Translate this page into:
Copy number polymorphism of glutathione-S-transferase genes (GSTM1 & GSTT1) in susceptibility to lung cancer in a high-risk population from north-east India
Reprint requests: Dr Sunita Saxena, Director, National Institute of Pathology (ICMR) Safdarjung Hospital Campus, New Delhi 110 029, India e-mail: sunita_saxena@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Genetic polymorphisms in glutathione-S-transferase genes (GSTM1 and GSTT1) have been studied intensively for their potential role in lung cancer susceptibility. However, most of the studies on association between the polymorphisms and lung cancer do not distinguish between genotypes with one or two copies of the genes. The present study investigates the gene dosage effects of GSTT1 and GSTM1 copy number and their environmental interactions to examine the association of lung cancer risk with trimodular genotypes of the GSTs in a high-risk population from north-east India.
Methods:
A total of 154 lung cancer cases and 154 age and sex matched controls from the high risk region of north-east India were analyzed by multiplex real-time PCR to determine the trimodal genotypes (+/+, +/- and -/-) in both the genes (GSTM1 and GSTT1).
Results:
No significant association and gene dosage effect of GSTM1 gene copy number with lung cancer risk (Ptrend=0.13) were found. However, absence of GSTT1 conferred 68 per cent (OR=0.32;95%CI=0.15-0.71; P=0.005) reduced risk compared to the two copy number of the gene. There was evidence of gene dosage effect of GSTT1 gene (Ptrend=0.006). Tobacco smoking was a major environmental risk factor to lung cancer (OR=3.03;95%CI=1.73-5.31; P<0.001). However, its interaction with null genotype of GSTT1 conferred significant reduced risk to lung cancer (OR=0.30;95%CI=0.10-0.91; P=0.03). Further in only tobacco smokers, null genotype was associated with increased reduced risk [0.03(0.001-0.78)0.03; Ptrend=0.006]. No effect modification of GSTM1 was observed with lung cancer risk by environmental risk factors.
Interpretation & conclusions:
The results suggest that absence of GSTT1 null genotype may be associated with a reduced risk of lung cancer and the effect remains unchanged after interaction with smoking.
Keywords
Copy number polymorphism
GSTM1
GSTT1
lung cancer
real-time PCR
Lung cancer is the leading cause of cancer-related deaths worldwide1. In India, it is the most frequent cancer amongst men2. In north eastern (NE) part of India lung cancer is among the ten leading sites of cancer, with the highest age-adjusted incidence rate (AAR) in Mizoram State (24.5 in males and 26.3 in females). Aizwal district alone showed an AAR of 36.0 in males and 38.7 in females which is almost three to ten times higher than Delhi3. High incidence rates suggest role of both genetic as well as environmental factors such as smoking, tobacco use and dietary carcinogen consumption.
Tobacco smoking remains the primary aetiological factor associated with the development of lung cancer accounting for nearly 80-90 per cent of the disease. Polycyclic aromatic hydrocarbons (PAHs) particularly benzo[a]pyrene (BaP) and nitrogen containing nitrosamines and aromatic amines are main carcinogens present in tobacco smoke that are implicated in lung carcinogenesis. Non-smoking tobacco and betel quid have also been implicated in lung carcinogenesis probably due to their accompanied consumption with smoking4. Deleterious effects of tobacco carcinogens are primarily mediated through DNA adduct formations following their activation in the detoxifying pathways. Activated PAHs and N-nitroso compounds produced by phase I xenobiotic metabolizing enzymes are substrates for the glutathione-S-transferase M1 and T1 (GSTM1 and GSTT1) phase II enzymes.
Evidences are available in literature on association of GSTM1 and GSTT1 genotypes with lung cancer56. Most of these studies have compared the “null” genotype with the “non-null” genotype and thus do not distinguish between one and two copy number of the genes. However, studies have reported a trimodal phenotype distribution for both GSTM1 and GSTT1 identifying homozygous wild type (+/+), hemizygous (+/-) and null (-/-) genotypes of the genes78. These studies suggest a gene dosage effect with three alleles corresponding to fast, intermediate and slow enzyme activity. Enzymatic activity of GSTT1 has been reported to be varying with the copy number of the gene9. Sprenger et al7 in their genotype-phenotype comparison showed correlation of significantly increased enzyme activity in individuals with two copy number of the GSTT1 compared to those with one copy number. Roodi et al10 showed that the relative risk of breast cancer increased with the present allele (+/- and +/+ genotypes) compared with -/- genotype, however, this trend was not significant.
Several methods (standard and long-range PCR) in the past have been used for distinguishing GSTM11011 and GSTT171112 alleles into three genotypes. These methods were primarily based on the fact that the two genes are flanked by highly homologous regions. Recent studies have used Taqman based real-time PCR assays to discriminate between the wild-type, hemizygous deletion, and homozygous deletion of the GSTM1 and GSTT1 genes1314. In our previous report on association of GST polymorphisms, comparing the null genotype (-/-) with combined non-null genotype (+/- and +/+) using traditional multiplex PCR-gel electrophoresis method in high risk north-east Indian population, we showed a significant protective effect of GSTM1 and GSTT1 null genotypes in lung cancer15. The present study was conducted to examine the relationship between GSTM1 and GSTT1 genes and lung cancer risk by assessing potential gene dosage effects and gene-environment interactions.
Material & Methods
Study subjects: This study consisted of 154 histopathologically diagnosed lung cancer cases registered at Dr. Bhubaneswar Borooah Cancer Institute, Guwahati, Assam and Civil Hospital, Aizawl, Mizoram and Sir Thutob Namgyal Memorial Hospital, Gangtok, Sikkim, the collaborating centers in north east India. Incident cases with lung as primary site of cancer, during the period of December 2006 to 2009 and willing to participate in the study were included. An equal number of voluntary, age and sex matched individuals with no history of any obvious disease were selected as controls (n=154) from the unrelated attendants who accompanied cancer patients. This provided a readily available and cooperative source of controls from the same socio-economic background as the cases reducing confounding biases.
The sample size was determined based on power calculation methods from evidences provided by our research group on association between GSTs and lung cancer15, the minimum sample size calculated was 144 at 5 per cent level of significance and 80 per cent power. Demographic data and characteristics such as age, sex, smoking habit, usage of tobacco, betel quid and alcohol, were obtained from subjects in a standard questionnaire used for all the centers, in an in-person interview by a trained data collector. A majority of cases and controls were literate with full primary schooling and some upto the college level. The occupational history of the study participants revealed that most of them were farm labourers or engaged in petty jobs with no exposure to any occupational hazards. Patients with only lung as their primary site of cancer were included. Any subject with history of familial malignancy or pulmonary infectious disease was excluded both from case and control. Final selected controls were included on the basis of no history of any obvious disease and those not taking any medication at the time of recruitment. All subjects provided written informed consent for participation in this research which was done under a protocol approved by the institutional ethics committee of Regional Medical Research Centre, North East Region, Guwahati (Indian Council of Medical Research). Smokers, chewers and drinkers were classified into two categories ever and never. For smoking, an individual who had never smoked or smoked less than 100 cigarettes in his/her lifetime and was not smoking at the time of reporting was considered never smoker or non-smokers. Ever smokers or smokers category included current smokers, and those who had quit within <1 year of reporting16. Blood sample (5 ml) was collected in EDTA vials and stored under -70°C until processed.
DNA isolation: Genomic DNA was isolated using Qiagen Blood DNA Isolation kit (Qiagen GmbH, Germany) and stored at -30°C till further analysis. DNA quantification was done using Nano-drop ND-1000 Full–spectrum UV/Vis spectrophotometer (NanoDrop Technologies, USA); 5 μl (20 ng) of DNA solution was used for each real-time PCR reaction.
Quantitative real-time TaqMan PCR for GSTM1 and GSTT1 copy number determination: GSTM1 and GSTT1 genotyping was performed using Taqman Gene Copy Number Assays purchased from Applied Biosystems (Foster City, California, USA). TaqMan gene copy number assays (GSTM1:Hs no Hs02595872_cn and GSTT1: Hs no Hs00817631_cn) were run simultaneously with a TaqMan Copy Number reference assay (RNase P: Part No. 4403326) in a duplex real-time PCR. Each 20 μl assay mixture containing 20ng of genomic DNA (5 μl) was prepared according to protocols developed by Applied Biosystems for copy number detection. Real-time PCR reactions were run on ABI PRISM 7000 Sequence Detection System (Applied Biosystems CA, USA) Each reaction was run in duplicates. In addition, a no-template control was also included in each run to rule out any contamination. Universal thermal cycling conditions were used, i.e 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C. Real-time data were collected by the SDS 1.0 software (Applied Biosystems, CA, USA). The number of copies of the target sequence in each test sample was determined by relative quantitation (RQ) using the comparative CT (ΔΔCT) method17. This method measures the CT difference (ΔCT) between target and reference gene, and then compares the ΔCT values of test samples to a calibrator sample which is known to have two copies of the target sequence. The copy number of the target is calculated to be two times the relative quantity. A sample homozygous for GSTM1 and GSTT1 wild type allele (2 copy number) was used as calibrator. The samples employed as calibrators were previously confirmed to have two copies of the genes examined.
Validations of copy number by PCR method: For validation of copy number estimation done through real-time PCR, 30 per cent of the samples were reanalyzed through PCR-gel electrophoresis methods. Copy number detection of GSTM1 was done through a two-step method. Samples showing presence of GSTM1 gene in multiplex PCR described in our previous report15 were reanalyzed for detection of null allele using primer described for GSTM1 null allele by Buchard et al11. Samples showing amplification of the 4748 bp null allele were considered as hemizygous genotype (1 copy) and those with no amplification were considered carrying two copies of GSTM1 gene. A sample with null GSTM1 genotype was included as positive control in each PCR. Genotypes of GSTT1 were detected through multiplex PCR described by Naito et al12. Amplification products of 566 and 458 bp represented null and present alleles of GSTT1 respectively. The results from PCR-gel electrophoresis method were in complete concordance with those from real-time PCR.
Statistical analysis: The association of GSTM1 and GSTT1 genotypes with lung cancer was evaluated by multivariate conditional logistic regression. The association of tobacco smoking, tobacco chewing, betel quid chewing, and alcohol intake with disease development was assessed by chi square/Fisher's exact test. Estimates of cancer risk imparted by GSTM1 and GSTT1 genotypes and other covariates such as tobacco smoking, chewing, betel quid chewing, and alcohol were determined by deriving the odds ratio (OR) and corresponding 95 per cent confidence intervals (95% CIs) using univariate and multivariate conditional logistic regression models. To evaluate the putative modifying effects of the GST genotypes on the effects of environmental factors, stratified analysis was performed for subjects positive for individual risk factors. For all the tests, a two-sided P<0.05 was considered significant. The data analysis was performed on STATA 8.0 software (State-Corp LP, College Station, TX, USA).
Results
A total of 154 lung cancer cases and 154 controls were successfully genotyped for polymorphism in GSTT1 and GSTM1. The distribution of gender and ethnicity was similar for cases and controls. Males were overrepresented in the study compared to females (M/F ratio: 3.05). Mean ages of cases and controls were 59.16 ± 9.95 (range 35-80 yr) and 60.39 ± 10.43 (range 38-85 yr), respectively (Table I). Distribution of both GSTM1 and GSTT1 genotypes was in agreement with Hardy-Weinberg equilibrium (HWE) in controls (P>0.05), however, significant deviation of GSTT1 genotypes from HWE was seen in cases (P=0.01).
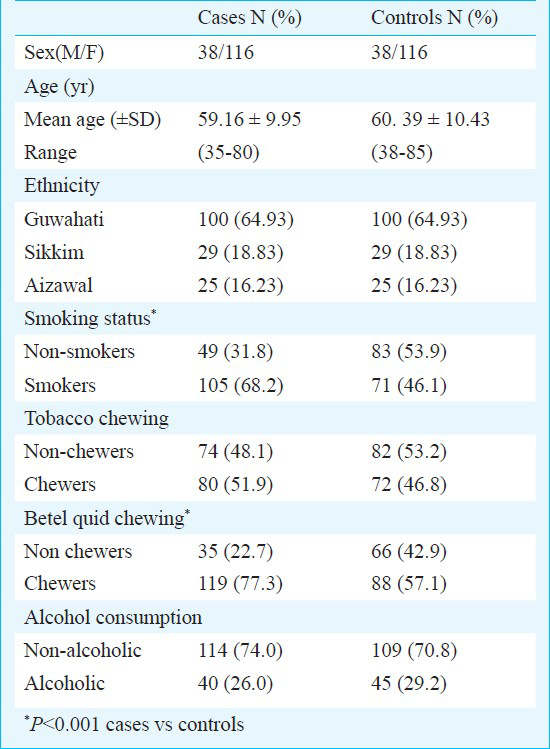
Association of genetic and environmental factors with lung cancer risk: The distribution of environmental risk factors and genotypes in cases and controls and their association with lung cancer risk are summarized in Table II. Risk habits such as smoking, tobacco chewing and betel quid chewing were more common among the cases compared to controls. Smokers constituted 68.2 per cent of the cases and 46.1 per cent of the controls, thus smoking was associated with a significant risk of lung cancer (OR=3.03; 95%CI=1.73-5.31; P<0.001). Betel quid chewing was present in 77.3 per cent of the cases and 57.1 per cent of controls, with the habit conferring greater than 2 fold risk to chewers compared to non-chewers (OR=2.39; 95% CI=1.38-4.16; P=0.002).
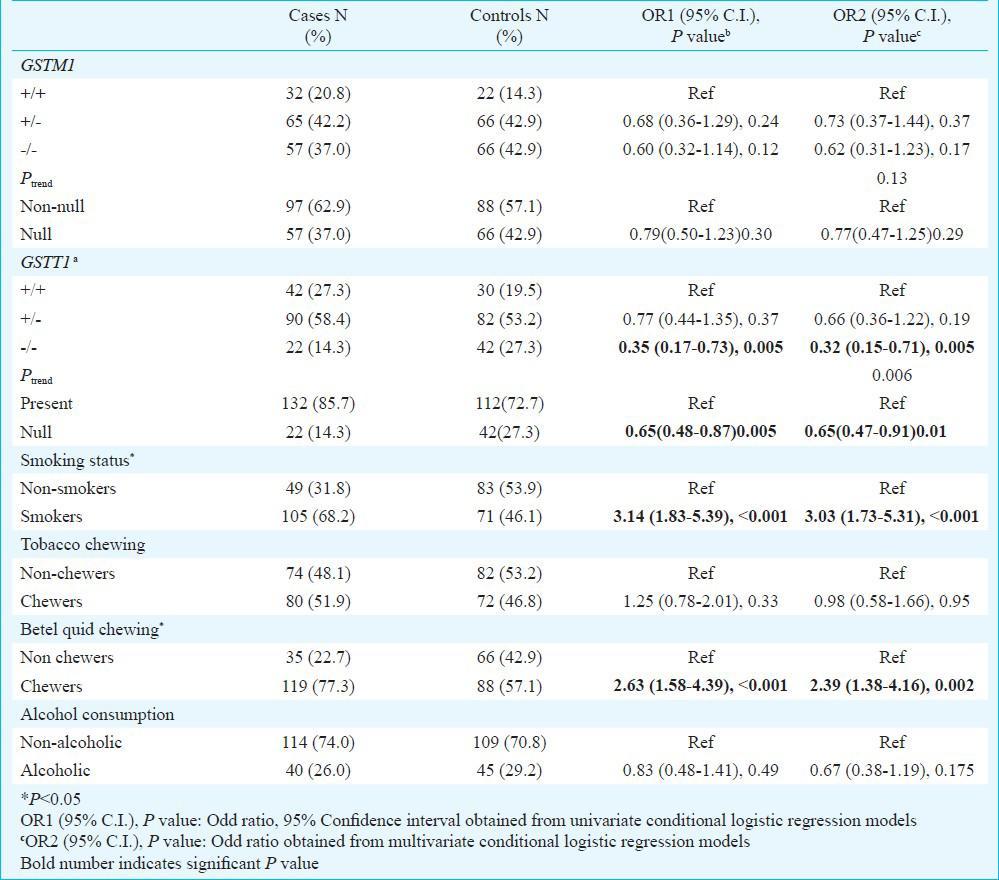
Frequency of GSTM1 wild-type and null alleles in the control population was 0.35 and 0.64, respectively. Distribution of wild type (+/+) two copy, hemizygous deletion (+/-) one copy and homozygous deletion (-/-) null copy of GSTM1 genotypes was 20.8, 42.2 and 37.0 per cent in cases and 14.3, 42.9 and 42.9 per cent in controls (Table II). Compared to individuals with two copy (+/+) genotype, the relative risk of lung cancer was 0.73 (95% CI = 0.37-1.44; P = 0.37) for the hemizygous genotype (+/-) and 0.62 (95% CI=0.31-1.23; P=0.17) for the null genotype (-/-). There was no evidence of gene dosage effect for GSTM1 (Ptrend=0.13). In contrast GSTT1 the wild type (+/+) two copy number and hemizygous one copy number genotype was more frequent in cases than controls (27.3 vs 19.5% and 58.4 vs 53.2%, respectively). Patients with null genotype conferred 68 per cent (OR = 0.32; 95% CI = 0.15-0.71; P=0.005) reduced risk compared to patients with two copy number of GSTT1. When risk associated with null genotype was compared with one copy number (hemizygous) of the gene it reduced to 51 per cent (OR = 0.49; 95% CI = 0.25-0.95; P = 0.03) (data not shown). Decreasing copy number of GSTT1 gene showed a positive dose relationship with lung cancer (Ptrend=0.006). A comparison of our data according to the classical ‘null’ versus the ‘non null’ genotype have attenuated the protective effect of GSTT1 null genotype from 68 to 45 per cent (OR = 0.55; 95% CI = 0.30-1.00; P=0.05) (Table II).
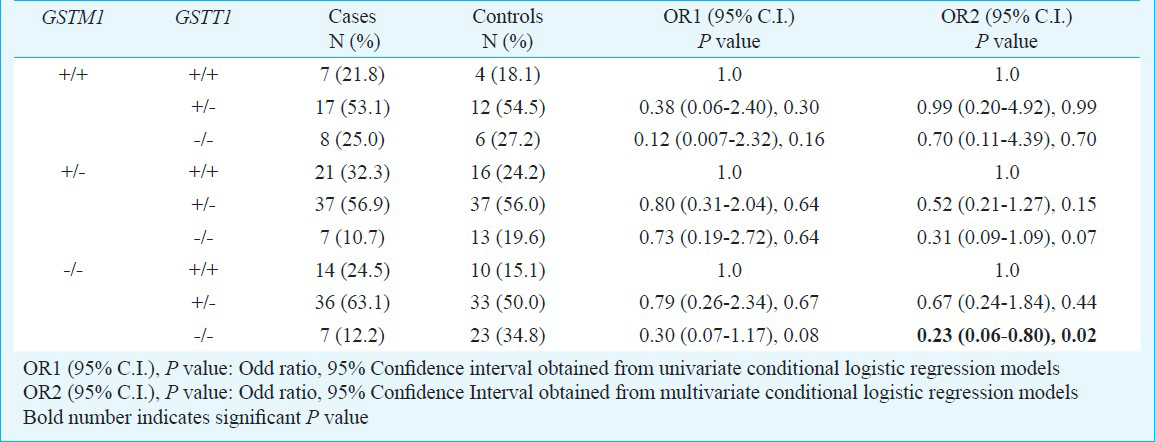
Gene-gene interaction: To elucidate gene-gene interactions associated with lung cancer, we investigated the role of these polymorphisms in combination (Table III). Interaction of all three GSTM1 genotypes with one or no copy of GSTT1 gene conferred reduced risk to lung cancer. However, most of these interactions were not significant. Only significant reduced lung cancer risk was observed for individuals with the combined GSTM1 and GSTT1 null genotype (OR=0.23; 95% CI = 0.06-0.80; P=0.02) which becomes insignificant after Bonferroni correction (PBonferroni correction=0.12).
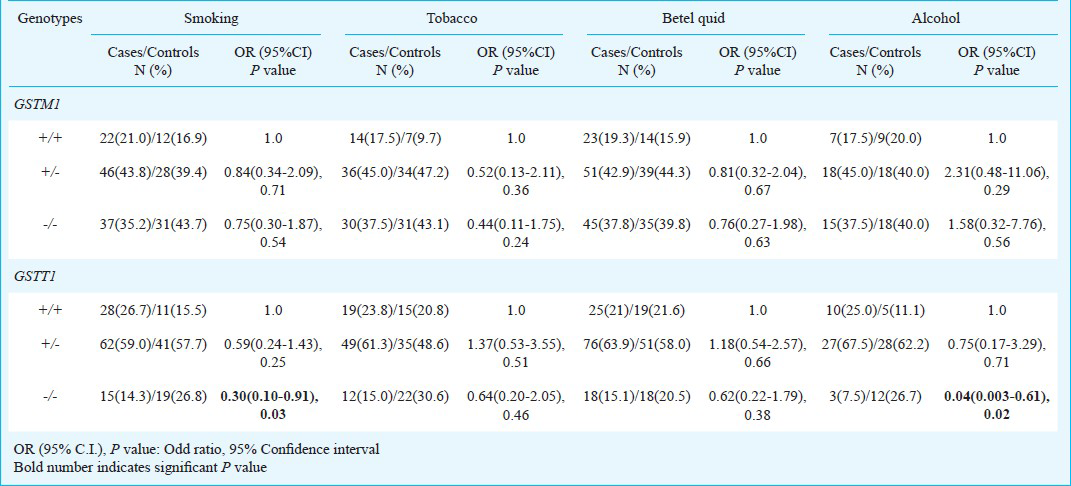
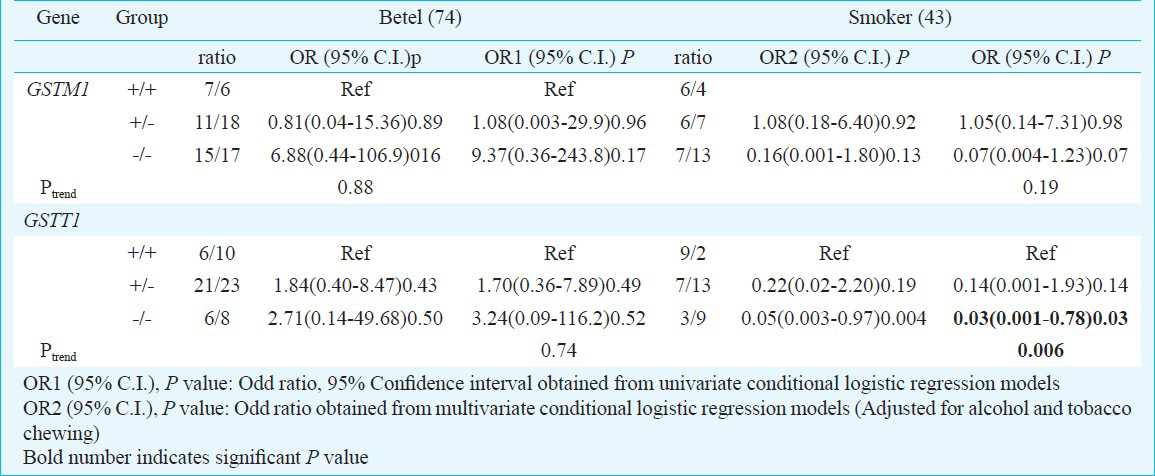
Gene-environment interaction: To evaluate the potential modifying effect of GSTM1 and GSTT1 genes on risk factors, stratified analysis was performed. Genotype distribution and association of GSTM1 and GSTT1 in cases and controls positive for smoking, tobacco chewing, betel quid chewing and alcohol consumption are given in Table IV. Interaction of risk factors with GSTM1 and GSTT1 genes imparted reduced risk with deletion in functional allele (+/- and -/-) compared to individuals with the presence of both allele (+/+). Smokers carrying GSTT1 null genotype showed significantly reduced risk (OR=0.30; 95%CI=0.10-0.91; P=0.03). Moreover, after stratifying the data further between exclusive smokers and betel nut chewers, protective effect of GSTT1 null genotype was more pronounced in smokers only (OR=0.030; 95% CI = 0.001-0.78; P=0.03, Ptrend=0.006) (Table V). Similar interaction was observed for alcohol, individuals with GSTT1 null genotype showed significantly reduced risk (OR=0.04; 95% CI=0.003-0.61; P=0.02) (Table IV). Interaction of risk habits with GSTM1 and GSTT1 genotypes showed carcinogen specificity. It was interesting to note that even though most of the results were not significant, yet interactions of risk habits with null genotype almost always yielded more protective effects than interactions with one copy number genotypes for both GSTM1 and GSTT1.
Discussion
Worldwide numerous studies have been conducted on the association between GSTT1 and GSTM1 polymorphism but with conflicting results. Although findings from India showed no significant association of GSTM1 and GSTT1 with lung cancer, but some studies suggest a possible interaction with smoking. Sreeja et al18 showed significant risk associated with GSTT1 null polymorphism. Similarly, Kumar et al19 have also found marginally significant risk associated with GSTT1 null genotype. However, these studies have investigated the risk for lung cancer in individuals with null genotype (no allele) compared with a combined group of individuals with either one or two functional alleles, thus underestimating the risk without accounting the effect of gene dosage of allele.
In the present study, there was no overall effect of GSTM1 polymorphism on lung cancer. For GSTT1 gene, the risk of lung cancer significantly decreased with the deletion of the functional allele i.e. from single copy of the gene (0.66) to the null genotype (0.32) compared to the two copy number of gene. However, the defined classical functions of GSTM1 and GSTT1 do not support protective roles for the null genotypes, but there has been precedent in studies on lung cancer particularly from outside India2021222324252627. Risch et al20 reported that GSTT1 null genotype was underrepresented among squamous cell carcinomas. Similarly in two other studies, GSTM1 null genotype was associated with reduced risk of lung cancer particularly in younger patients of 50-60 yr and in squamous cell carcinomas2122. Some other studies have also found moderately decreased although non-significant, risk associated with GSTT1 null genotype232425. In the light of the above results it is easy to speculate a dual role of the GSTs depending upon the conditions of stress. Studies have explained this duality on the basis of metabolite toxicity26 and population genetics10.
When analyzing joint effects of the two GST genes, combination of null genotypes of both the genes imparted significant reduced risk though insignificant after Bonferroni correction. This suggests that an increased glutathione conjugation by the present alleles of both genes imparts increased risk to cancer. Combined conjugation activities by GSTs deplete the level of glutathione in the cell impairing xenobiotic defense and thereby exposing it to oxidative damage and induced mutagenesis27. The formation of glutathione conjugates generally causes the electrophiles to be less toxic and readily excreted. However, this conjugation might also act as transporter molecule by releasing reversibly bound electrophilic compounds.
Tobacco smoking was a strong risk factor in the study. Tobacco is consumed both in smoking and smokeless forms. In India, tobacco is smoked as cigarettes or in the form of bidi. Tobacco smoke comprises nearly 60 carcinogenic compounds whereas its unburned form contains 16 identified carcinogens28. Among these, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and polycyclic aromatic hydrocarbons (PAH) are considered to be the most important causative agents for the development of lung cancer. PAH require metabolic activation and subsequent binding to DNA (forming bulky “PAH-DNA adducts”) to exert their carcinogenic action29. Similar activation of one of the N-nitrosamines, NNK, by the P450 system produces metabolites that form methyl and pyridyloxobutyl DNA adducts. Detoxification of these toxic metabolites occurs via the action of multiple Phase II enzymes, most notably the glutathione-S-transferases.
In the present study, interaction of smoking with GSTT1 +/- and -/- genotypes compared to the +/+ genotype was protective in nature indicating a genetic modulation of the risk imparted by smoking. Similar result was reflected in a meta-analysis by Raimondi et al5, where a negative trend of the odds ratios for GSTT1 null allele was observed with increasing amount of lifetime smoking for both Caucasians and Asians subjects. Further, studies have indicated adverse association between smoking and lung cancer among individuals with GSTT1 null genotype particularly in non-smokers. Alexandrie et al24 found that GSTT1 null genotype was associated with a decreased risk for lung cancer in heavy smokers. Although not significant, Wenzlaff et al30 also found that never smokers with GSTT1 null genotype with no household environmental tobacco smoke were at one-third the risk of lung cancer compared with GSTT1 present genotype. Further, a possible protective effect of being GSTT1 null in non-smoker has also been reported31.
The discrepancy in results could be explained by different ethnic population, differences in categorization of smokers and different habit of dietary compounds. As there are several dietary compounds, particularly intake of crucifreous vegetables that need to be controlled to fully elucidate true gene-environment interactions related to lung caner risk. London et al32 found that individuals with detectable level of isothiocyanate (ITC) were at reduced risk of lung cancer with the null genotype of both GSTM1 and GSTT1. Isothiocyanates found in cruciferous vegetables, are substrates for GSTs and are associated with reduced cancer risk. The present study lacks information on both dietary status and the pack years of the smokers.
There might be some more limitations to this study. The sample size of our study was relatively small. GSTT1 genotypes showed deviation from HWE in lung cancer cases. After ruling out false positive associations and genotyping errors perhaps population stratification, could be a reason for this deviation. However, the cases were incident, and thus, the data did not show report or recall bias. Also case-control matching was done in reference to age, gender, and ethnicity, thereby controlling for any confounding effect accounted by these variables. Estimation of interactive OR in this study in some cases yielded small subgroup sizes which limit the reliability of estimating gene-environment effects. Thus these results should be considered empirical observations for further studies on larger number of samples. In summary, trimodular genotypes of GSTM1 and GSTT1 were determined and gene dosage effect was observed with GSTT1 copy number. Our results indicated that null genotype of GSTT1 might be associated with a reduced risk of lung cancer risk. Furthermore, protective effect of GSTT1 was strongly associated with smokers only.
Our results were in contrast with some previous reports on lung cancer1422 which observed no significant association of hemizygous and homozygous genotypes of GSTM1 and GSTT1 when compared with homozygous wild type genotype. This might be due to the difference in sample size, however, in contrast to many of these studies, the homogeneity of our population from an ethnically isolated north-eastern part of India allowed the detection of small inherited variations in metabolism. Thus, the present results indicate that ethnicity and carcinogen exposure along with trimodal distribution of GST enzymes can be a major determinant of risk of lung cancer.
Acknowledgment
This study was supported by grants from Indian Council of Medical Research (ICMR), (49/4/RMRC/NE/2005-NCD-II/III), New Delhi, and University Grants Commission (UGC), Government of India, New Delhi, India (10-2(5)/2005(ii)-E.U.II).
References
- GLOBOCAN 2012 v10, Cancer incidence and mortality worldwide: IRAC cancer base no 11. 2013. Lyon, France: International Agency for Research on Cancer; Available from: http://globocan.iarc.fr
- [Google Scholar]
- Population Based Cancer Registry, Mizoram. In: Three-year report of Population Based Cancer Registries, 2006-2008. First Report of 20 PBCRs in India. Bangalore: National Cancer Registry Programme Indian Council of Medical Research; 2010. p. :330-80.
- [Google Scholar]
- Cancer risks from betel quid chewing beyond oral cancer: a multiple-site carcinogen when acting with smoking. Cancer Causes Control. 2010;21:1427-35.
- [Google Scholar]
- Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164:1027-42.
- [Google Scholar]
- Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol. 2008;167:759-74.
- [Google Scholar]
- Characterization of the glutathione S-transferase GSTT1 deletion: discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics. 2000;10:557-65.
- [Google Scholar]
- Hereditary interindividual differences in the glutathione transferase activity towards trans-stilbene oxide in resting human mononuclear leukocytes are due to a particular isozyme(s) Carcinogenesis. 1985;6:1211-6.
- [Google Scholar]
- Xenobiotic-metabolizing genes and small-for-gestational-age births: interaction with maternal smoking. Epidemiology. 2006;17:38-46.
- [Google Scholar]
- Association of homozygous wild-type glutathione S-transferase M1 genotype with increased breast cancer risk. Cancer Res. 2004;64:1233-6.
- [Google Scholar]
- Multiplex PCR detection of GSTM1, GSTT1, and GSTP1 gene variants: simultaneously detecting GSTM1 and GSTT1 gene copy number and the allelic status of the GSTP1 Ile105Val genetic variant. J Mol Diagn. 2007;9:612-7.
- [Google Scholar]
- Alternative genotyping method of GSTT1 null/present polymorphism. Expert Rev Mol Diagn. 2006;6:873-7.
- [Google Scholar]
- A multiplex real-time PCR method for detection of GSTM1 and GSTT1 copy numbers. Clin Biochem. 2009;42:500-9.
- [Google Scholar]
- Copy number variants of GSTM1 and GSTT1 in relation to lung cancer risk in a prospective cohort study. Ann Epidemiol. 2009;19:546-52.
- [Google Scholar]
- Polymorphisms of glutathione-S-transferase genes and the risk of aerodigestive tract cancers in the Northeast Indian population. Genet Test Mol Biomarkers. 2010;14:715-23.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Method. 2001;25:402-8.
- [Google Scholar]
- Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene polymorphisms in lung cancer susceptibility in a South Indian population. J Hum Genet. 2005;50:618-27.
- [Google Scholar]
- Lung cancer risk in north Indian population: role of genetic polymorphisms and smoking. Mol Cell Biochem. 2009;322:73-9.
- [Google Scholar]
- Glutathione-S-transferase M1, M3, T1 and P1 polymorphisms and susceptibility to non-small-cell lung cancer subtypes and hamartomas. Pharmacogenetics. 2001;11:757-64.
- [Google Scholar]
- GSTM1, GSTT1 and GSTP1 polymorphisms and lung cancer risk. Cancer Lett. 2002;180:165-71.
- [Google Scholar]
- Interactions between GSTM1, GSTT1 and GSTP1 polymorphisms and smoking and intake of fruit and vegetables in relation to lung cancer. Lung Cancer. 2007;55:137-44.
- [Google Scholar]
- Genetic polymorphisms of glutathione S-transferases as modulators of lung cancer susceptibility. Carcinogenesis. 2002;23:1475-81.
- [Google Scholar]
- Influence of CYP1A1, GSTM1, GSTT1, and NQO1 genotypes and cumulative smoking dose on lung cancer risk in a Swedish population. Cancer Epidemiol Biomarkers Prev. 2004;13:908-14.
- [Google Scholar]
- Genetic polymorphisms in CYP1A1, GSTM1, GSTP1 and GSTT1 metabolic genes and risk of lung cancer in Asturias. BMC Cancer. 2012;12:433.
- [Google Scholar]
- Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994;300:271-6.
- [Google Scholar]
- Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 1990;30:603-31.
- [Google Scholar]
- Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733-44.
- [Google Scholar]
- Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogens. In: Anders MW, ed. Bioactivation of foreign compounds. Orlando (FL): Academic Press; 1985. p. :177-42.
- [Google Scholar]
- GSTM1, GSTT1 and GSTP1 polymorphisms, environmental tobacco smoke exposure and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26:395-401.
- [Google Scholar]
- Glutathione S-transferase T1-null genotype interacts synergistically with heavy smoking on lung cancer risk. Environ Mol Mutagen. 2001;38:83-6.
- [Google Scholar]
- Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724-9.
- [Google Scholar]






