Translate this page into:
Controversies in the use & implementation of drug-eluting stent technology
Reprint requests: Dr Somjot S. Brar, Director of Vascular Medicine, 4867 Sunset Blvd., Rm. 3755, Los Angeles, CA 90027, USA e-mail: SBrar@cvri.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The introduction of drug eluting stents has resulted in dramatic reductions in the rates of restenosis and the need for repeat revascularization. In the last several years, concern has been raised regarding the long-term safety of this technology, particularly in the area of late restenosis and stent thrombosis. The development of newer anti-restenotic drug coatings, biodegradable polymers and even completely bioabsorbable stents offer the potential to address these limitations. Additional questions that have recently come to the forefront include the optimal duration of dual antiplatelet therapy, the use of platelet reactivity assays and genetic testing and drug eluting stent use in the treatment of acute myocardial infarction. This article will attempt to address these and other areas of controversy in the use and implementation of drug eluting stents.
Keywords
Bare metal stents (BMS)
drug eluting stents (DES)
rates of restenosis
revascularization
Introduction
Since being introduced in 1999, drug eluting stents (DES) have revolutionized the treatment of coronary artery disease. Compared to bare metal stents (BMS), DES have led to dramatic reductions in the rates of restenosis and repeat revascularizations, while improving target lesion revascularization (TLR)1–4. In recent years, concern has been raised regarding the long-term safety of DES and the risk of late restenosis and stent thrombosis (ST). This potential increased risk remains an area of uncertainty in the field of cardiology. The goal of this review will be to help clarify some of the controversies surrounding DES use, including newer stent technology, in-stent restenosis, ST risk, the role of genetic testing and platelet function assays and outcomes in “off-label” indications.
First generation DES
The first generation DES include Cypher (Cordis, Bridgewater, NJ) and Taxus stents (Boston Scientific, Nantick, MA). These stents consist of a stainless steel platform, a non-erodible permanent polymer coating and an anti-restenotic drug. The Cypher stents release sirolimus, a macrolide antibiotic that inhibits neointimal proliferation, while the Taxus stents release paclitaxel, a chemotherapeutic agent that suppresses cell mitosis56.
The landmark RAVEL (Randomized Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with De Novo Native Coronary Artery Lesions) trial was the first randomized study comparing sirolimus-eluting stents (SES) with BMS in patients undergoing percutaneous intervention (PCI) for simple lesions. At 1 year, the rates of restenosis were 0% for the SES group and 26.6 per cent for the BMS group7. These findings were confirmed in the SIRIUS trial, which showed a dramatic reduction in restenosis rates in more complex lesions treated with SES compared to BMS (3.2 vs. 35.4%; P<0.001)8.
Similar to the SES, randomized trials have demonstrated the superiority of paclitaxel-eluting stents (PES) over BMS. The pivotal approval trial, TAXUS IV, found significantly lower rates of in-stent restenosis in single lesions treated with PES compared to BMS at 9 month angiographic follow up (5.5 vs. 24.4%; P<0.0001)9. Subsequent randomized trials and meta-analyses have confirmed these findings across different patient subgroups and lesion characteristics239–13.
These extraordinary results demonstrating profound reductions in restenosis rates associated with SES and PES led to the widespread adoption of the first generation DES for both “on-label” and “off-label use.” In the mid 2000s however, reports began to surface regarding increased mortality associated with DES compared to BMS. Results from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), reported significantly increased mortality rates associated with DES from 6 month to 3 year follow up (adjusted RR 1.32; 95% CI: 1.11-1.57)14. These results, along with reports of ST, a rare but serious complication of coronary artery stents, raised concern regarding the overall safety profile of DES15. Originally presented in 2005 and later published in 2006, Barvy and colleagues16 reported a significantly increased risk of late ST associated with DES use. This meta-analysis, which included 14 clinical trials consisting of almost 6675 patients with relatively simple lesions, noted a 4 to 5-fold increased late ST risk in DES compared to BMS (RR = 5.02; 95% CI: 1.29-35.45; P=0.02)16. The median time for ST was also significantly later in the DES group compared to the BMS group (18 vs. 3.5 months; P=0.0003). These findings were confirmed by Stone and colleagues4, who performed a pooled analysis from randomized, double blind trials comparing the first generation DES with BMS. From stent placement to 4 year follow up, the overall rates of ST among SES (1.2 vs. 0.6%; P=0.20) and PES (1.3 vs. 0.9%; P=0.30) were similar to BMS. Between 1 and 4 years however, the rates of ST in both the SES (0.6 vs. 0%; P=0.025) and PES (0.7 vs. 0.2%; P=0.028) groups were significantly higher compared to BMS4 (Fig. 1).
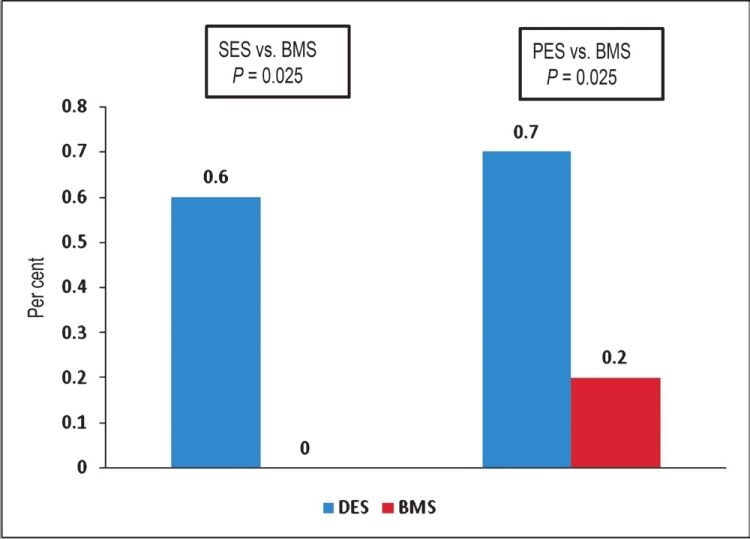
- Rates of stent thrombosis from years 1-4 after SES and PES implantation compared to BMS. Source: Ref. 4.
Other studies have corroborated the increased ST risk after 1 year associated with the first generation DES. In a large, collaborative meta-analysis using the Academic Research Consortium (ARC) definitions of ST, no difference was noted in the rates of early ST (<30 days) or late ST (30 days to 1 year) between the first generation DES and BMS. The rates of very late ST (>1 yr), however, was significantly higher in SES and PES when compared to BMS (0.7 vs. 0.1%; P= 0.006)17. In the j-Cypher study, a multicenter prospective registry of consecutive patients undergoing SES implantation in Japan, the overall cumulative rates of definite ST with SES were low (30 day =0.3%; 1 yr = 0.6%; and 5 yr =1.6%). The rates of late and very late ST, however, continued to rise without attenuation out to 5 years of follow up18.
One of the proposed mechanisms associated with increased ST risk beyond 1 year in the first generation DES, surrounds the permanent polymer coatings that assist in anti-restenotic drug release. Histopathology studies have demonstrated these permanent polymers result in delayed healing, impaired endothelialization and hypersensitivity reactions that likely contribute to the increased risk of ST19–23. Over the last 5 to 7 years, this increased ST risk associated with DES, has been a cause for concern in the field of interventional cardiology.
In addition to the risk of ST, recent concern regarding DES efficacy has focused on the rates of late and very late restenosis. The initial trials comparing the first generation DES with BMS found less than 6 per cent restenosis in patients treated with SES or PES89. Despite these remarkable results, attention has been directed to the area of long-term outcomes associated with restenosis. A prospective, systematic study by Bryne and colleagues24, reported rates of delayed, in-segment binary restenosis of 12.1 per cent in lesions treated with SES and 17.2 per cent in lesions treated with PES at 2 year angiographic follow up24. Other single center and registry studies also reported rates of late restenosis greater than 10 per cent in patients treated with first generation DES24–27. A recently published study from the j-Cypher registry18, found that patients treated with SES demonstrated cumulative indices of TLR of 15.9 per cent at 5 years. From the time of stent implantation out to 5 years, these restenosis events rose at a rate of 2.2 per cent per year without attenuation18. These results from the j-Cypher registry differs from the clinical course of BMS, which demonstrate an early restenosis phase (until 6 months), an intermediate regression phase (6 months to 3 years) and a late luminal re-narrowing phase (beyond 4 years)28. In an attempt to improve the long term safety and efficacy of DES, research has focused on the development of new polymers and stent platforms to minimize restenosis and reduce ST risk.
Second generation DES
The second generation DES include the Endeavor (Medtronic, Minneapolis, MN, USA), Resolute (Medtronic), Xience V (Abbot Vascular, Santa Clara, CA, USA) and Promus (Boston Scientific, USA) stents and utilize a more biocompatible, non-erodible polymer. The Endeavor, second generation stents utilize a cobalt-chromium (CoCr) platform and a permanent phorylcholine polymer that facilitates the release of the sirolimus analogue, zotarolimus. Although not biodegradable, this biocompatible polymer releases 95 per cent of zotarolimus within 14 days. Both animal and in vivo studies have demonstrated less inflammation and greater endothelial stent strut coverage with zotarolimus eluting stents (ZES) compared with SES and PES29–31. Clinical data from the ENDEAVOR I and II studies reported significantly lower in-stent late loss, binary restenosis and TLR with ZES compared to BMS3233. Additionally, rates of death, myocardial infarction (MI) and ST remained similar to BMS out to 5 years of follow up.
The Resolute ZES utilizes a CoCr stent platform designed in a continuous, sinusoidal-helical pattern. Additionally, a biocompatible BioLinx polymer, facilitates the extended release of 85 per cent of zotarolimus within 60 days and almost 100 per cent by 180 days34. The results from the prospective, observational RESOLUTE US trial, which enrolled 1402 patients with de novo native coronary lesions, demonstrated 12 month rates of target lesion failure (TLF) of 3.7 per cent in patients receiving the Resolute ZES compared to 6.5 per cent in historical Endeavor ZES controls (pnoninferiority < 0.001; post-hoc psuperiority = 0.002). Overall clinical outcomes additionally demonstrated low rates of cardiac death (0.4%), MI (1.3%), TLR (2.0%) and ST (0.1%). The prevalence of diabetes in the RESOLUTE US population was 34.4 per cent, with prespecified diabetic subgroup analysis demonstrating low rates of TLF (4.3%), cardiac death (0.5%), MI (0.8%) and TLR (3.0%)35. These findings were confirmed in the RESOLUTE All Comers trial and the TWENTE trial, which reported non-inferiority between the Resolute ZES and everolimus-eluting stents (EES) at 1 and 2 year follow up36–38. Based on these findings, on February 17, 2012, the U.S. Food and Drug Administration (FDA) approved the Resolute ZES for use in de novo coronary lesions and diabetic patients, becoming the first stent gaining an “on-label” indication for this population39.
Xience V and Promus stents elute everolimus, an antiproliferative agent in the same family as sirolimus. Attached to a CoCr or platinum-chromium (PtCr) stent platform, is a biocompatible polymer that assists in the release of approximately 80 per cent of everolimus within 30 days and almost 100 per cent within 4 months5.
The safety and efficacy of the EES when compared to BMS, have been demonstrated in the SPIRIT I, II and III randomized trials40–42. Additionally, in the recently published SPIRIT IV trial which randomized 3690 patients undergoing PCI to either Xience V EES or Taxus Express PES, the EES arm demonstrated significantly better rates of TLF (4.2 vs. 6.8%; P=0.001) and major adverse cardiac events (MACE) (4.2 vs. 6.9%; P= 0.0009) at 1 year43. The superiority of EES was also demonstrated in the area of ischaemia-driven TLR (2.5 vs. 4.6%; P=001), target vessel MI (1.8 vs. 2.9%; P=0.04) and definite/probable ST (0.29 vs. 1.10%; P=0.004) (Fig. 2)42.
The strength of evidence supporting the clinical efficacy of EES is also supported by data from the COMPARE trial, which randomized 1800 patients undergoing coronary intervention to receive either Xience V EES or Taxus Liberte PES44. This real world study reported better rates of MACE (6.2 vs. 9.1%; P=0.02) and target vessel revascularization (TVR) (2 vs. 6%; P=0.0001), again demonstrating the superiority of EES. Confirming these findings was a recently published meta-analysis by Baber and colleagues44, which identified 13 randomized trials comparing EES and non-EES DES. At a mean follow up of 21.7 months (range of 9-48 months), EES were superior to non-EES DES in the area of definite/probable ST (0.7 vs. 1.5%; P=0.001), MI (2.9 vs. 3.9%; P=0.002) and TVR (5.7 vs. 7.7%; P= 0.004), with no difference in cardiac mortality (1.6 vs. 1.9%; P=0.38). In sensitivity analyses, the overall superiority of EES persisted for the endpoints of ST, TVR and MI regardless of clopidogrel duration (6 or 12 months) or duration of follow up (≤1 yr or >1 yr)45.
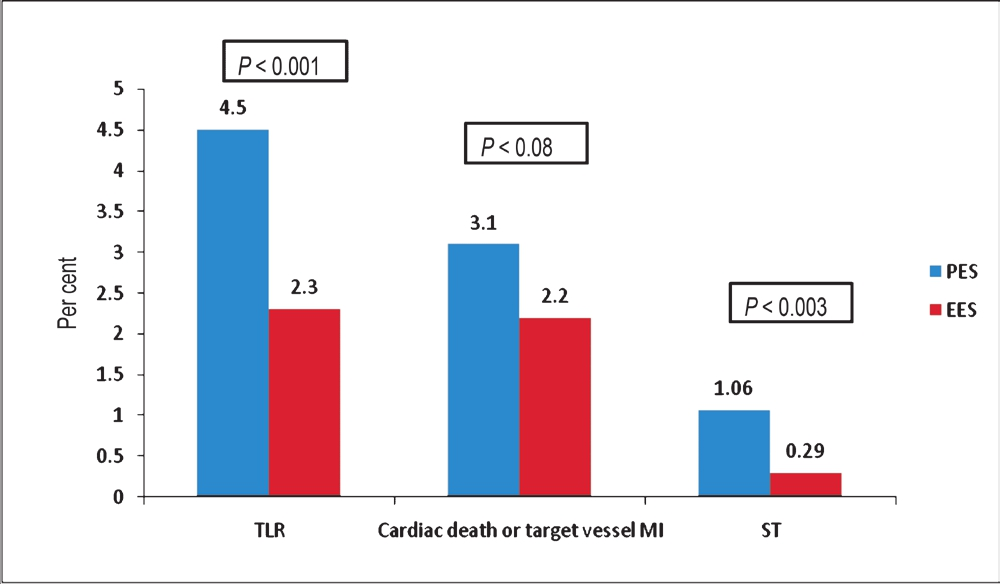
- Twelve month follow up comparing PES with EES in the end-points of target lesion revascularization (TLR), cardiac death or target vessel MI or stent thrombosis (ST) from the SPIRIT IV trial. Source: Ref. 43.
A recently published network meta-analysis that involved 49 randomized trials and 50,844 patients provided further evidence into the superiority of EES, particularly those with CoCr stent platforms46. When compared to BMS, rates of ST were significantly lower at 1 year (OR: 023; 95% CI: 0.13-0.41) and 2 year follow up (OR: 0.35; 95% CI: 0.17-0.69). These findings are in stark contrast to the widely held notion regarding increased late and very late ST risk associated with DES. Additionally, CoCr-EES were also associated with less ST at 1 year compared to PES (OR: 028; 95% CI: 0.16-0.48), SES (OR: 0.41; 95% CI: 0.24-0.0.70) and ZES (OR: 0.21; 95% CI: 0.10-0.44). The second generation DES, particularly CoCr-EES appear to have improved safety and efficacy profiles compared to not only BMS but also the first generation DES.
Bioabsorbable polymers
The biocompatible polymer coatings on the first and second generation DES are effective in facilitating anti-proliferative drug release. It is thought that the more biocompatible polymers on the second generation DES offer less inflammation and a greater degree of re-endothelialization compared with the first generation DES. Despite these improvements, the second generation polymers persist for many years and may play a significant role in the occurrence of late and very late ST events47–49. Because of this, extensive research is currently underway in the development of fully bioabsorbable polymers that not only offer control of drug delivery but also avoid the long-term issues related to the presence of residual polymers. There have been many biodegradable polymers developed over the last few years, but questions and controversy remain regarding if this technology will result in improved clinical outcomes.
The all-comers Limus Eluted from a Durable Versus Erodible Stent Coating (LEADERS) trial4950, evaluated the BioMatrix stent (Biosensors International, Singapore), which utilizes a sirolimus analogue (Biolimus A9) and a biodegradable polylactic acid (PLA) polymer that completely dissolves over a 6 to 9 month period. This trial, which enrolled 1707 patients, demonstrated that the BioMatrix stent was non-inferior to Cypher SES in the area of MACE, (10.6 vs. 12.0%; P=0.37)5051. A second trial, NEVO RES-ELUTION I52, evaluated the NEVO sirolimus-eluting stent system with a bioabsorbable polylactic-glycolic acid (PLGA) polymer (Cordis, Bridgewater, NJ, USA) that completely degrades in 3 to 4 months. This study, which randomized patients to either the NEVO SES or Taxus Liberte PES, demonstrated significantly lower rates of in-stent late loss in patients treated with the bioabsorbable polymer (0.13 ± 0.31 vs. 0.36 ± 0.46 mm; P<0.001)52.
Additionally, the recently published Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents-4 (ISAR TEST 4) trial reported outcomes among 2603 patients randomized to receive either a SES with a novel bioabsorbable polymer, a standard Cypher SES or a Xience V EES. Three-year follow up data found non-inferiority in the combined endpoints of cardiac death, target vessel MI or TLR in the bioabsorbable polymer compared to Cypher or Xience V stents (20.1 vs. 20.9%; P=0.59). There also was no statistically significant difference in the endpoints of definite/probable ST (1.2 vs. 1.7%; P=0.32) or TLR (13.9 vs. 14.2%; P=0.79)5354 (Fig. 3).
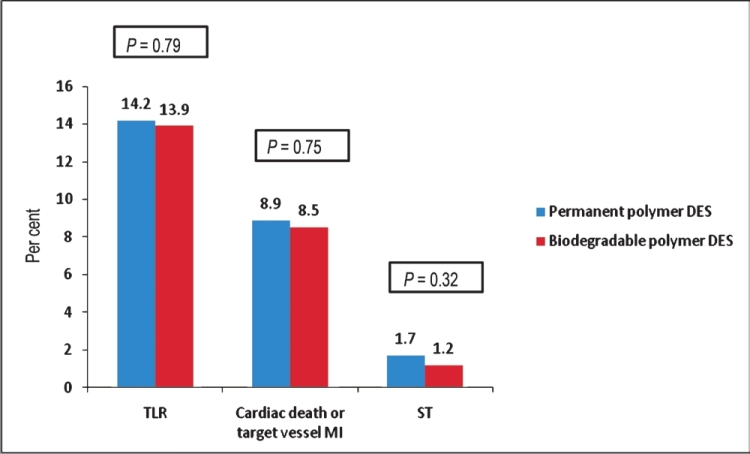
- Thirty six month follow up comparing permanent polymer DES with biodegradable polymer DES in the end-points of target lesion revascularization (TLR), cardiac death or target vessel MI or stent thrombosis (ST) from the ISAR-TEST 4 Trial. Source: Ref. 53.
Newer technology including polymer-free and fully bioabsorbable DES are currently under various stages of development with future studies upcoming. This future step forward represents the next generation of DES and offer the possibility of minimizing ST risk and the need for long-term dual antiplatelet therapy (DAPT), while maximizing revascularization for coronary artery disease.
Antiplatelet therapy
Current guidelines on the use of DES, recommend DAPT consisting of aspirin and a thienopyridine. Clopidogrel, the most widely used thienopyridine, works by inhibiting the P2Y12 platelet receptor, thereby interfering with ADP-induced platelet activation and aggregation55. Multiple studies have demonstrated that treatment with DAPT significantly reduce cardiac events after DES implantation56–59. In addition to clopidogrel, other currently approved thienopyridines include prasugrel and ticagrelor.
One controversy that has come to attention since the development and implementation of DES is related to the optimal duration of DAPT. Initial studies demonstrating the efficacy of the first generation DES maintained clopidogrel therapy for 3 to 6 months after stent implantation89. Soon after these landmark trials were published, case reports began to surface of angiographically documented ST that occurred 343 to 442 days after DES implantation60. In all cases, ST occurred soon after antiplatelet therapy was discontinued60. Further retrospective studies revealed that the discontinuation of clopidogrel, even more than 6 months after DES implantation, was associated with increased ST risk60–63. These findings were supported by results from a substudy of the Basel Stent Kosten Effetivtats Trial- Late Thrombotic Events (BASKET-LATE) trial, which was conducted to determine the incidence of late clinical events in patients treated with DES compared to BMS. After clopidogrel discontinuation, late ST-related events were 2 to 3 times more frequent in the DES group compared to the BMS group64. Based on these studies, the ACCF/AHA/SCAI released guidelines for percutaneous coronary interventions in 2011 that recommend 12 months of thienopyridine treatment after DES implantation65. Currently, no guidelines exist regarding the continuation of thienopyridine therapy in patients with DES beyond 1 year.
Results from several recent trials involving the Endeavor ZES have demonstrated favourable safety and efficacy profiles despite shorter durations of DAPT. In the DATE (Duration of Dual Antiplatelet Therapy after Implantation of Endeavor Stent) Registry, 823 patients were enrolled to either 3 months or greater than 3 months of DAPT after PCI with the Endeavor ZES66. At 1 year follow up, there were no significant difference in the composite endpoint of cardiac death, MI or ST between both treatment groups (0.5 vs. 0.7%; P=0.93). The results of the larger RESET (Real Safety and Efficacy of a 3-Month Dual Antiplatelet Therapy Following Zotarolimus-Eluting Stent Implantation) trial, which assessed shorter durations of DAPT was presented at the American College of Cardiology (ACC) 2012 Scientific Sessions67. This study, which randomized 2148 patient undergoing PCI with ZES to either 3 months or 12 months of DAPT, found no significant difference in the composite endpoint of all-cause mortality, MI or ST (1.3 vs. 0.8%; P=0.48)67. Additional findings noted no difference in rates of ST (0.2 vs. 0.3%; P=0.65), MI (0.2 vs. 0.4%; P=0.41), TVR (3.9 vs. 3.7%; P=0.70) or major/minor bleeding (0.5 vs. 1.0%; P=0.20) between both the short (3 months) and standard (12 month) duration of DAPT. The results of these studies suggest that a shorter duration of DAPT may be appropriate in relatively lower risk patients who undergo stenting with ZES. These findings have important implications especially in patients with increased bleeding risks, medication compliance issues or those who require future surgery or procedures that require interruption of DAPT.
In order to better understand the optimal duration of antiplatelet therapy, Mauri and colleagues are currently conducting a trial that will randomize over 15,000 patients to receive either 12 or 30 months of aspirin plus thienopyridine therapy after PCI. The goal of the Dual Antiplatelet Therapy (DAPT) study is to determine the safety and effectiveness of continuing antiplatelet therapy beyond the recommended 12 month duration68. Another study currently in progress utilizes platelet responsiveness testing in order to determine the optimal duration of antiplatelet therapy68. The goal of the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) registry will be to determine the relationship between platelet responsiveness testing using the VerifyNow P2Y12 assay and the occurrence of late and very late ST events in patients who have continued or discontinued thienopyridine and aspirin therapy after DES implantation. The 30 day results of the ADAPT-DES registry were recently presented at the 2011 Transcatheter Cardiovascular Therapeutics (TCT) Scientific Symposium69. At 30 days, the absolute and relative levels of platelet responsiveness were independent predictors of ST with a significant number of events directly attributed to clopidogrel hypo-responsiveness. Given the generally low levels of ST along with the relatively modest sensitivity and specificity of platelet function assays, the authors concluded that platelet responsiveness was unlikely to provide information to guide decision making for the prevention of ST at 30 days.
Other antiplatelet agents currently under investigation include the intravenous P2Y12 receptor antagonist, cangrelor and the protease-activated receptor (PAR-1) inhibitor, vorapaxar. Pharmacologic studies of cangrelor demonstrate immediate and potent platelet inhibition70. Because of a relatively short half-life (3-6 min), this agent has the added benefit of rapid reversibility71. The safety and efficacy of cangrelor was recently assessed in two trials that resulted from the CHAMPION (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) program. The CHAMPION PLATFORM trial randomized 5362 patients presenting with ACS to intravenous cangrelor or placebo at the time of PCI71. All patients received 600 mg of clopidogrel, in addition to standard MI care. At 48 h, there was no significant difference in the composite endpoint of death, MI or ischaemia-driven revascularization between both treatment groups (7.0 vs. 8.0%; P= 0.17). The cangrelor group however, demonstrated lower rates of the pre-specified secondary endpoints of ST (0.2 vs. 0.6%; OR: 0.31; 95% CI 0.11-0.85; P=0.02) and all-cause mortality (0.2 vs. 0.7%; OR: 0.33; 95% CI 0.13-0.83; P=0.02) with no difference in the rate of blood transfusions (0.6 vs. 1.0%; P=0.13). Complimentary to the CHAMPION PLATFORM, the CHAMPION PCI trial randomized 8877 patients to intravenous cangrelor or oral clopidogrel (600 mg) prior to PCI72. At 48 h, there was no difference in the composite endpoint of death, MI or ischaemia-driven revascularization (7.5 vs. 7.1%; OR: 1.05; 95% CI 0.88-1.24; P=0.59), need for blood transfusions (1.1 vs. 1.0%; P=0.68), groin hematoma >5 cm (1.9 vs. 1.7%; P=0.48) or Thrombolysis in Myocardial Infraction (TIMI) major bleeding (0.4 vs. 0.3%; P=0.35) between both groups. One of the controversies regarding the results of the CHAMPION PLATFORM and CHAMPION PCI trials was with the challenges identifying post-procedure MIs. Because of this, White and colleagues71 performed a pooled analysis of the CHAMPION trials using the universal MI definition73. This definition requires stable cardiac biomarkers prior to PCI with a new or recurrent myocardial infarction identified by a subsequent rise in cardiac troponin, creatine kinase (CK) or CK-MB isozyme >3 times the 99th percentile upper limit of normal. Using this universal definition, cangrelor was associated with a significant reduction in the composite endpoint of death, MI or ischaemia-driven revascularization at 48 h compared to a 600 mg loading dose of clopidogrel given either before or early after PCI in patients presenting with acute coronary syndrome (ACS) (OR: 0.82; 95% CI 0.68-0.99; P=0.037). Additionally, there was a significant reduction in ST (OR: 0.44; 95% CI 0.22-0.87; P=0.018) with no difference in TIMI major bleeding (0.3 vs. 0.3%; OR: 1.0; 95% CI 0.56-1.78; P=0.99) or need for blood transfusions (1.0 vs. 0.8%; OR: 1.24; 95% CI 0.88-1.76; P=0.22).
Given the short half-life and rapid reversibility of cangrelor, there has been substantial interest in using this antiplatelet agent prior to surgery. In order to assess the effect of cangrelor for bridging thienopyridine-treated patients awaiting cardiac surgery, Angiolillo and colleagues performed an open-label, prospective, randomized trial comparing this intravenous P2Y12 receptor antagonist to placebo in patients with ACS or treated with a coronary stent awaiting coronary artery bypass graft (CABG)75. The results of the BRIDGE (Maintenance of Platelet Inhibition with Cangrelor after Discontinuation of Thienopyridines in Patients Undergoing Surgery) Trial, reported a significantly greater degree of platelet inhibition in the cangrelor group with no difference in major bleeding prior to CABG. Cangrelor, although not yet FDA approved, has the potential to provide potent platelet inhibition, especially in patients unable to take oral thienopyridines or those requiring rapid reversibility prior to early CABG or other surgery71–73.
Vorapaxar, a PAR-1 inhibitor represents a novel class of antiplatelet agent that inhibits thrombin-mediated platelet activation. Preclinical and phase 2 studies in patients with CAD support the potential of these drugs to improve clinical outcomes75. The results of the large, multinational, double blind, placebo-controlled trial evaluating vorapaxar in the prevention of adverse cardiac events was recently presented at the ACC 2012 Scientific Sessions76. The TRA 2P-TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events- Thrombolysis in Myocardial Infarction 50) trial, involved 26,449 patients with underlying cardiovascular disease. At 3 years, patients treated with vorapaxar demonstrated a significant reduction in the composite endpoint of cardiovascular death, MI or stroke compared to placebo (9.3 vs. 10.5%; HR: 0.87; 95% CI 0.80-0.94; P<0.001). Treatment with vorapaxar however, resulted in significantly more bleeding events compared to the placebo (4.2 vs. 2.5%; HR 1.66; 95% CI 1.43-1.93; P<0.001)77. A second trial assessing vorapaxar in the treatment of ACS found no significant difference in the composite endpoint of cardiovascular death, MI, stroke, recurrent ischaemia or urgent coronary revascularization compared to placebo (18.5 vs. 19.9%; HR: 0.92; 95% CI 0.85-1.01; P=0.07). Rates of moderate and severe bleeding were additionally higher in the vorapaxar group compared to the control group (7.2 vs. 5.2%; HR: 1.35; 95% CI 1.16-1.58; P<0.001)78. The specific role of PAR-1 inhibitors in the era of DES are currently under investigation and offer a potential to expand treatment options for patients undergoing coronary intervention. Future large scale trials are needed to define the specific role of vorapaxar and other PAR-1 receptor inhibitors.
Genetic testing
Clopidogrel is a prodrug, activated by cytochrome P450 2C19 (CYP2C19) to an active metabolite that inhibits ADP-induced platelet aggregation80. Pharmacogenetic and pharmacodynamic studies have demonstrated wide inter-individual variability in the metabolism and degree of platelet inhibition associated with clopidogrel79–82. Additionally, many genetic variations affecting clopidogrel metabolism have been discovered83–86. It is estimated that 5 to 30 per cent of all patients are poor metabolizers of clopidogrel, which could lead to adverse events after stent placement8087. Patients with high on-treatment platelet reactivity despite clopidogrel, therefore, may have an increased risk of cardiovascular events including ST after PCI88.
To evaluate this concern, Mega and colleagues performed a meta-analysis that identified nine genetic studies evaluating the CYP2C19 genotype in patients treated with clopidogrel89. Among the 9685 patients included in this study, 6937 (71.5%) were non-carriers, 2544 (26.3%) had one reduced-function CYP2C19 allele and 218 (2.2%) had two reduced function CYP2C19 alleles. Compared to non-carriers, patients with one reduced function allele had a significantly increased risk of the composite endpoint of cardiovascular death, myocardial infarction or stroke (HR: 1.55; 95% CI: 1.11-2.17; P=0.01). In patients who carried two reduced function alleles, there was an even greater risk of the composite endpoint compared to non-carriers (HR: 1.76; 95% CI: 1.24-2.50; P=0.002). In terms of ST, carriers of one reduced function CYP2C19 allele (HR: 2.67; 95% CI: 1.67-4.22; P=0.001) and carriers of two alleles (HR: 3.97; 95% CI: 1.75-9.02; P=0.001) were at significantly increased risk for ST when compared to non-carriers. Based on this meta-analysis and other studies that identified an increased cardiovascular risk associated with carriers of the CYP2C19 genotype, the FDA issued an alert recommending the consideration of CYP2C19 genetic testing in patients undergoing PCI and clopidogrel therapy90.
To further appraise the data on the association between clopidogrel responsiveness and CYP2C19 polymorphisms, Holmes and colleagues performed a systematic review and meta-analysis that came to different conclusions from the study by Mega et al91. This study identified 32 trials that included 42,016 patients who underwent genetic testing for the loss of function CYP2C19 genotype. In a treatment-only analysis of 22 prospective observational studies and four randomized trials, individuals with any copy of a reduced function CYP2C19 allele had a significantly increased risk of cardiovascular events (RR: 1.18; 95% CI: 1.09-1.28). Due to evidence of small study bias, the analysis was restricted to larger studies (>200 outcome events), with subsequent analysis demonstrating a smaller and non-significant effect associated with the CYP2C19 genotype (RR: 0.97; 95% CI: 0.86-1.09). Of all clinical outcomes, ST was most strongly associated with one or more loss of function CYP2C19 alleles (RR: 1.75; 95% CI: 1.5-2.03). The authors concluded that although an association between the CYP2C19 genotype and responsiveness to clopidogrel exists, there was no association between this polymorphism and cardiovascular events with the exception of ST.
These results appear to contradict the earlier meta-analysis published by Mega et al, and has subsequently generated considerable controversy regarding the interpretation and conclusions reached. Some of the major concerns relate to the inclusion of a large number of patients who did not undergo coronary stenting, the varied clopidogrel dosing (particularly loading dose) and the clinical endpoints included in this meta-analysis. The decreased emphasis on ST risk was also a problem to some, given the devastating consequences of ST. Although the absolute risk increase of 14 events per 1000 individuals in clopidogrel non-responders may seem relatively low, if this number is extrapolated to all the patients undergoing PCI, there could be thousands of additional ST events per year92. Given these conflicting findings and to assist healthcare providers incorporate the FDA warning into clinical practice, the ACC and AHA released a clinical alert that argued against routine genetic testing prior to clopidogrel initiation due to lack of evidence93. These recommendations differ from the FDA advisory and therefore, the use of genetic testing to identify individuals with the CYP2C19 genotype remains an area of uncertainty in the field of cardiology.
Platelet function testing
Several small studies have suggested that platelet reactivity assays are superior to genetic testing in identifying patients who are poor clopidogrel responders94. These assays utilize light transmission aggregometry (LTA) to test platelet aggregation and activation in response to an ADP agonist95. The POPULAR (Do Platelet Function Assays Predict Clinical Outcomes in Clopidogrel- Pretreated Patients Undergoing Elective PCI) study was designed to evaluate the ability of six platelet function tests to predict thrombotic events including ST, in clopidogrel treated patient undergoing coronary interventions. Of the assays evaluated, high on-treatment platelet reactivity evaluated by the VerifyNow P2Y12 (Accumetrics, San Diego, CA, USA), Plateletworks (Helena Laboratories, Beaumont, TX, USA) and Innovance PFA P2Y system (Siemens, Tarrytown, NY, USA), were associated with the composite endpoint of all-cause mortality, non-fatal MI, ST and ischaemic CVA96.
Further evaluation of the efficacy of platelet function assays were carried out by Brar and colleges, who identified six studies utilizing the VerifyNow P2Y12 assay shortly after percutaneous intervention97. After grouping platelet reactivity unit (PRU) values into quartiles (quartile I representing the lowest on-treatment platelet reactivity and quartile IV representing the highest on-treatment platelet reactivity), pair-wise comparisons were performed using quartile I as the reference. Subjects with the highest on-treatment PRU values (quartile III and quartile IV), demonstrated the greatest risk of the composite endpoint of death, MI or ST (quartile III- HR: 1.82; 95% CI: 1.2-2.75 and quartile IV- HR 2.62; 95% CI: 1.77-3.87). When analysis was performed on a continuous scale, every 10 unit increase in PRU value was associated with a 4 per cent increase in the composite endpoint. In terms of ST, event rates were significantly greater in quartile IV compared to quartile I (3.4% vs. 0.4%; P=0.002). Utilizing receiver operating characteristic (ROC) analysis, a PRU value of 230 units or more was most accurate in predicting the composite endpoint of death, MI or ST. When this value was used to group patients into high (PRU ≥ 230) or normal (PRU <230) on-treatment platelet reactivity, the high reactivity group had significantly greater event rates (HR: 2.10; 95% CI: 1.62-2.73, P<0.0001) and mortality rates (HR: 1.66; 95% CI: 1.50-6.46; P=0.002) when compared to the normal platelet reactivity group. This increased risk associated with high on-treatment platelet reactivity remained significant, even after adjusting for age, gender, the presence of ACS or stent choice (DES or BMS)94.
The GRAVITAS (Gauging Responsiveness with A VerifyNow Assay- Impact on Thrombosis and Safety) trial was designed to determine whether increasing the maintenance dose of clopidogrel can overcome platelet non-responsiveness based on the VerifyNow assay98. This trial, randomized 2214 patients undergoing PCI with high on-treatment platelet reactivity (PRU ≥ 230) to standard (75 mg daily) versus high (150 mg daily) clopidogrel maintenance dosing. At 6 month follow up, the reduction in platelet reactivity based on the VerifyNow assay was significantly greater in the high clopidogrel dosing group compared to standard clopidogrel dosing group (80 vs. 37 PRU; P<0.0001). Despite the reduction in platelet reactivity, there was no significant difference in the composite endpoint of cardiovascular death, MI or ST (2.3 vs. 2.3%; P=0.98).
These findings were supported by the TRIGGER PCI (Testing Platelet Reactivity in Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy with Prasugrel) trial, which was presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2011 Scientific Session99. This trial, which randomized patients with high on-treatment platelet reactivity undergoing PCI to receive either standard clopidogrel dosing or the novel, more potent P2Y12 receptor antagonist, prasugrel (10 mg daily), was terminated early due to a paucity of clinical events. After 6 months, only one clinical endpoint, a periprocedural MI, was observed.
Both the GRAVITAS and TRIGGER PCI trials have important limitations to keep in mind when analyzing the impact of platelet function testing in coronary interventions. In the GRAVITAS trial, event rates for both the standard and high clopidogrel dosing groups were significantly lower than predicted, which led to underpowering for the composite endpoint of death, MI or ST. Additionally, the TRIGGER PCI trial was stopped after only one clinical event was observed at the time of the first interim analysis. These findings suggest that higher risk patients, such as those undergoing complicated or unsuccessful PCI procedures, were excluded from enrollment in the GRAVITAS and TRIGGER PCI trials. The follow up duration in these studies were also likely too short to identify clinically meaningful differences in endpoints. In the patient level meta-analysis by Brar and colleagues, Kaplan-Meier curves did not appear to diverge until after 180 days98. Endpoints from the GRAVITAS and TRIGGER PCI trials were obtained at 180 days, which may be too early to detect significant differences in event rates. These observations suggest that the design of future trials assessing the modification of antiplatelet therapy based on platelet function testing, should consider larger cohorts, longer follow up duration and the recruitment of higher-risk patients.
Analogous to INR monitoring for warfarin treatment, there may be a therapeutic range which best balances the efficacy and safety of dual antiplatelet therapy after PCI. The Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty- Platelet Reactivity Predicts Outcome (ARMYDA-PRO) trial and other studies have established efficacy thresholds of platelet inhibition to minimize cardiac events after coronary interventions (240 PRU by the VerifyNow assay)100–103. These values are similar to the 230 PRU cut-off identified by Brar and colleagues in the previously mentioned patient-level meta-analysis99. In the future, platelet function testing may be used to identify individuals who are poor metabolizers of clopidogrel and therefore, at increased risk for adverse cardiovascular events. Conversely, testing could identify individuals with an enhanced clopidogrel response, which could lead to increased bleeding complications. Results from ARMYDA study group noted that the 30 day incidence of major bleeding was directly related to pre-PCI platelet reactivity. Using the VerifyNow P2Y12 assay, patients within the lowest quartile of platelet reactivity (high clopidogrel responsiveness) were at 4.5-fold increased risk of major bleeding when compared to the highest quartile of platelet reactivity (lowest clopidogrel responsiveness)104. Using these studies, there may be an ideal range for platelet reactivity that maximizes the benefits while minimizing the risks of antiplatelet therapy after coronary stenting. Additional research is necessary to better understand and incorporate the data from genetic and platelet function testing into clinical practice.
DES in acute myocardial infarction
Based on the findings from the landmark SIRIUS and TAXUS IV trials, the FDA initially approved the use of DES for discrete, de novo lesions in native coronary vessels with diameters of 2.5 to 3.5 mm105106. As more data demonstrating the efficacy of DES became available, the use of this technology for more diverse patient populations and clinical situations rapidly increased. Many of these applications were for “off-label” indications, including the treatment of ACS. There have been concerns however, that the use of DES in this setting may be associated with higher risks of ST61107108. Some of the proposed mechanisms for this phenomenon relate to possible stent placement within a necrotic arterial wall core, thrombus trapping behind the stent struts, delayed endothelialization due to the anti-restenotic drug or significantly increased risks associated with discontinuing dual antiplatelet therapy after acute myocardial infarction6. Although the benefits of DES for FDA-approved indications are well established, whether this applies for primary PCI, particularly in the setting of ST-segment myocardial infarction (STEMI) remains controversial.
As the pivotal DES approval trials excluded patients with AMI, clinical data regarding procedural choices and outcomes remain limited. Previous registry and randomized trials comparing DES to BMS in STEMI, have reported conflicting results regarding late safety profiles and other clinical outcomes. The GRACE (Global Registry of Acute Coronary Events) study, which collected data on 5093 patients with STEMI, reported higher mortality associated with DES compared to BMS during 6 month to 2 year follow up (HR 4.9; P=0.01)109. These observations of increased late mortality with DES questioned the safety of DES during STEMI and led to randomized trials addressing these concerns. The TYPHOON (Trial to Assess the Use of Cypher Sirolimus-Eluting Coronary Stent in Acute Myocardial Infarction Treated with Balloon Angioplasty) trial, randomized 712 patients presenting with STEMI to either SES or BMS during primary PCI. At 4 year follow up, the SES group demonstrated significantly better freedom from TLR (92.4 vs. 85.1%; P=0.002). There was additionally no significant difference in freedom from cardiac death (97.6 vs. 95.9%; P=0.37), freedom from repeat MI (94.8 vs. 95.6%; P= 0.85) or definite/probable ST (4.4 vs. 4.8%; P=0.83)110. The 5 year follow up data from the PASSION (Paclitaxel-Eluting Versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation) trial also demonstrated no significant difference in MACE between PES and BMS108. This trial, which randomized 619 patients to either PES or BMS at the time of primary PCI, noted no significant difference in the composite of cardiac death, recurrent MI or TLR (18.6 vs. 21.8%; HR 0.82; 95% CI: 0.58-1.18; P=0.28) or definite/probable ST (4.2 vs. 3.4%; HR 1.19; 95% CI: 0.51-276; P= 0.68)111. These results differ from the results reported in the DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) trial, which randomized 626 patients presenting with STEMI to receive either DES (SES, PES or ZES) or BMS with or without distal protection during primary PCI. At 3 year follow up, the DES group demonstrated significantly better rates of TLR (6.1 vs. 16.3%; P<0.001) but increased rates of cardiac death (6.1 vs. 1.9%; P=0.01) compared to the BMS group113.
The conflicting results of these trials could be related to limitations arising from the relatively small study sizes and subsequent underpowering to detect low frequency adverse events. The largest randomized trial to date, the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial randomized 3006 patients presenting with STEMI to receive either PES or BMS during primary PCI13. At 12 month follow up, patients who received PES had significantly lower rates of ischaemia-driven TLR (4.5 vs. 7.5%; HR 0.59; 95% CI: 0.43-0.83; P=0.002) and TVR (5.8 vs. 8.7%; HR 0.69; 95% CI: 0.48-0.89; P=0.006) when compared to BMS. Additionally, there was no significant difference in the primary safety endpoint of death, MI, stroke or ST (PES 8.1 vs. BMS 8.0%; HR 1.02; 95% CI: 0.76-1.36; P=0.92), rates of death or re-infarction (PES 6.8 vs. BMS 7.9%; HR 0.97; 95% CI: 0.7-1.32; P=0.83) and ST (PES 3.2 vs. BMS 3.4%; HR 0.93; 95% CI: 0.59-1.47; P=0.77) (Fig. 4). These results were confirmed at 3 year follow up, with patients who received PES demonstrating significantly lower rates of ischaemia driven TLR (9.4 vs. 15.1%; HR 0.60; 95% CI: 0.48-0.76; P<0.0001) compared to BMS. Rates of all-cause mortality (PES 5.6 vs. BMS 6.6%; HR 0.84; 95% CI: 0.6-1.17; P=0.31) and definite or probable ST (PES 4.8 vs. BMS 4.3%; HR 1.10; HR 1.10; 95% CI: 0.74-1.65; P=0.63) were similar in both the PES and BMS cohorts at 3 year follow up113.
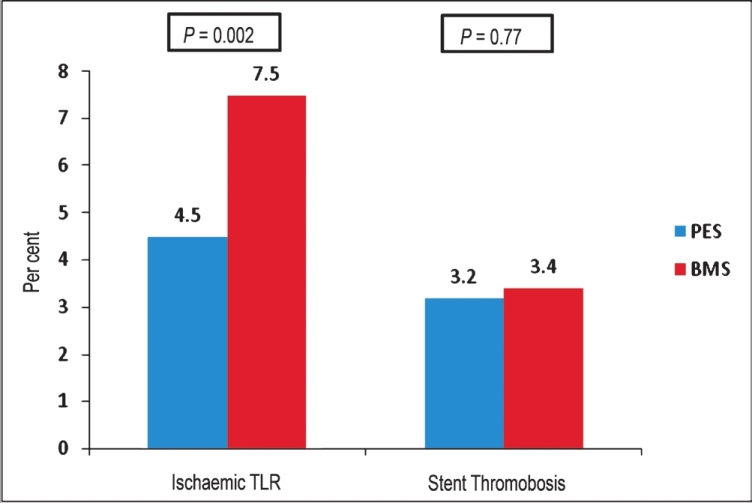
- Twelve month event rates in patients with STEMI treated with PES and BMS from the HORIZONS-AMI trial. Source: Ref. 13.
To better compare the efficacy and safety of DES in STEMI, Kastrati and colleagues performed a meta-analysis, which identified eight randomized trials consisting of 2786 patients114. After a mean follow up of 12 to 24.2 months, patients treated with DES compared to BMS, demonstrated better rates of TLR (HR 0.38; 95% CI: 0.29-0.50; P<0.001), with no significant difference in death (HR 0.76; 95% CI: 0.53-1.10; P=0.14), recurrent MI (HR 0.72; 95% CI: 0.48-1.08; P=0.11) or ST (HR 0.80; 95% CI: 0.46-1.39; P=0.43). A more recent and larger meta-analysis was conducted by Brar and colleagues113, which included 13 randomized trials consisting of 7352 patients and 18 registry studies consisting of 26,521 patients115. At a maximum follow-up of 2 years, patients treated with DES compared to BMS, demonstrated a significantly reduced need for repeat revascularization (5.3 vs. 11.5%; RR 0.44; 95% CI: 0.35-0.55; P<0.001). Additionally, there were no significant differences in terms of mortality (3.7 vs. 4.3%; RR 0.89; 95% CI: 0.70-1.14; P=0.36) or definite/probable ST (2.7 vs. 2.6%; RR 0.96; 95% CI: 0.72-1.30; P=0.81) between the DES and BMS, out to 2 years of follow up. Based on these recently published randomized trials and meta-analyses, DES in the setting of AMI, particularly STEMI, appears both safe and efficacious when compared to BMS.
Based on these findings, on February 22, 2012, the FDA approved both the Taxus Liberte (Boston Scientific) and Taxus ION (Boston Scientific) stents for the treatment of patients presenting with AMI116. Initially approved in 2009 for use in de novo, native vessel coronary lesions, the Taxus Liberte PES, offers thinner struts and a continuous cell architecture that provides a lower profile and greater flexibility117118. The Taxus ION PES on the other hand, was approved in 2011 and is composed of a novel PtCr stent platform that offers increased strength, deliverability and visibility compared to steal or cobalt alloys119. The Taxus Liberte PES and Taxus ION PES are currently the only stents in the U.S. with an approved indication for AMI. Future studies with longer follow up and involving the newer generation DES are required to determine the stability and strength of these findings.
Conclusions
DES have transformed the field of cardiology, allowing for higher risk coronary interventions and an alternative for patients who would otherwise require coronary artery bypass surgery. These stents provide advantages over BMS in the area of improved TLR and decreased rates of repeat revascularization. Some of the concerns and controversies surrounding DES involve the long-term risk of restenosis and ST, especially after DAPT is interrupted. Current and future technology, including the newer generation EES and ZES, biodegradable polymers and even completely biodegradable stents offer the potential for improvement in the care of patients undergoing coronary stenting. Additional areas including “off-label” indications, platelet reactivity assays and genetic testing to detect CYP2C19 polymorphisms have the potential to maximize the benefits of DES while minimizing the risks.
References
- Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030-9.
- [Google Scholar]
- A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:989-97.
- [Google Scholar]
- Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-48.
- [Google Scholar]
- Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998-1008.
- [Google Scholar]
- Current and future drug-eluting coronary stent technology. Expert Rev Cardiovasc Ther. 2011;9:485-503.
- [Google Scholar]
- A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773-80.
- [Google Scholar]
- Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315-23.
- [Google Scholar]
- A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221-31.
- [Google Scholar]
- Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112:3306-13.
- [Google Scholar]
- A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J Am Coll Cardiol. 2007;50:491-7.
- [Google Scholar]
- Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215-23.
- [Google Scholar]
- Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946-59.
- [Google Scholar]
- Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009-19.
- [Google Scholar]
- Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056-61.
- [Google Scholar]
- Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am J Med. 2009;122:581 e1-10.
- [Google Scholar]
- Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation. Five-Year outcome of the j-Cyper Registry. Circulation. 2012;125:584-91.
- [Google Scholar]
- Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
- [Google Scholar]
- Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333-42.
- [Google Scholar]
- Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175-81.
- [Google Scholar]
- Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94:1690-7.
- [Google Scholar]
- Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701-5.
- [Google Scholar]
- Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291-9.
- [Google Scholar]
- In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897-907.
- [Google Scholar]
- Long-term clinical outcomes after drug-eluting and bare-metal stenting in Massachusetts. Circulation. 2008;118:1817-27.
- [Google Scholar]
- Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter German Cypher Stent Registry) Am J Cardiol. 2005;95:1302-8.
- [Google Scholar]
- Very long-term (15 to 20 years) clinical and angiographic outcome after coronary bare metal stent implantation. Circ Cardiovasc Interv. 2010;3:468-75.
- [Google Scholar]
- Angioscopic comparison of neointimal coverage between zotarolimus- and sirolimus-eluting stents. J Am Coll Cardiol. 2008;52:789-90.
- [Google Scholar]
- Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc Interv. 2009;2:1240-7.
- [Google Scholar]
- A prospective, randomized, 6-month comparison of the coronary vasomotor response associated with a zotarolimus- versus a sirolimus-eluting stent: differential recovery of coronary endothelial dysfunction. J Am Coll Cardiol. 2009;53:1653-9.
- [Google Scholar]
- ENDEAVOR I & II Clinical Program Long Term Follow-up. 2005. Presented at Transcatheter Cariovascular Therapeutics (TCT). Available from: http://www.tctmd.com/show.aspx?id=57840
- [Google Scholar]
- Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798-806.
- [Google Scholar]
- In vivo performance of a novel co-polymer system for extended release of zotarolimus in a second generation drug eluting stent. Presented at Transcatheter Therapeutics 2006. Washington D.C. October 22-27. Available from: http://www.tctmd.com/show.aspx?id=55876
- [Google Scholar]
- Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011;57:1778-83.
- [Google Scholar]
- Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136-46.
- [Google Scholar]
- Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet. 2011;377:1241-7.
- [Google Scholar]
- A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59:1350-61.
- [Google Scholar]
- Medtronic Resolute Integrity Drug-Eluting Stent Obtains FDA Approval for Treating Coronary Artery Disease. Feb. 17, 2012. Available from: http://investorrelations.medtronic.com/phoenix.zhtml?c=76126&p=irol-newsArticle&id=1662613
- [Google Scholar]
- A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention. 2005;1:58-65.
- [Google Scholar]
- A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention. 2006;2:286-94.
- [Google Scholar]
- Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903-13.
- [Google Scholar]
- Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663-74.
- [Google Scholar]
- 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial) J Am Coll Cardiol. 2011;58:11-8.
- [Google Scholar]
- Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol. 2011;58:1569-77.
- [Google Scholar]
- Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393-402.
- [Google Scholar]
- Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention. 2009;5:157-65.
- [Google Scholar]
- A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786-95.
- [Google Scholar]
- Polymer-free cerivastatin-eluting stent shows superior neointimal inhibition with preserved vasomotor function compared to polymer-based paclitaxel-eluting stent in rabbit iliac arteries. EuroIntervention. 2010;6:126-33.
- [Google Scholar]
- Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet. 2011;378:1940-8.
- [Google Scholar]
- Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163-73.
- [Google Scholar]
- Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: a randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the TAXUS Liberte paclitaxel-eluting stent in de novo native coronary artery lesions. Circ Cardiovasc Interv. 2010;3:556-64.
- [Google Scholar]
- Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58:1325-31.
- [Google Scholar]
- Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation. 2006;113:273-9.
- [Google Scholar]
- Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol. 2002;39:9-14.
- [Google Scholar]
- Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary stent implantation. Circulation. 1999;99:2364-6.
- [Google Scholar]
- A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation. 2000;101:590-3.
- [Google Scholar]
- Randomized comparison of ticlopidine and clopidogrel after intracoronary stent implantation in a broad patient population. Circulation. 2001;104:539-43.
- [Google Scholar]
- Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519-21.
- [Google Scholar]
- Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-30.
- [Google Scholar]
- Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitats Trial (BASKET) Lancet. 2005;366:921-9.
- [Google Scholar]
- Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352-6.
- [Google Scholar]
- Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584-91.
- [Google Scholar]
- 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44-122.
- [Google Scholar]
- Three-month dual antiplatelet therapy after implantation of zotarolimus-eluting stents: the DATE (Duration of Dual Antiplatelet Therapy AfterImplantation of Endeavor Stent) registry. Circ J. 2010;74:2314-21.
- [Google Scholar]
- A New strategy for discontinuation of dual antiplatelet therapy: Real safety and Efficacy of 3-month dual antiplatelet therapy following zotarolimus-Eluting stent implantation: RESET Trial. Presented at the American College of Cardiology (ACC) Scientific Session 2012. March 24-27. Chicago, IL. Available from: http://www.cardiosource.org/Science-And-Quality/Clinical-Trials/R/RESET-Trial.aspx?w_nav=Search&WT.oss=hong%20RESET&WT.oss_r=11&
- [Google Scholar]
- Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035-41.
- [Google Scholar]
- ADAPT-DES: Assessment of dual antiplatelet therapy with drug-eluting stents. Presented at Transcatheter Cariovascular Therapeutics (TCT) 2011. November 7-11. San Francisco, CA. Available from: http://www.tctmd.com/show.aspx?id=109583
- [Google Scholar]
- The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110:925-34.
- [Google Scholar]
- Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330-41.
- [Google Scholar]
- Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318-29.
- [Google Scholar]
- Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163:182-90 e184.
- [Google Scholar]
- Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012;307:265-74.
- [Google Scholar]
- Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009;373:919-28.
- [Google Scholar]
- TRA 2°P-TIMI 50: Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombosis. Presented at the American College of Cardiology (ACC) Scientific Session 2012. March 24-27. Chicago, IL. Available from: http://www.cardiosource.org/Science-And-Quality/Clinical-Images/_ACC12/TRA-2P-TIMI-50-Presentation-Slides.aspx?w_nav=Search&WT.oss=tra%202p%20timi%2050&WT.oss_r=12%
- [Google Scholar]
- Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404-13.
- [Google Scholar]
- Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20-33.
- [Google Scholar]
- Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92-9.
- [Google Scholar]
- Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908-13.
- [Google Scholar]
- Onset and offset of platelet inhibition after high-dose clopidogrel loading and standard daily therapy measured by a point-of-care assay in healthy volunteers. Am J Cardiol. 2006;98:681-4.
- [Google Scholar]
- Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation. 2005;112:2946-50.
- [Google Scholar]
- Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429-36.
- [Google Scholar]
- Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735-41.
- [Google Scholar]
- Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J Thromb Haemost. 2007;5:2153-5.
- [Google Scholar]
- Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244-7.
- [Google Scholar]
- Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003;89:783-7.
- [Google Scholar]
- Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919-33.
- [Google Scholar]
- Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821-30.
- [Google Scholar]
- US Food and Drug Administration. FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. March 12, 2010. Retrieved January 15, 2012. Available from: www.fda.gov/drugs/drugsafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm
- [Google Scholar]
- CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704-14.
- [Google Scholar]
- An important miscue in clopidogrel pharmacogenomics. 2011. Available from: http://blogs.theheart.org/topolog/2011/12/27/miscue-inclopidogrel-pharmacogenomics
- [Google Scholar]
- ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122:537-57.
- [Google Scholar]
- Level of adenosine diphosphate receptor P2Y12 blockade during percutaneous coronary intervention predicts the extent of endothelial injury, assessed by circulating endothelial cell measurement. J Am Coll Cardiol. 2010;56:1024-31.
- [Google Scholar]
- Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754-62.
- [Google Scholar]
- Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58:1945-54.
- [Google Scholar]
- Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097-105.
- [Google Scholar]
- TRIGGER-PCI: A Prospective, Randomized Trial of Prasugrel vs Clopidogrel in Clopidogrel-Hyporesponsive Patients with Stable Ischemic Heart Disease Undergoing Percutaneous Coronary Intervention. Presented at Transcatheter Cariovascular Therapeutics (TCT) 2011. November 7-11. San Francisco, CA. Available from: http://www.tctmd.com/show.aspx?id=108799
- [Google Scholar]
- Point-of-care assessment of platelet reactivity after clopidogrel to predict myonecrosis in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2010;3:318-23.
- [Google Scholar]
- Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237-42.
- [Google Scholar]
- Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52:1128-33.
- [Google Scholar]
- Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29:992-1000.
- [Google Scholar]
- Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study) Am J Cardiol. 2011;107:995-1000.
- [Google Scholar]
- U.S. Food And Drug Administration. CYPHER Sirolimuseluting coronary stent- P020026 approval letter, April 24, 2003. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf3/P020026a.pdf
- [Google Scholar]
- U.S. Food And Drug Administration. TAXUS Express2 Paclitaxel-Eluting Coronary Stent System- P030025 approval letter, March 4, 2004. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf3/p030035a.pdf
- [Google Scholar]
- Trading restenosis for thrombosis. New questions about drug-eluting stents? N Engl J Med. 2006;355:1949-52.
- [Google Scholar]
- Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573-83.
- [Google Scholar]
- Mortality following placement of drug-eluting and bare-metal stents for ST-segment elevation acute myocardial infarction in the Global Registry of Acute Coronary Events. Eur Heart J. 2009;30:321-9.
- [Google Scholar]
- Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty) JACC Cardiovasc Interv. 2011;4:14-23.
- [Google Scholar]
- 5-year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction: a follow-up study of the PASSION (Paclitaxel-Eluting Versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation) trial. JACC Cardiovasc Interv. 2011;4:24-9.
- [Google Scholar]
- Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2010;56:641-5.
- [Google Scholar]
- Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377:2193-204.
- [Google Scholar]
- Meta-analysis of randomized trials on drug-eluting stents vs.bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28:2706-13.
- [Google Scholar]
- Use of drug-eluting stents in acute myocardial infarction: a systematic review and meta-analysis. J Am Coll Cardiol. 2009;53:1677-89.
- [Google Scholar]
- Boston Scientific. Boston Scientific receives industry's first FDA approval for drug-eluting coronary stent use in heart appack patients [press release]. February 22, 2012. Available from: http://www.bostonscientific.mediaroom.com/index.php?s=24889&item=122390
- [Google Scholar]
- U.S. Food and Drug Administration. TAXUS Liberte Atom (2.25 mm) Paclitaxel-Eluting Coronary Stent System (Monorail and Over-the Wire Delivery Systems)- P060008/S008 approval letter, May 21, 2009. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf6/p060008S008a.pdf
- [Google Scholar]
- U.S. Food and Drug Administration. TAXUS Liberte Long (2.75-4.00 mm × 38 mm) Paclitaxel-Eluting Coronary Stent System (Monorail and Over-the Wire Delivery Systems)-P060008/S011 approval letter, July 13, 2009. Available from: http://www.accessdata.fda.gov/ cdrh_docs/pdf6/p060008S011a.pdf
- [Google Scholar]
- U.S. Food and Drug Administration. ION™ Paclitaxel-Eluting Coronary Stent System - P100023 approval letter, April 22, 2011. Available from: http://www.accessdata.fda.gov/ cdrh_docs/pdf10/p100023a.pdf
- [Google Scholar]






