Translate this page into:
CTX-M15 type ESBL producing Salmonella from a paediatric patient in Chennai, India
For correspondence: +skk.microbes@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Resistance to 3rd generation cephalosporins in Salmonella has been attributed to the production of extended spectrum β-lactamases (ESBLs), but AmpC and KPC or metallo β-lactamase (MBL) mediated resistance have also been reported1. ESBLs reported in Salmonella spp. are the derivatives of TEM, SHV, CTX-M and PER type β-lactamase families1. We isolated Salmonella enterica ser. Typhimurium (B9903) from the blood culture of a 6-month old baby admitted to a paediatric hospital in Chennai in October, 2009. The patient was febrile, lethargic and refused intake. The baby was diagnosed with haemophagocytic syndrome with NK cell deficiency, dengue hemorrhagic fever grade IV, sepsis, steroid induced hypertension. The baby responded to ciprofloxacin and co-trimoxazole along with intravenous immunoglobin and steroids. The child was well at the time of discharge after 17 days in hospital. There was no history of travel or any animal contact.
Species identification and antibiotic susceptibility were performed by automated system (VITEK-2, France). The isolate was further confirmed by serotyping using specific antiserum. Antigenic structure of the isolate was identified as O4, 12: H phase 1 – i: phase 2 – 1, 2 at National Salmonella & Escherichia Centre, Central Research Institute, Kasauli, India. The antibiotic susceptibility testing was interpreted according to CLSI guidelines2. The isolate was resistant to ampicillin, cefepime, ceftriaxone and ceftazidime but susceptible to piperacillin/tazobactam, ciprofloxacin, co-trimoxazole, and tetracycline. The ESBL phenotype was determined by combined disc synergy test 2 and further confirmed by using E-strip (AB bioMerieux, Solna, Sweden) with cefotaxime and clavulanic acid.
The strain was identified as belonging to Salmonella phage type group E. The phage typing was carried out at National Salmonella Phage Typing Centre at Lady Hardinge Medical College, New Delhi.
PCR-assays for the genes encoding β-lactamases3 showed that S. Typhimurium B9903 was found to carry the ESBL and narrow-spectrum β-lactamase genes namely, blaCTX-M-15 and blaTEM-1. The regions located upstream of the blaCTX-M were amplified with primers annealing to ISEcp1 and to orf513 of ISCR1 together with reverse primer of blaCTX-M4. The sequences located downstream of the blaCTX-M gene were amplified with forward primer of blaCTX-M and reverse primer of IS903 Bint4.
Amplification with primers ISEcp1 and blaCTX-M yielded a PCR product of about 1050 bp. Sequencing analysis of PCR products from original isolate and transconjugant revealed that insertion element ISEcp1 was located upstream of blaCTX-M-15 gene similar to the E. coli isolate previously reported in India (Gen Bank accession number AY044436)5. Amplification with primers TEM F and TEM R yielded a 861 bp product with a sequence consistent with that of blaTEM-1.
Plasmid analysis revealed that S. Typhimurium B9903 harboured a plasmid with size of 25 kb, using E. coli NCTC 50192 harbouring four plasmids of 154, 66, 38 and 7 kb as a reference marker6. To determine if the ESBL-phenotype was transferable, liquid mating-out experiment was attempted with azide-resistant E. coli J53 as a recipient. Transconjugants were selected on Mac Conkey agar plates containing sodium azide (200 μg/ml) and cefotaxime (4 μg/ml)6. The ESBL-phenotype was successfully transferred in conjugation and transconjugation efficiency was very high about 5 × 10-1 per donor cells. Plasmids purified from the original strain and transconjugant were typed by PCR-based replicon typing7.
The MICs of various antibiotics were determined by using broth macro dilution method according to CLSI guidelines (Table). The MICs of all the β-lactams except carbapenems and cefoxitin for S. Typhimurium B9903 and its transconjugant were higher than that of recipient strain. There was a reduction in the MICs of β-lactams when tested with clavulanic acid/tazobactam.
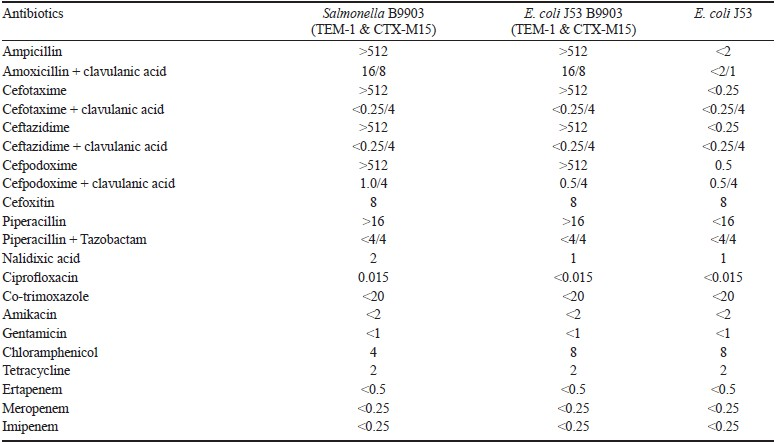
Plasmid analysis revealed that the ESBL-positive transconjugant harboured a 25 kb plasmid that belonged to IncI1 type. It was found to carry the blaCTX-M-15 gene together with blaTEM-1 by PCR.
ESBL producing non-typhoidal Salmonellae are an increasing concern worldwide. CTX-M15 producing S. enterica has been reported from Indian and Pakistani patients hospitalized in Kuwait8. blaCTX-M-15 gene linked with IncI1 type plasmids have been identified in various strains in different geographic locations around the world viz., S. Typhimurium, S. Ohio, S. Enteritidis, E. coli in UK; S. Anatum in Pakistan; E. coli in France and Australia and S. Infantis in Honduras9–11. We report here a CTX-M-15 type ESBL producing Salmonella isolated from the blood culture of a paediatric patient with haemophagocytic syndrome.
The finding of the blaCTX-M gene in Salmonella in association with ISEcp1 is a matter of concern, as this insertion element facilitates the mobilization and expression of CTX-M mediated resistance, thereby limiting the therapeutic options available for typhoid fever and curtailing the use of cephalosporins. Measures such as improved food and personal hygiene could help prevent the potential spread of such resistant strains.
The sequences of the blaCTX-M15 associated with ISEcp1 has been submitted to the GenBank database and assigned accession number HM117627.
Authors thank Shrimati Priya Goodwill and Shri A. Ramesh, Apollo Hospital, Chennai, for their technical assistance, and National Salmonella & Escherichia Centre, Central Research Institute, Kasauli, India for serotyping. The authors acknowledge the help of Dr Alessandra Carattoli, Department of Infectious, Parasitic, Immune-mediated Diseases, Istituto Superiore di Sanità, Rome, Italy towards plasmid typing in this study.
References
- Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella.Int. J Antimicrob Agents. 2004;23:547-55.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing: 20th Informational Supplement M100-S20. Wayne, PA, USA: CLSI; 2010.
- [Google Scholar]
- A set of multiplex PCRs for genotypic detection of extended-spectrum β-lactamases, carbapenemases, plasmid-mediated AmpC β-lactamases and OXA β-lactamases. Int J Antimicrob Agents. 2011;37:356-9.
- [Google Scholar]
- Extended-spectrum beta-lactamases of the CTX-M type now in Switzerland. Antimicrob Agents Chemother. 2007;51:2855-60.
- [Google Scholar]
- Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol Lett. 2001;201:237-41.
- [Google Scholar]
- Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob Agents Chemother. 2005;49:71-6.
- [Google Scholar]
- Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219-28.
- [Google Scholar]
- Emergence of CTX-M-15 types extended-spectrum beta-lactamase-producing Salmonella spp. in Kuwait and the United Arab Emirates. J Med Microbiol. 2008;57:881-6.
- [Google Scholar]
- Dominance of blaCTX-M within an Australian extended-spectrum beta-lactamase gene pool. Antimicrob Agents Chemother. 2008;52:4198-202.
- [Google Scholar]
- Replicon typing of plasmids carrying CTX-M or CMY β-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob Agents Chemother. 2006;50:3203-6.
- [Google Scholar]
- Replicon typing of plasmids in Escherichia coli producing extended-spectrum β-lactamases. J Antimicrob Chemother. 2009;63:67-71.
- [Google Scholar]





