Translate this page into:
Covert airflow obstruction dominates the overt ones in interstitial lung disease: An appraisal
For correspondence: Dr Parthasarathi Bhattacharyya, Department of Pulmonary Medicine, Institute of Pulmocare and Research (IPCR), Kolkata 700 156, India e-mail: parthachest@yahoo.com
-
Received: ,
Abstract
Background & objectives
The co-presence of non-emphysematous airflow obstruction in interstitial Lung disease (ILD) is not elaborated. The present study aims the job with spirometry.
Methods
ILD affected individuals with or without airflow obstruction (FEV1/FVC<0.7 or >0.7) on spirometry were compared in terms of FEV1 and FEF25-75 derived variables [FEF25-75 (%-predicted), FEV1-FEF25-75 distance, reversibility of FEV1 and FEF25-75 to salbutamol and change in FEV1 and FEF25-75 in %-predicted values]. Those showing significant difference (P=0.0001) suggesting obstruction were selected to draw respective receiver operating curve (ROC) curves to identify the best cut-off value for individual parameters. The efficacy of each surrogate was tested to identify airflow obstruction in both the initial ‘overlap’ as well as the ‘unmixed’ ILD affected individual for the presence of airflow obstruction.
Results
FEV1/FVC identified 30 overlap from 235 ILDs. The FEF25-75 (%-predicted), FEV1-FEF25-75 distance, FEF25-75 reversibility (in ml) and FEV1 (%-predicted) were significantly (P<0.0001) different between the two groups. Of these, the FEF25-75 (%-predicted) had high specificity and sensitivity (93.33 and 79.47%) to identify airflow limitation in the initial unmixed ILD-group. The surrogates with their cut off values identified 92 extra individuals making it 122/235 (51.91%) of ILD having airflow obstruction. The ‘unmixed’ group showed higher frequency and degree of FEV1 reversibility.
Interpretation & conclusions
The findings of this study suggest that the airflow obstruction in ILD involves both the intrathoracic large and small airways. Although seemingly parallel, their relative status (qualitative and quantitative) needs research especially in light of the a etio pathology and the extent of involvement of ILD.
Keywords
Airflow obstruction
FEF25-75
FEV1-FEF25-75 distance
interstitial lung disease
obstructive airway disease
small airway disease
Bronchial involvement in interstitial lung disease (ILD) is well known, but its functional consequences are seldom discussed. ILD encompasses a diffuse pro-fibrotic or fibrotic physiology of lungs derived from more than 200 aetiologies where the bronchial involvement is essentially marked by traction bronchiectasis and bronchiolectasis1. Such traction bronchiectasis is different from the usual bronchiectasis, which is a state of irreversible dilation of bronchi and distortion of the bronchial wall, from other causes that develops as a direct sequel of airway inflammation. Traction bronchiectasis evolves from the fibrotic tissue pulling on the bronchi2. While bronchiectasis and its cardinal HRCT (high-resolution computed tomography) features are found to have an association only with less severe airflow obstruction3, the traction bronchiectasis is unlikely to cause any airflow limitation for its pulling effect on the airways.
The abundance of literature and discussion on the presence and importance of traction bronchiectasis has overshadowed the possibility of a co-presence of airflow limitation from an obstructive airway disease (OAD) in ILD. An OAD can involve the relatively proximal airways as well as the small airways. The frequency of small airway involvement is more common in unmixed diseases of airflow obstruction as asthma and chronic obstructive pulmonary disease (COPD)4. Such airflow obstruction in ILD can be secondary to these coexisting OADs (asthma and COPD) or can evolve from the ILD pathobiology itself. The airflow limitation in ILD may have therapeutic or prognostic implications. CPFE (combined pulmonary fibrosis and emphysema), a recognized pattern of a mixture of ILD and emphysema (a type of COPD) has shown a relatively worse prognosis than ILD alone5.
The diagnosis of ILD is based primarily on clinical, physiological, morphological qualities manifested in HRCT chest, while that of OAD is spirometry-based functional elaborations6,7. Hence, a spirometry-based understanding of airflow limitation in ILD may be worthwhile to unfold the presence of the concomitant airflow obstruction syndromes including asthma or COPD or overlap or airflow limitation in small airways.
Globally, the FEV1/FVC<0.7 (forwarded by the ‘GOLD’) is regarded as a marker of airflow limitation8. This criterion has been found to reveal OAD in 12.93 per cent of cases in our recent experience9. The FEV1 and FEF25-75 are the two common parameters seen to be affected primarily in airflow limitation; the latter been claimed to reflect the airflow in small airways10. Hence, we wished to see the presence of airflow limitations in ILD using the parameters related to these two variables. The present study aimed at revealing OAD in ILD using the accepted (GOLD) criteria as FEV1/FVC and its prospective surrogates formed of FEV1 and FEF25-75.
Material & Methods
This study was undertaken at the department of Pleuro-parenchymal Diseases, Institute of Pulmocare and Research, Kolkata, India after clearance from the Institute Ethics Committee between September ‘2021 to October’ 2023. Criteria for defining ILD were based on the concurrence between the independent observations of one experienced pulmonologist and a radiologist diagnosing ILD on HRCT chest. Other investigations (lung function test with assessment of hypoxemia and best possible aetiological evaluation depending on the real-world feasibility) were incorporated in the diagnostic process. All the study participants underwent spirometry (performed observing the ATS/ERS guideline)
Study participants characteristics:
Inclusion/exclusion criteria
Individuals with ILD with age between 30-70 yr (n=235) were consecutively included in this study after obtaining a written informed consent from each. The inability to perform spirometry, the presence of any other concomitant lung disease or complications of the ILD revealed on necessary investigations (radiological, microbiological, cytological or histological) in real-world practice, and the presence of any significant systemic comorbidity that can influence the performance of spirometry according to the investigator were excluded from the study (Figure).
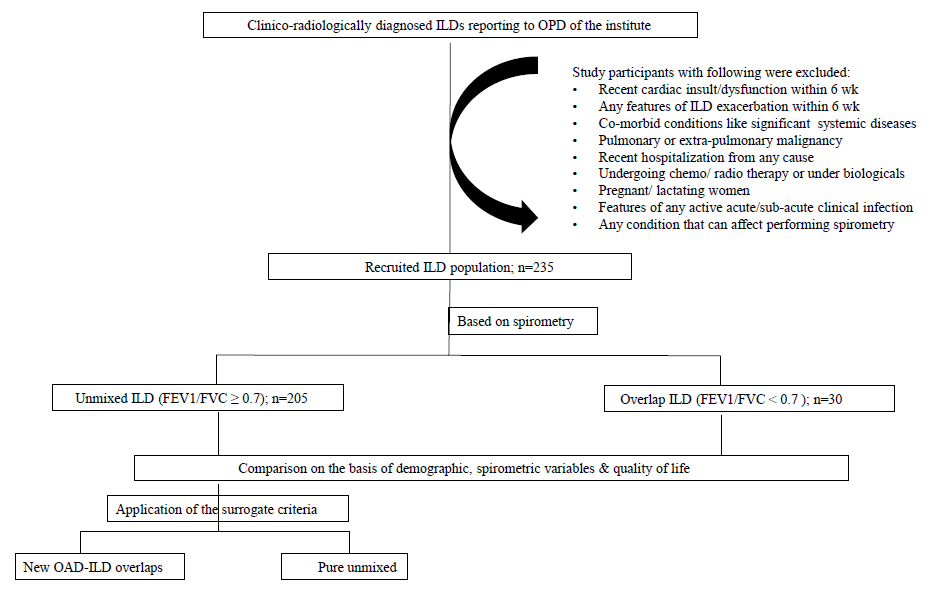
- Flowchart of participants selection and research methodology.
Study subject classification
We used the GOLD criteria (FEV1/FVC<0.7) to identify airflow limitation. When applied, it divided the population into two groups, namely (i) ILD ‘unmixed’ and (ii) ILD with OAD or the ‘overlaps’. The two groups were compared statistically using the common spirometric variables such as FEV1, FVC, FEV1/FVC, FEF25-75 and the surrogate derived from these [(%-predicted value), FEV1 to FEF25-75 distance (%-predicted values), reversibility of FEV1 and FEF25-75 to salbutamol inhalation (described in %-predicted values) and FEF25-75 reversibility (in ml) as shown in Figure. The derived variables considered were FEF25-75 reversibility and FEV1-FEF25-75 distance.
Statistical analysis
Parametric un-paired t-test was used for variables like FEV1 and FVC, while Mann-Whitney test was used for the rest of the non-parametric variables. Subsequently, ROC (receiver operating characteristics) curves were drawn with all those selected parameters that showed highly significant difference (P=0.0001) between the two groups (overlap-ILD and unmixed ILD. The identification of the best discriminative parameters was attempted through calculating cut-off values for optimal specificity and sensitivity for all of them. Those chosen cut-offs were applied independently to both the groups to see their performance to unveil airflow limitation in both of these (Figure).
The sample size was checked ‘post- using G power software version 3.1 incorporating the average prevalence of airflow obstruction made with different parameters from available studies. Firstly, apriori analysis was performed to generate the sample size; followed by performing the same using post-hoc analysis to achieve the power as 0.9. This revealed the sample size of 225 to yield the desired power (Supplementary Table I).
Results
Demography and exposures
A total of 235 individuals with ILD were recruited in this study. Of these 30 had FEV1/FVC<0.7 suggesting a diagnosis of OAD with ILD; the rest (n=205) were marked as unmixed ILD. The mean age of the two groups were 64.35±7.65 and 61.07±10.75 yr, respectively with the mean male: female ratio being 2:1 and 1:1.05 respectively. On multinomial regression, out of all the demographic variables and exposures such as tobacco, which may influence airway obstruction, we found an adjusted odds ratio of 4.69 for the overlap-ILD group, while all other variables like age and sex had no significant contribution (Supplementary Table II and III).
Lung function and comparative analysis
The FEF25-75 (%-predicted), FEV1-FEF25-75 distance, FEV1 (%-predicted), and FEF25-75 reversibility (in ml) stood out as prospective surrogates of FEV1/FVC<0.7 (Table I) displaying significant (P≤0.0001) difference between the two groups.
| Variables | ILD+OAD overlap (n=30) | Unmixed ILD (n=205) |
|---|---|---|
| Age (Mean±SD) | 64.35±7.51 | 61.07±10.75 |
| Sex (M:F) | 20:10 | 100:105 |
| FVC (%-predicted) | 66.37±17.76 | 65.38±17.04 |
| FEV1 (%-predicted) | 49.77±16.45 | 69.58±18.9* |
| FEF25-75 (%-predicted) | 19.63±15.14 | 71.14±35.56* |
| FEV1/FVC (absolute value) | 0.591±0.143 | 0.839±0.072* |
| FEV1 – FEF25-75 distance (with %-predicted values) | 26.59±16.76 | -2.14 ± 30.18* |
| FEV1 reversibility (in ml) | 48.41±82.37 | 52.52±79.79 |
| % change FEV1 | -8.22±9.18 | -3.97±5.72* |
| FEF25-75 reversibility (in ml) | 35.67±136.5 | 244.1±395.9* |
| % change FEF25-75 | 8.65±20.5 | 17.74±29.2* |
| FEV1 reversibility ≥200ml+12% | n=2 | n=8 |
| FEV1 reversibility ≥100ml | n=7 | n=29 |
| FVC (%-change) | 5.61±7.19 | 1.95±5.81* |
| FVC reversibility (in ml) | 110.7±131.9 | 30.53±94.41* |
P*<0.05. ILD, interstitial lung disease; OAD, obstructive airway disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second, FEF25-75, forced expiratory flow between 25 and 75% of the forced vital capacity
Of the proposed parameters, the parameters that showed a significant difference (P≤0.0001) were FEV1 (%-predicted), FEF25-75 (% predicted), FEV1-FEF25-75 distance, and reversibility of FEF25-75 (in ml) to salbutamol inhalation. Simultaneously, the frequency of FEV1 changes [asthma (≥200ml+12%) and minimum perceptible difference of FEV1 (≥100ml) in one participant with COPD] and FVC change (in ml and %-predicted) were recorded.
ROC curves with these selected variables (surrogates of FEV1/FVC<0.7) were used to help to find the optimal cut-off values with their corresponding sensitivity and specificity (Table II). Of these, FEF25-75 (%-predicted) was found to have the best sensitivity and specificity (Table II).
| Spirometric parameters | ||||
|---|---|---|---|---|
| FEF25-75 (%-predicted) | FEV1-FEF25-75 distance (using %-predicted) | FEF25-75 reversibility (in ml) | FEV1 (%-predicted) | |
| Cut-off | >41.5 | >15.5 | >155.0 | >54.5 |
| Sensitivity | 79.47 | 73.68 | 59.21 | 78.81 |
| Specificity | 93.33 | 90.0 | 93.33 | 63.33 |
| Positive Predictive Value (PPV) | 98.36 | 97.39 | 96.77 | 91.53 |
| Negative Predictive Value (NPV) | 47.54 | 67.5 | 30.33 | 37.25 |
The performances of the surrogate parameters with respect to the identification of airflow obstruction in the initial overlap-ILD and the unmixed-ILD groups are presented in Table III. The derived cut-off value of FEF25-75 (%-predicted) as 41.5 could identify 93.3 per cent of overlap cases (28 of 30) and 34 new cases of OAD in the unmixed-ICD group. The FEV1-FEF25-75 distance, however, could independently identify another extra 15 individuals with OAD in the overlap-ILD group. The third parameter, FEF25-75 reversibility and the fourth one [FEV1 (%-predicted)] could also independently identify 36 and seven new ILD individuals with airflow obstruction, respectively. Three out of four surrogates showed over 90 per cent diagnostic accuracy to identify the initial overlap-ILD group with obstruction.
| Criterion | Cut-off values | Specificity (%) | Sensitivity (%) | OAD+ILD group (n=30) | Unmixed ILD (n=205) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of cases identified | Diagnostic accuracy (%) | No. of cases identified | Overlap with %-predicted FEF cut-off | Overlap with FEV1-FEF25-75 distance | No. of independent diagnosis in the ‘unmixed’ group | ||||
| FEV1/FVC (GOLD guidelines) | <0.7 | 100 | 100 | 26 | 100 | NA | NA | NA | NA |
| FEF25-75 (%-predicted) | >41.5 | 93.33 | 79.47 | 28 | 93.3 | 36 | -- | 2 | 34 |
| FEV1-FEF25-75 distance | >15.5 | 90 | 73.68 | 27 | 90 | 64 | 49 | -- | 15 |
| FEF25-75 reversibility | >155 | 59.21 | 93.33 | 27 | 90 | 62 | 16 | 20 | 36 |
| FEV1 (%-predicted) | >54.5 | 78.81 | 63.33 | 19 | 63.33 | 31 | 17 | 7 | 7 |
FEF, forced expiratory flow; NA, not available
Discussion
Thirty out of 235 (12.76%) of the study participants (all had ILD) showed airflow obstruction according to the GOLD criterion8. The prospective surrogates of FEV1/FVC<0.7 were chosen using either FEV1 [%-predicted and reversibility to salbutamol inhalation], or FEF25-75 [%-predicted and salbutamol-reversibility] and the both as FEV1-FEF25-75 distance. These surrogates have highly significant difference (P≤0.0001) between the groups. ROC curves drawn with the best discriminants showed that FEF25-75 (%-predicted), had an optimal sensitivity (79.47%) and good specificity (93.33%) to identify the presence of airflow obstruction in ILD with a cut-off value as 41.5 (Figure, Table II). An available information showed FEF25-75 (<50%-predicted) correlated well with RV/TLC ratio to suggest airflow obstruction in ILD11.
The FEF25-75, was apparently the best-evolved surrogate of FEV1/FVC<0.7 (the GOLD narrated marker of airflow obstruction), which successfully identified airflow obstruction in about 93.3 per cent (28 of 30) of overlap ILDs and 34 out of 205 participants in unmixed- ILD group independently (Table III). The other two parameters FEV1-FEF25-75 distance, and FEF25-75 reversibility could identify an extra 15 and 36 study participants not picked up by the FEF25-75 cut-off value. Thus, altogether using the derived surrogate criteria, we diagnosed 34, 15, 36 and 7 adding up to 92 (44.87%) participants with airflow limitations in our initial ‘unmixed’ ILD group making the total frequency of airflow obstruction including those diagnosed by the original GOLD criterion (n=30) as 122 out of 235 (51.91%).
The surrogate parameters were mainly chosen from FEF25-75 that measured the expiratory airflow during the mid-expiratory phase of FVC. Since the FEF25-75 is thought to mirror the impairment of airflow in small airways12, all the surrogate parameters were either FEF25-75 or its derivatives likely to represent the same. The involvement of FEF25-75 is reportedly reduced in early stages of diseases of small airways13 and is the site for origin of primary airflow obstruction in many individuals of OAD (obstructive airway disease)14. Low FEF25-75 in individuals with otherwise normal lung function has emerged as a useful predictor for the development of COPD15. In COPD, decreased FEF25-75 (%-predicted) is observed frequently16; reflecting the impact of inflammation of small airways including remodelling as a cardinal feature of cigarette smoking induced COPD17. The reduction of FEF25-75 also suggests severe form of asthma or airway disease18.
The reason for choosing the FEV1-FEF25-75 distance as a surrogate has theoretical grounds. Studies have shown that FEF25-75 (% predicted) values <65 per cent may have clinical relevance especially when FEV1 values are normal19. A cross-sectional study involving 234 individuals with respiratory symptoms found that while FEV1 and FEV1/FVC did not predict airway hyper-reactivity, FEF25-75 did20. Reduced FEF25-75 might precede impairment of FEV1, so indicating early asthma and poor prognosis in early asthma21,22. Studies in allergic rhinitis have shown that reduced FEF25-75 could be linked to bronchial hyper-reactivity23 and positive response to bronchodilator testing24. People regard COPD as a disease of small airways11. Thus, FEF25-75 can represent primarily the early change in small airways that precedes and exceeds the change in FEV1 and the parameter is thought to reflect a differentially predominant small airway obstruction in a case of OAD12. Therefore, the FEV1-FEF25-75 distance is likely to increase in early airway diseases. Theoretically, for any OAD, differential regional involvement in either small (distal) and relatively large (proximal) part of the intrathoracic airways can be FEF25-75 and FEV1 respectively. In this study, the FEV1-FEF25-75 distance was studied to understand the early airflow obstruction and a predominantly small airway changes inthe study participants.
We presume that FEF25-75 represents airflow mostly at bronchi proximal to the respiratory units to manifest reversibility as observed in our practice since for the lack of smooth muscles in the walls; the bronchioles distally should lose the potential for dynamic behaviour of bronchodilator responsiveness. The FEF25-75 actually represents airflow in a region of smaller airways and not some of the exact divisions of airway ramifications. Given this, change in FEF25-75 should be contemplated when there is a concomitant change in FEV1 and/ or FVC. The change in FEF25-75 (%-predicted) correlated well to the bronchodilator responsiveness in asthmatic children24. It helps to appreciate the relevant reversible airflow obstruction25. The reversibility potential of the FEF25-75 was apparent in a study when the significant bronchodilator reversibility was described by PEF, FEF25-75, and per cent of sGAW26.
However, literature is lacking on combined asthma and ILD. Both ILD and COPD are diseases of the adulthood and late adulthood; hence, COPD is more likely the OAD in ILD. In this study, the FEF25-75 related parameters unearthed a far higher number of airflow obstruction than those by the FEV1/FVC criterion. Particularly, participants with, reference standard of airflow obstruction as FEV1/FVC <0.7, had the statistically lesser FEF25-75 from those without airflow limitation (Table I). Findings of this study suggest that individuals diagnosed based on FEF25-75 are likely to have a predominant small airway disease to start with as is apparent from both the initial (OAD+ILD) overlap and the unmixed-ILD group having lower FEF25-75 in common. This observation also supports the concept that that FEF25-75 (%-predicted) can be an earlier marker for COPD than FEV115. The impact of COPD or early COPD in individuals with ILD is hence a research issue worth exploring. We feel that the reduction of FEF25-75 (%-predicted) and other surrogate markers of FEV1/FVC ratio (<0.7) were determinants of COPD in the study participants.
The airflow changes in ILD are mostly in the peripheral bronchial axis that includes the small airways and we have reported earlier that FEF25-75 may even be increased compared to FVC in ILD27. In face of the interstitial fibrosis imparting traction over to the cartilage-free small airways, the FEF25-75 change may be minimal or even reversed. Thus, a reduction in FEF25-75 is possibly a more plausible marker of airflow obstruction in ILD. Since the chronic hypersensitivity pneumonitis (cHP) remains the frequent-most aetiology of ILD in India28, it is possible that small airway involvement in ILD could have caused the airflow obstruction reflected by change in FEF25-75 itself or the parameters derived of it.
Bronchodilator responsiveness (in terms of FEV1 reversibility) signifies a dynamic constriction of airway lumen. In our cohort, it is significantly lower in the overlap group compared to the ‘unmixed’ patients of ILD with the former showing more severe airflow obstruction [FEV1/FVC ratio of 41.15±211.9 vs. 57.70±80.77; P=0.04] possibly from depleted reversibility potential because of remodelling effect of OAD. The better reversibility /responsiveness of FEV1 or FEF25-75 in the ‘unmixed’ ILD patients (with preserved FEV1/FVC ratio) suggests the limitation of the FEV1/FVC as the sole criteria to understand airflow obstruction. The story signifies that the level of affection (peripheral and central), airway remodelling, and the type of affection (predominantly fibrotic or inflammatory/constrictive) concomitantly influence the status of FEF25-75 in ILD. The issue demands intricate research.
The presence of coexisting OAD appears therapeutically important in ILD. A FEV1-reversibility of 100-ml or the minimum clinically important difference (MCID) in COPD29 may signify a positive and perceptible treatment-effect on wellbeing and the health related quality of life in COPD30. Such a change in FEV1 can improve FVC that acts as prognostic marker and an end-point in therapeutic trials in ILD31. Incidentally, the change in FVC was higher in our original ‘overlap’ group (Table I).
This study was not without limitations. This was a single-centre based observation. A concomitant analysis of DLCO and lung volumes in this relatively small number of participants could possibly yield much important information. Similarly, concomitant use of impulse oscillometry could have been a good and insightful adjunct. It would have been worthwhile to look for the a etiological association in the exercise since the aetiology of ILD varies from place to place, the relative frequency of airflow obstruction in ILD can vary in different geographical areas. However, the concept will be applicable everywhere.
Overall, this study opens a new domain for consideration in ILD. The implication of the observation demands further research to guide the decision of treatment of OAD in ILD.
Acknowledgment
The authors are thankful to the technicians and staff members of the institute who supported in accomplishing this study.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Traction bronchiectasis/bronchiolectasis is associated with interstitial lung abnormality mortality. Eur J Radiol. 2020;129:109073.
- [Google Scholar]
- Fleischner society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
- [Google Scholar]
- Airflow obstruction in bronchiectasis: Correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198-204.
- [Google Scholar]
- Small airways obstruction and its risk factors in the burden of obstructive lung disease (BOLD) study: A multinational cross-sectional study. Lancet Glob Health. 2023;11:e69-82.
- [Google Scholar]
- Combined pulmonary fibrosis and emphysema: A distinct underrecognized entity. Eur Respir J. 2005;26:586-93.
- [Google Scholar]
- Diagnostic assessment of patients with interstitial lung disease. Pri Care Resp J. 2011;20:120-7.
- [Google Scholar]
- Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Pri Care Resp J. 2012;21:167-73.
- [Google Scholar]
- 2023. Available from: https://goldcopd.org/2023-gold-report-2/, accessed on November 19, 2023.
- Overt and covert airflow limitations in DPLD: A spirometric appraisal. Eur Respir J. 2023;62:PA2926.
- [Google Scholar]
- Small airways in children with allergic rhinoconjunctivitis: The potential role of a multicomponent nutraceutical. Acta Biomed. 2020;91:350.
- [Google Scholar]
- Lung volumes and airway resistance in patients with a possible restrictive pattern on spirometry. J Bras Pneumol. 2016;42:341-7.
- [Google Scholar]
- The pragmatic role of FEF25-75 in asymptomatic subjects, allergic rhinitis, asthma, and in military setting. Expert Rev Respir Med. 2019;13:1147-51.
- [Google Scholar]
- Role of FEF25–75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol. 2006;20:641-7.
- [Google Scholar]
- FEF25-75% values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:2913-21.
- [Google Scholar]
- Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung. 2011;189:121-9.
- [Google Scholar]
- The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res. 2019;20:49.
- [Google Scholar]
- FEF25-75% is a more sensitive measure reflecting airway dysfunction in patients with asthma: A comparison study using FEF25-75% and FEV1%. J Allergy Clin Immunol Pract. 2021;9:3649-59.
- [Google Scholar]
- Forced expiratory flow at 25–75% as a marker for airway hyper responsiveness in adult patients with asthma-like symptoms. Tanaffos. 2018;17:90.
- [Google Scholar]
- FEF25-75 and FEV1/FVC in relation to clinical and physiologic parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527.
- [Google Scholar]
- Poor symptom control is associated with reduced CT scan segmental airway lumen area in smokers with asthma. Chest. 2015;147:735-44.
- [Google Scholar]
- Role of FEF25%–75% as a predictor of bronchial hyperreactivity in allergic patients. Ann Allergy Asthma Immunol. 2006;96:692-700.
- [Google Scholar]
- Impaired FEF25-75 values may predict bronchial reversibility in allergic children with rhinitis or asthma. J Biol Regul Homeost Agents. 2012;26:S19-25.
- [Google Scholar]
- FEF25-75 and FEV1/FVC in relation to clinical and physiologic parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527.
- [Google Scholar]
- Alternative functional criteria to assess airflow-limitation reversibility in asthma. Rev Port Pneumol. 2015;21:69-75.
- [Google Scholar]
- An efficient diagnosis of diffuse parenchymal lung disease from spirometry exploring the novel role of FEF25–75. J Med Paediatr Oncol. 2022;43:A002.
- [Google Scholar]
- Hypersensitivity pneumonitis: Clinical manifestations–Prospective data from the interstitial lung disease-India registry. Lung India. 2019;36:476-82.
- [Google Scholar]
- Minimal clinically important differences in COPD lung function. COPD. 2005;2:111-24.
- [Google Scholar]
- Health-related quality of life associates with change in FEV 1 in COPD: Results from the COSYCONET cohort. BMC Pulm Med. 2020;20:1-2.
- [Google Scholar]
- Forced vital capacity as a primary end point in idiopathic pulmonary fibrosis treatment trials: Making a silk purse from a sow’s ear. Thorax. 2013;68:309-10.
- [Google Scholar]






