targets currently being investigated are listed in Table II.
Conclusions
Current treatment of SCLC involves chemotherapy with the addition of concurrent thoracic radiation for patients with limited disease. The most common chemotherapy regimen includes four cycles of etoposide and platinum. Addition of a third agent or intensifying therapy further has not demonstrated a survival benefit. Prophylactic cranial irradiation decreases the incidence of brain metastases and improves overall survival. Newer targeted agents, based on our understanding of the molecular carcinogenesis of SCLC are currently in clinical trials.
References
- Small cell bronchogenic carcinoma: a distinct clinicopathologic entity. Semin Oncol. 1978;5:234-43.
- [Google Scholar]
- Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539-44.
- [Google Scholar]
- Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24:4526-7.
- [Google Scholar]
- The nature of the “oat-celled sarcoma” of the mediastinum. J Pathol Bacteriol. 1926;29:241-4.
- [Google Scholar]
- World Health Organization Classification of tumours. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. p. :31-4.
- [Google Scholar]
- Lung tumours with neuroendocrine differentiation. Eur J Cancer. 2009;45(Suppl 1):251-66.
- [Google Scholar]
- Carcinoma of the lung. In: Pathology of the lungs. London: England Churchill Livingstone; 2000. p. :475-8.
- [Google Scholar]
- Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26:1184-97.
- [Google Scholar]
- Neuroendocrine neoplasms of the lung. In: Leslie KO, Wick MR, eds. Practical pulmonary pathology. London England: Churchill Livingstone; 2005. p. :423-63.
- [Google Scholar]
- Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710-7.
- [Google Scholar]
- A new international staging system for lung cancer. Chest. 1986;89(Suppl 4):225S-33S.
- [Google Scholar]
- Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer - what limits limited disease? Lung Cancer. 2002;37:271-6.
- [Google Scholar]
- Staging and clinical prognostic factors for small-cell lung cancer. Cancer J. 2001;7:437-47.
- [Google Scholar]
- The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067-77.
- [Google Scholar]
- The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049-59.
- [Google Scholar]
- Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316:912-8.
- [Google Scholar]
- Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group. J Clin Oncol. 1997;15:3030-7.
- [Google Scholar]
- Chemotherapy alone or chemotherapy with chest radiation therapy in limited stage small cell lung cancer. A prospective, randomized trial. Ann Intern Med. 1987;106:655-62.
- [Google Scholar]
- Outcome of patients with small-cell lung cancer: effect of changes in staging procedures and imaging technology on prognostic factors over 14 years. J Clin Oncol. 1990;8:1042-9.
- [Google Scholar]
- Isolated pleural effusion in small cell lung carcinoma: favorable prognosis. A review of the Southwest Oncology Group experience. Chest. 1982;81:208-11.
- [Google Scholar]
- Prognostic factors in small-cell carcinoma of the lung: an analysis of 1,521 patients. J Clin Oncol. 1989;7:344-54.
- [Google Scholar]
- Prognostic significance of supraclavicular lymph nodes in small cell lung cancer: a study from four consecutive clinical trials, including 1,370 patients. “Petites Cellules” Group. Chest. 1998;114:1538-41.
- [Google Scholar]
- Importance of clinical staging in limited small-cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol. 1993;11:1592-7.
- [Google Scholar]
- Small cell lung cancer 1973-1983: early progress and recent obstacles. Int J Radiat Oncol Biol Phys. 1984;10:515-39.
- [Google Scholar]
- Small-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group study. Ann Intern Med. 1978;88:194-9.
- [Google Scholar]
- Combined modality induction therapy without maintenance chemotherapy for small cell carcinoma of the lung. J Clin Oncol. 1984;2:294-304.
- [Google Scholar]
- Combined modality treatment of small cell carcinoma of the lung. Arch Intern Med. 1981;141:469-73.
- [Google Scholar]
- Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665-72.
- [Google Scholar]
- Canadian multicenter randomized trial comparing sequential and alternating administration of two non-cross-resistant chemotherapy combinations in patients with limited small-cell carcinoma of the lung. J Clin Oncol. 1987;5:1401-9.
- [Google Scholar]
- Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst. 1991;83:855-61.
- [Google Scholar]
- Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282-91.
- [Google Scholar]
- Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol. 1994;5:601-7.
- [Google Scholar]
- Long-term follow-up of limited stage small cell lung cancer patients treated with carboplatin-based chemotherapy and radiotherapy by the Minnie Pearl Cancer Research Network (MPCRN) ASCO Annnual Meeting Abstracts. J Clin Oncol. 2004;22(Suppl 14):7222.
- [Google Scholar]
- Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91.
- [Google Scholar]
- Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038-43.
- [Google Scholar]
- Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530-5.
- [Google Scholar]
- A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21:1810-6.
- [Google Scholar]
- A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol. 2011;22:1798-804.
- [Google Scholar]
- Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593--a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114-22.
- [Google Scholar]
- Maintenance daily oral etoposide versus no further therapy following induction chemotherapy with etoposide plus ifosfamide plus cisplatin in extensive small-cell lung cancer: a Hoosier Oncology Group randomized study. Ann Oncol. 2002;13:95-102.
- [Google Scholar]
- Does maintenance/consolidation chemotherapy have a role in the management of small cell lung cancer (SCLC)? A metaanalysis of the published controlled trials. Cancer. 2005;104:2650-7.
- [Google Scholar]
- A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618-24.
- [Google Scholar]
- Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890-5.
- [Google Scholar]
- Population-based outcomes for small cell lung cancer: impact of standard management policies in British Columbia. Lung Cancer. 2004;43:7-16.
- [Google Scholar]
- The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys. 1988;14:219-26.
- [Google Scholar]
- Alternating radiotherapy and chemotherapy in 173 consecutive patients with limited small cell lung carcinoma. GROP and the French Cancer Center's Lung Group. Int J Radiat Oncol Biol Phys. 1990;19:1135-8.
- [Google Scholar]
- Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265-71.
- [Google Scholar]
- Long term survival data from CALGB 8837: radiation dose escalation and concurrent chemotherapy (CT) in limited stage small cell lung cancer (LD-SCLC). Possible radiation dose-survival relationship. Proc Am Soc Clin Oncol. 2002;21:A-1190. Abstract
- [Google Scholar]
- Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: an update of Cancer and Leukemia Group B Study 8083. J Clin Oncol. 1998;16:2466-7.
- [Google Scholar]
- Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG) Ann Oncol. 2001;12:1231-8.
- [Google Scholar]
- Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336-44.
- [Google Scholar]
- Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol. 1997;15:893-900.
- [Google Scholar]
- Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054-60.
- [Google Scholar]
- Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823-30.
- [Google Scholar]
- Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. 2006;17:543-52.
- [Google Scholar]
- Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63-5.
- [Google Scholar]
- Clinical biology of small cell carcinoma: relationship to surgical therapy. Semin Oncol. 1978;5:272-9.
- [Google Scholar]
- Small cell lung cancer presenting as a solitary pulmonary nodule. Chest. 1992;101:225-31.
- [Google Scholar]
- Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg. 2000;70:1615-9.
- [Google Scholar]
- Results of surgery in small cell carcinoma of the lung. Lung Cancer. 2006;52:299-304.
- [Google Scholar]
- A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106(Suppl 6):320S-3S.
- [Google Scholar]
- Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J Neurooncol. 2000;48:243-8.
- [Google Scholar]
- Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183-90.
- [Google Scholar]
- Prophylactic Cranial Irradiation: More Questions Than Answers. Semin Radiat Oncolo. 1995;5:61-8.
- [Google Scholar]
- Social consequences of brain or liver relapse in small cell carcinoma of the bronchus. Radiother Oncol. 1985;4:335-9.
- [Google Scholar]
- Prophylactic cranial irradiation in small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:355-72.
- [Google Scholar]
- Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5.
- [Google Scholar]
- Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-84.
- [Google Scholar]
- Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-72.
- [Google Scholar]
- Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467-74.
- [Google Scholar]
- Prophylactic cranial irradiation revisited: cost-effectiveness and quality of life in small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:68-74.
- [Google Scholar]
- Decision analysis for prophylactic cranial irradiation for patients with small-cell lung cancer. J Clin Oncol. 2006;24:3597-603.
- [Google Scholar]
- Small-cell carcinoma of lung: reinduction therapy after late relapse. Ann Intern Med. 1983;98:472-4.
- [Google Scholar]
- Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol. 1987;23:1697-9.
- [Google Scholar]
- Treatment of relapse of small cell lung cancer in selected patients with the initial combination chemotherapy carboplatin, etoposide, and epirubicin. Thorax. 1992;47:369-71.
- [Google Scholar]
- Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658-67.
- [Google Scholar]
- Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086-92.
- [Google Scholar]
- Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26:5401-6.
- [Google Scholar]
- Randomized phase III trial of amrubicin versus topotecan (Topo) as second-line treatment for small cell lung cancer (SCLC). ASCO Annual Meeting Abstracts. J Clin Oncol. 2011;29(Suppl 15) Abstract No. 7000
- [Google Scholar]






