Translate this page into:
Cross-neutralization between three mumps viruses & mapping of haemagglutinin-neuraminidase (HN) epitopes
Reprint requests: Dr Sunil R. Vaidya, WHO National Measles Reference Laboratory, National Institute of Virology (ICMR) 20-A, Dr Ambedkar Road, Post Box 11, Pune 411 001, Maharashtra, India e-mail: vaidyasr@icmr.org.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The reports from the countries where mumps vaccine is given as routine immunization suggest differences in mumps virus neutralizing antibody titres when tested with vaccine and wild type viruses. Such reports are unavailable from countries like India where mumps vaccine is not included in routine immunization. We, therefore, undertook this study to understand the cross-neutralization activity of Indian mumps viruses.
Methods:
By using commercial mumps IgG enzyme immunoassay (EIA) and a rapid focus reduction neutralization test (FRNT), a panel of serum samples was tested. The panel consisted of 14 acute and 14 convalescent serum samples collected during a mumps outbreak and 18 archived serum samples. Two wild types (genotypes C and G) and Leningrad-Zagreb vaccine strain (genotype N) were used for the challenge experiments and FRNT titres were determined and further compared. The HN protein sequence of three mumps viruses was analyzed for the presence of key epitopes.
Results:
All serum samples effectively neutralized mumps virus wild types and a vaccine strain. However, significantly lower FRNT titres were noted to wild types than to vaccine strain (P<0.05). The comparison between EIA and FRNT results revealed 95.6 per cent agreement. No amino acid changes were seen in the epitopes in the Indian wild type strains. All potential N-linked glycosylation sites were observed in Indian strains.
Interpretation & conclusions:
Good cross-neutralization activity was observed for three mumps virus strains, however, higher level of FRNT titres was detected for mumps virus vaccine strain compared to Indian wild type isolates.
Keywords
Cross-neutralization
India
mumps wild types
mumps Leningrad-Zagreb vaccine strain
Mumps virus (MuV) is a member of the Paramyxoviridae family, subfamily Paramyxovirinae and belongs to the genus Rubulavirus, which only infects humans1. MuV has a single-stranded, negative sense RNA genome consisting of 15,384 nucleotides. The mumps virus genome encodes two surface glycoproteins, fusion (F) and haemagglutinin-neuraminidase (HN); four core proteins, nucleoprotein (N), phospho (P), matrix (M) and large protein (L); and the membrane associated small hydrophobic (SH) protein2. Within the MuV genome, the most sequence variation is found in the SH gene, and this region has been recommended for the genotypic classification3. A standard naming convention has been proposed for mumps virus genotypes that differentiated these into 12 genotypes, i.e. A-N, except for E or M4. However, serological tests with human serum indicate presence of a single serotype. Circulation of mumps virus genotype C has been reported from the States of Maharashtra and Tamil Nadu56, and that of mumps genotype G from the States of Maharashtra and Punjab57.
The HN is the major antigenic protein, known to elicit neutralizing antibodies, which may be critical for generating a protective host humoral immune response89. A study on mumps HN sequences showed antigenic divergence between vaccine (Leningrad-Zagreb, Urabe AM9 and Jeryl Lynn-5) and wild-type mumps (genotypes C, D and G) viruses10. We investigated a mumps outbreak in 2012 in an unimmunized population from Osmanabad, Maharashtra, India where circulation of two different mumps viruses (genotypes- C and G) was noted in nearby villages5. Therefore, a study was designed to understand the cross-neutralization activity of mumps viruses isolated from two villages. In addition, mumps Leningrad-Zagreb vaccine strain (MuV-LZ) was included in the cross-neutralization study.
Material & Methods
This study was conducted in the WHO National Measles Reference Laboratory, National Institute of Virology (NIV), Pune, Mahrashtra, India, during December 2013-May 2014. A panel of 46 serum samples consisting of 14 acute and 14 convalescent serum samples collected during 2012 mumps outbreak from Osmanabad district, Maharashtra, India5 and 18 stored serum samples referred for either measles laboratory diagnosis or measles immunity testing at NIV were included. The history of clinical mumps was not available for 18 stored serum samples. All subjects were likely unimmunized for mumps, since mumps vaccine is not used in universal immunization programme in India. Additionally, during investigation detailed information about the vaccination history was taken from the patient's parents or immunization records available at primary health centres. All samples were tested using commercial mumps IgG antibody enzyme immuno assay (EIA) (Siemens Healthcare Diagnostics Products GmbH, Germany). In addition, 14 acute and 14 convalescent samples were also tested by using commercial mumps IgM antibody EIA (Siemens Healthcare Diagnostics Products GmbH, Germany) and mumps focus reduction neutralization test (FRNT) standardized at NIV.
Mumps wild type and vaccine strains used in FRNT: A wild type mumps virus was isolated from a throat swab collected from an 11 yr old female patient presented with fever and bilateral parotitis from Apsinga village in Osmanabad district, India5. This patient showed presence of mumps IgM antibodies in serum sample and serologically confirmed as a mumps case. Mumps SH gene reverse transcriptase (RT)-PCR was performed as per the protocol described previously5, and it revealed mumps virus genotype C (MuV-C) and sequence deposited in GenBank (KF305773) (www.ncbi.nlm.nih.gov/genbank/).
Another wild type mumps virus was isolated from throat swabs collected from a 6 yr old female presented with fever and bilateral parotitis from Sangavi, Pune (Unpublished data). This patient showed presence of mumps IgM antibodies in serum sample and serologically confirmed as a mumps case. Mumps SH gene RT-PCR revealed mumps virus genotype G (MuV-G) and sequence deposited in GenBank (JX 442438). Due to unavailability of mumps genotype G isolate from the Osmanabad outbreak, MuV Pune strain was used for the challenge experiment in FRNT.
Virus stocks were prepared in Vero cells and 0.5 ml aliquots were prepared by adjusting foetal bovine serum (FBS) concentration to 10 per cent. Aliquots were stored at -80°C. For each challenge experiment, a new aliquot was used.
Mumps Leningrad-Zagreb vaccine strain (MuV-LZ) was obtained from the Serum Institute of India (SII) Limited, Pune. Aliquots of 0.5 ml were prepared by adding extra 10 per cent FBS and stock vials were stored at -80°C.
Mumps focus reduction neutralization test (FRNT): Mumps FRNT was standardized with three mumps viruses as described previously11. A minor modification was made in FRNT protocol where the volume of primary/secondary antibody/substrate was reduced (50 μl); plates were overlaid with 0.8 per cent carboxy methyl cellulose (CMC) and washed manually. After development, blue stained foci were counted by eye and 50 per cent focus reduction neutralization titres were deduced using the Kärber formula11. A mumps neutralizing antibody titre >1:4 is considered as positive as described earlier11. The FRNT titres obtained by challenging with three viruses were log10 transformed and statistical analysis was undertaken.
Analysis of HN gene sequences of wild types and vaccine strains: The complete HN gene sequencing of mumps isolates (i.e. stock virus used for the challenge experiments in FRNT) were performed (GenBank accession numbers; KF843895 & KF738114). HN gene sequence of MuV-LZ was retrieved from GenBank (AY685920) and multiple alignments of nucleotide and amino acid sequences were undertaken using MEGA version 5 standard procedure. N-linked glycosylation sites were located in amino acid sequences with pattern N-X-[S or T], where X represents any amino acid, followed by a serine (S) or threonine (T) except a proline.
Statistical analysis: Descriptive statistics were reported for continuous variables. The titres were compared using Student t-test.
Results
Qualitative comparison between EIA and FRNT: Forty six serum samples were tested using commercial mumps IgG EIA and FRNT. Of these 46 serum samples, 37 (80.4%) were positive by mumps IgG EIA and 35 (76%) by FRNT using all three mumps virus strains i.e. MuV-C, MuV-G and MuV-LZ. Overall, a good concordance was noted between EIA and FRNT results with a concordance of 95.6 per cent (35 positive and 9 negative by both tests) and discordant results observed for two samples. These samples were positive in mumps IgG EIA but negative in FRNT.
Of the 14 acute serum samples, mumps IgM antibodies were detected in 13 and mumps IgG antibodies detected in 12 samples. However, 10 of 14 samples showed the presence of neutralizing antibodies by FRNT using MuV-C, MuV-G and MuV-LZ strains. In 14 convalescent samples, mumps IgM antibody was detected in 13 and mumps IgG antibody was detected in all 14 samples. As expected, all 14 convalescent serum samples showed presence of neutralizing antibodies in FRNT using all three mumps virus strains. Of the 18 stored samples, 11 showed positive results in mumps IgG EIA and in FRNT with all three mumps strains indicating past exposure to MuV.
Comparative neutralizing antibody titres to three mumps viruses: Forty six serum samples were tested in FRNT using three challenge viruses, i.e. MuV-C, MuV -G and MuV-LZ (Table). The quantitative correlations (R2 of neutralizing antibody titres between MuV-LZ vs. MuV-C, MuV-LZ vs. MuV-G, and MuV-C vs. MuV-G were 0.91, 0.96 and 0.94, respectively. However, 21 samples showed more than two-fold difference in neutralizing antibody titres to MuV-C and MuV-LZ; 18 samples showed more than two-fold difference in titres to MuV-G and MuV-LZ. Two samples showed more than two-fold difference between FRNT titres to MuV-C and MuV-G strains.
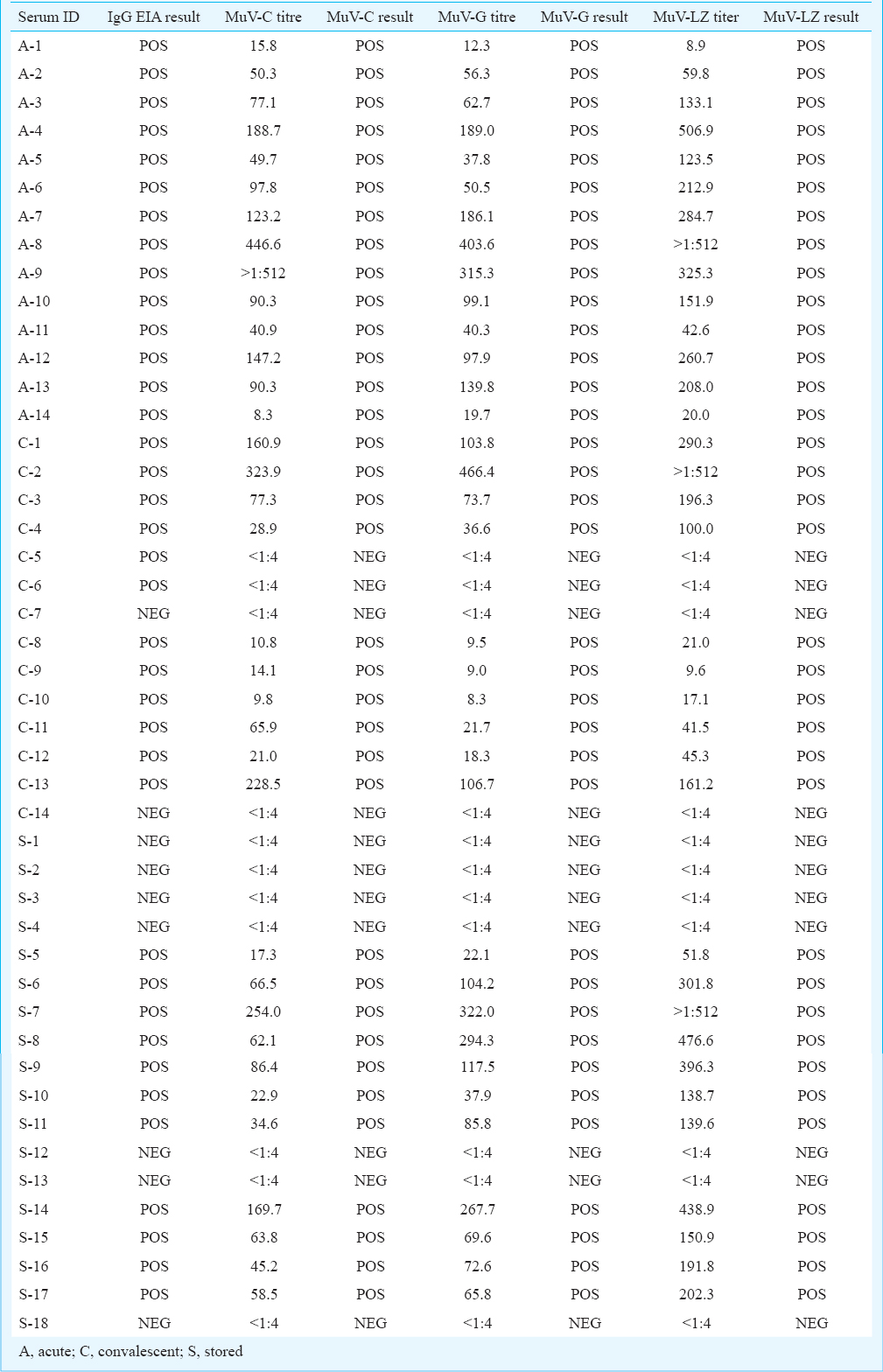
When mean FRNT titres (GMT) to three-challenge viruses were statistically compared, MuV-LZ vaccine showed higher titres than wild types (MuV-C and MuV-G) in all the samples (P<0.05, paired t test). The mean (± SE) FRNT titres to MuV-C, MuV-G and MuV-LZ were 1.80±0.08, 1.83 ± 0.08) and 2.11 ± 0.08, respectively.
When individual groups of serum samples were compared for FRNT titres, 14 acute serum samples showed significantly higher FRNT titres to MuV-LZ than MuV-C and MuV-G but FRNT titre differences amongst wild types were not evident. Similar observations were noted for 14 convalescent samples and 18 stored serum samples.
Mapping of amino acid changes in HN gene: Majority of neutralizing epitopes are localized in the HN gene of mumps virus; therefore, sequences of wild type and vaccine strains were analyzed. Indian wild types showed eight potential N-linked glycosylation sites (at amino acid positions; 127, 284, 329, 400, 448, 464, 507 and 514). Overall, no changes were detected in known neutralizing epitopes in Indian wild type strains. Additional regions for escaping neutralizing antibodies as reported in the Croatian wild types10 were also seen in Indian wild types (Figure).
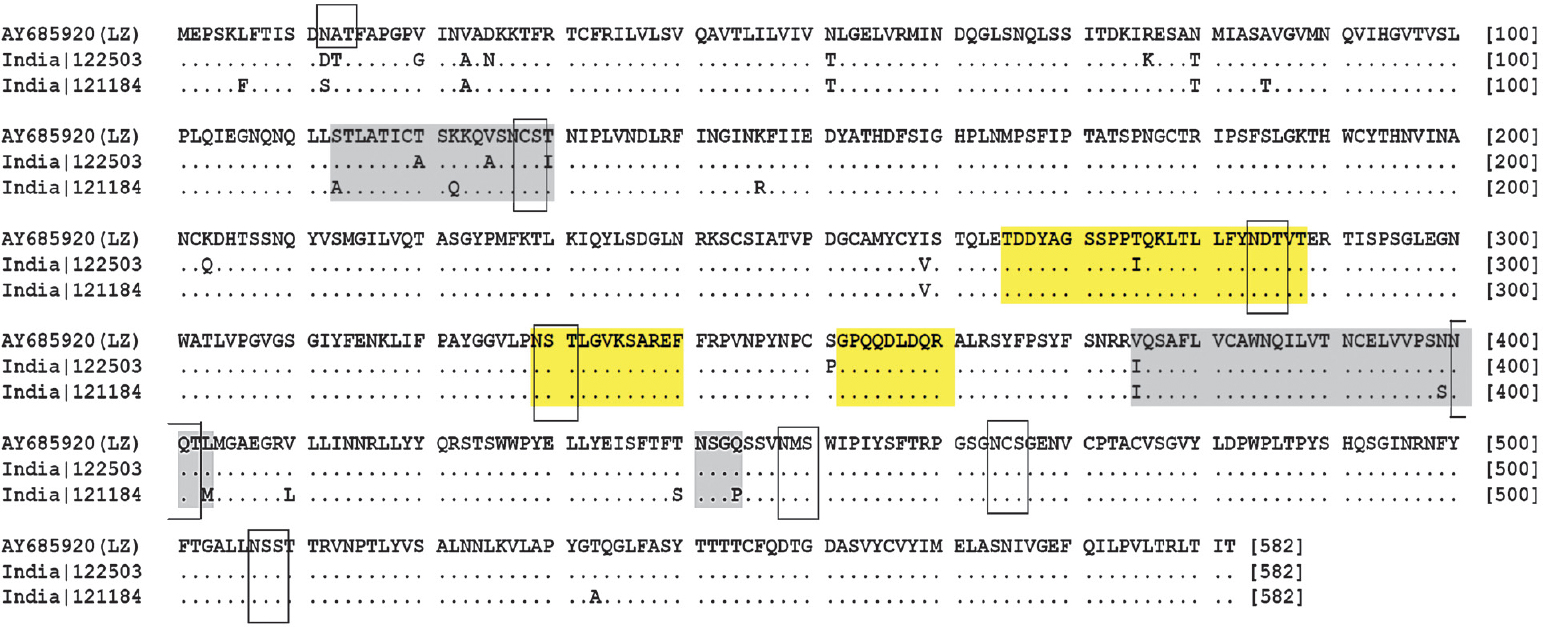
- Deduced amino acid sequence of HN of mumps viruses isolated from India and LZ vaccine strain. Potential glycosylation sites are marked by rectangles. Regions highlighted in yellow colour indicate epitopes. Gray coloured regions are involved in viral escape from neutralizing antibodies as suggested in earlier study10.
Discussion
The comparison of mean FRNT titres for MuV-C, MuV-G and MuV-LZ showed higher titres for vaccine strain (MuV-LZ) compared to wild type viruses (MuV-C and MuV-G). This observation needs further study to confirm in individuals vaccinated with mumps Leningrad-Zagreb strain. Our study confirmed the findings from other countries that documented lower neutralization titres to wild-type mumps strains compared to the particular mumps vaccine strains, i.e. JL, Urabe, Hoshino and L-31213141516.
Interestingly, 14 convalescent serum samples collected from two villages showed good cross-reactivity to both wild type (genotypes C and G) viruses and mumps LZ-vaccine virus (genotype N). A study conducted in USA among vaccinated individuals suggested neutralization activity to genetically diverse mumps virus strains (Jeryl Lynn/USA63, Urabe-AM9/JPN73, Enders/USA45, Odate-1/JPN, Iowa-G/USA06, Lo1/UK88 and 88-1961/USA88) and indicated no evidence of mumps immune escape16. In the present study similar finding was observed in unvaccinated population from India.
Previous report showed that antibodies induced by immunization with Jeryl Lynn mumps vaccine effectively neutralized heterologous virus strains (genotype G) and higher neutralizing antibody titres were observed to Jeryl Lynn compared to genotype G virus15. We observed that serum samples collected from naturally infected or unimmunized population from India effectively neutralized mumps genotype C, genotype G and genotype N vaccine strain. However, higher neutralizing antibody titres to mumps vaccine strain were noted.
HN protein is a major target for humoral immune response in mumps virus infection as it elicits neutralizing antibodies89. The amino acid positions 265-288, 329-340 and 352-360 of HN protein have been reported to evoke immune response and responsible for virulence8917. Indian mumps virus isolates did not show any change at these amino acid positions. The region 329-340 is reported to have the ability to induce neutralizing antibodies not only to attenuated virus strains but also to wild types6. This region is well conserved in Indian wild types and vaccine virus LZ. The known motifs viz. leucine-zipper, neuraminidase (240-NRKSCS-245) and receptor-binding site of haemagglutinin (405-GAEGRV-410) were reported as conserved in mumps viruses18. All potential N-linked glycosylation sites were observed in Indian wild type viruses except at position 12. Mutation of N to D/S at position 12 resulted in loss of a potential glycosylation site in both Indian wild type strains. As reported earlier, the gain or loss of a carbohydrate could affect neutralization epitopes, reduce accessibility, and may facilitate immune escape19. This may be the possible reason for differences in neutralizing antibody titres against three challenge viruses.
Overall, good cross-neutralization activity was observed between three mumps viruses. However, MuV-LZ strain showed higher levels of neutralizing antibody titres than wild types isolates from India.
Acknowledgment
Authors acknowledge Shri Vivek Vaidya from Serum Institute of India Limited, Pune, for providing mumps Leningrad-Zagreb vaccine strain, and thank Shrimati Neelakshi Kumbhar for standardization of FRNTs and Shrimati Deepika Chowdhury for HN gene sequencing work. The statistical assistance provided by Shri Atul Walimbe is acknowledged.
Conflicts of Interest: None.
References
- Protective effects of glycoprotein-specific monoclonal antibodies on the course of experimental mumps virus meningoencephalitis. J Virol. 1985;53:727-34.
- [Google Scholar]
- Molecular cloning and characterization of six genes, determination of gene order and intergenic sequences and leader sequence of mumps virus. J Gen Virol. 1988;69:2893-900.
- [Google Scholar]
- Proposal for genetic characterization of wild-type mumps strains: preliminary standardization of the nomenclature. Arch Virol. 2005;150:1903-9.
- [Google Scholar]
- Circulation of two mumps virus genotypes in an unimmunized population in India. J Med Virol. 2013;85:1426-32.
- [Google Scholar]
- Characterisation of mumps virus genotype C among patients with mumps in India. Indian J Med Microbiol. 2013;31:290-2.
- [Google Scholar]
- Genotyping and subtyping of mumps virus isolates from the Indian subcontinent. Arch Virol. 2013;158:2359-63.
- [Google Scholar]
- Characterization of genotype-specific epitopes of the HN protein of mumps virus. J Gen Virol. 1997;78:3187-93.
- [Google Scholar]
- Localization of a new neutralizing epitope on the mumps virus hemagglutinin-neuraminidase protein. Virus Res. 2001;74:133-7.
- [Google Scholar]
- Antigenic differences between vaccine and circulating wild-type mumps viruses decreases neutralization capacity of vaccine-induced antibodies. Epidemiol Infect. 2013;141:1298-309.
- [Google Scholar]
- Development of a focus reduction neutralization test (FRNT) for detection of MuV neutralizing antibodies. J Virol Methods. 2010;163:153-6.
- [Google Scholar]
- Antigenic and genetic variation of the HN protein of mumps virus strains. J Gen Virol. 1996;77:2491-7.
- [Google Scholar]
- Antigenic relationships between six genotypes of the small hydrophobic protein gene of mumps virus. J Gen Virol. 2002;83:2489-96.
- [Google Scholar]
- Molecular epidemiology of mumps virus in Japan and proposal of two new genotypes. J Med Virol. 2004;73:97-104.
- [Google Scholar]
- Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis. 2008;198:508-15.
- [Google Scholar]
- Recent mumps outbreaks in vaccinated populations: no evidence of immune escape. J Virol. 2012;86:615-20.
- [Google Scholar]
- Hemagglutinin-neuraminidase (HN) amino acid alterations in neutralization escape mutants of Kilham mumps virus. Virus Res. 1990;17:119-29.
- [Google Scholar]
- Hemagglutinin-neuraminidase sequence and phylogenetic analyses of mumps virus isolates from a vaccinated population in Singapore. J Med Virol. 2003;70:287-92.
- [Google Scholar]
- Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J Virol. 2000;74:11955-62.
- [Google Scholar]






