Translate this page into:
Contribution of insulin resistance to decreased baroreceptor sensitivity & cardiometabolic risks in pre-obesity & obesity
For correspondence: Dr Gopal Krushna Pal, Department of Physiology, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry 605 006, India e-mail: drgkpal@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Although insulin resistance (IR) is a known complication in obesity, the physiological mechanisms linking IR with cardiometabolic risks in obesity have not been well studied. This study was conducted to assess the difference in cardiovascular (CV) risk profile in IR and non-IR (NIR) conditions, and contribution of IR to cardiometabolic risks in pre-obese and obese individuals.
Methods:
Basal CV, blood pressure variability, autonomic function test and cardiometabolic parameters were recorded in pre-obese (n=86) and obese (n=77) individuals during 2012 and 2015. The association of altered cardiometabolic parameters with homeostatic model for IR (HOMA-IR) in pre-obese and obese groups and with baroreceptor sensitivity (BRS) in IR and NIR groups was calculated by appropriate statistical analysis.
Results:
Decreased BRS, a known CV risk and cardiometabolic parameters were significant in IR (pre-obese and obese) group compared to the NIR group. Sympathovagal imbalance in the form of increased sympathetic and decreased parasympathetic activities was observed in individuals with IR. There was no significant difference in the level of independent contribution of HOMA-IR to cardiometabolic parameters in pre-obese and obese groups. Adiponectin and inflammatory markers had an independent contribution to BRS in IR group.
Interpretation & conclusions:
Findings of the present study demonstrated that the intensity of cardiometabolic derangements and CV risk were comparable between IR, pre-obese and obese individuals. Pro-inflammatory state, dyslipidaemia and hypoadiponectinaemia might contribute to CV risk in these individuals with IR. IR could possibly be the link between altered metabolic profile and increased CV risks in these individuals independent of the adiposity status.
Keywords
Baroreceptor sensitivity
cardiometabolic risks
insulin resistance
non-insulin resistance
obesity
pre-obesity
Obesity has become a global epidemic and is among the important risk factors contributing to the overall disease burden1. Obesity is associated with metabolic complications and cardiovascular (CV) morbidities and mortality234. Insulin resistance (IR) has been reported to be a critical mediator in the association between obesity and its co-morbidities including pro-inflammatory state, dyslipidaemia, diabetes, hypertension and CV diseases5. Presence of IR, which is strongly associated with cardiometabolic problems, has been used to categorize individuals at high risk for future CV morbidities45. South-Asians have been reported to be more insulin resistant compared to their Caucasian counterparts6. However, the impact of IR on cardiometabolic risk factors in normoglycaemic, normotensive obese adult Indian population has not been established.
Increased sympathetic activity is one of the established pathophysiological mechanisms for CV diseases associated with obesity7. Sympathovagal imbalance (SVI) in the form of increased sympathetic activity and reactivity and decreased parasympathetic activity and reactivity has been reported in obesity8. Decreased heart rate variability (HRV) representing the autonomic dysfunction has been associated with increased CV morbidity9. Further, studies have reported significant improvement in the indices of HRV with decreased IR in obese individuals independent of the degree of obesity1011. However, the difference in the nature and magnitude of SVI among age and body mass index (BMI) matched obese individuals, as distinguished by their IR status has not been clearly elucidated.
Decreased baroreceptor sensitivity (BRS) has been documented as a marker for risk stratification in patients with cardiac diseases12 including myocardial infarction13 and heart failure14. Although studies have reported decreased BRS in individuals with metabolic syndrome and IR15, the status of BRS in IR and non-IR (NIR) pre-obese and obese individuals has not been assessed. Therefore, present study was aimed to assess the association of decreased BRS with cardiometabolic risk factors in IR and NIR young obese adults. Although IR is considered to be the link between obesity and its associated co-morbidities such as diabetes, hypertension and other CV risks, reports suggest that obese individuals can also be insulin sensitive1617. Therefore, the present study was also aimed to assess the difference in CV risks between the IR and NIR, pre-obese and obese individuals, and the contribution of IR to CV risk profile in these individuals.
Material & Methods
This cross-sectional study was conducted in the department of Physiology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. The study protocol was approved by the Research and Ethics Committees of JIPMER, and written informed consent was obtained from all the participants before initiation of the study.
The sample size was calculated using OpenEpi, version 3 (www.OpenEpi.com), open source calculator. Based on the previous report18, the sample size was calculated with 90 per cent power to detect the difference (2.44) between means and standard deviation (SD) of 2.23 in group 1 and 1.81 in group 2 of homeostatic model for IR (HOMA-IR) with a significance level of (alpha) 0.05. The minimum sample size was calculated to be 15 in each group. However, since BPV indices including BRS and HRV are highly variable parameters even in healthy population, the sample size was increased to above 35 in the present study.
Inclusion & exclusion criteria: A total of 163 apparently healthy young adults having BMI 23.00 kg/m2 or above and aged between 18 and 40 yr were enrolled from among the attendants or relatives of patients who had accompanied patients attending the Medicine OPD, JIPMER and were willing voluntarily to be the participants in the study from 2012 to 2015. Those on antihypertensive therapy or receiving any medication, with a history of smoking and/or alcoholism, with acute or chronic ailments and known cases of diabetes mellitus, hypertension, cardiac diseases, kidney disease or any endocrinal disorder were excluded. As the level of physical fitness is a major determinant of vagal tone, those performing regular athletic activities, body-building exercises and yoga1920 were also excluded.
Grouping of participants: Height and body weight were measured, and BMI was calculated.
Fasting blood glucose (FBG) and serum insulin were measured to calculate IR21. The 163 participants, based on the BMI classification of the WHO for Asian population22 and HOMA-IR values, were divided into following four groups:
-
(i)
Pre-obese NIR group: Participants having BMI 23.00 - 27.49 kg/m2 and HOMA-IR <2.5 (n=49);
-
(ii)
Pre-obese IR group: Participants having BMI 23.00 - 27.49 kg/m2 and HOMA-IR ≥2.5 (n=37);
-
(iii)
Obese NIR group: Participants having BMI 27.50 kg/m2 or above and HOMA-IR <2.5 (n=41);
-
(iv)
Obese IR group: Participants having BMI 27.50 kg/m2 or above and HOMA-IR ≥2.5 (n=36).
Brief procedure: All the participants reported to the autonomic function testing laboratory between 0800-0900 h for the following recordings:
Baseline cardiovascular parameters and baroreceptor sensitivity: After 10 min of supine rest, BRS and other CV parameters such as basal heart rate (BHR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, interbeat interval, stroke volume, cardiac output and total peripheral resistance were measured by continuous blood pressure variability method using Finapres (Finometer version 1.22a; Finapres Medical Systems BV, Amsterdam, the Netherlands) based on the principle of Penaz and Wesseling23. Rate-Pressure Product (RPP), a determinant of myocardial oxygen consumption and workload were calculated using the formula, RPP = 10–2 × (BHR×SBP)24.
Recording of heart rate variability (HRV) and conventional autonomic function test (CAFT): For the recording of short-term HRV, the recommendation of the Task Force on HRV was followed25, using BIOPAC MP 100 data acquisition system (BIOPAC Inc., USA) following the method as described earlier26. Different frequency domain indices (FDI) such as total power (TP), low frequency (LF) component expressed as normalized unit (LFnu), high frequency (HF) component expressed as normalized unit (HFnu) and LF/HF ratio; and time domain indices (TDI) such as mean RR, square root of the mean squared differences of successive normal to normal intervals (RMSSD), standard deviation of normal to normal interval (SDNN) and the number of interval differences of successive NN intervals >50 ms (NN50) were recorded.
Following three conventional autonomic function tests (CAFT) were performed using the standard procedures as described earlier27: (i) Lying to standing test (30:15 ratio); (ii) Deep breathing test (E:I ratio); and (iii) Isometric handgrip test (ΔDBPIHG).
Measurement of biochemical parameters: Fasting blood sample (10 ml) was collected from all participants. FBG was estimated by colorimetric, enzymatic method with glucose oxidase and peroxidase (Genuine Biosystem; Chennai) and insulin was measured using enzyme-linked immunosorbent assay (ELISA) method (DiaMetra, Italy). HOMA-IR was calculated using the formula (HOMA-IR = FBG (mMol) × Insulin (μIU/l)/22.5) and for insulin sensitivity, HOMA 2 per cent S was calculated28.
Lipid profile such as total cholesterol (TC), triglycerides, high-density lipoproteins (HDL), serum total proteins, serum albumin and globulin was assessed using fully automated analyzer (AU400, Olympus, USA). Low-density lipoproteins (LDL) and very LDL were calculated using Friedwalds equation8. Atherogenic index (AI) was calculated using the formula: AI=(TC-HDL)/HDL8.
High-sensitive C-reactive protein (hsCRP) and leptin were quantified using the commercial kits available from DBC Diagnostics Biochem Canada Inc., Canada, and the serum neopterin concentration was quantified using the ELISA kit from DRG, USA. Other inflammatory markers such as interleukin 6 (IL-6), tumour necrosis factor alpha (TNF-α), interferon gamma and adiponectin were estimated using ELISA kits from Orgenium, Tiilitie, Finland. The inter- and intra-assay coefficients of variation for measuring biochemical parameters such as serum insulin, adiponectin, leptin and inflammatory markers were found to be <10 per cent.
Total antioxidant (TAO) and oxidant thiobarbituric acid reactive substance (TBARS) levels were estimated using ELISA kits available from Cayman Chemical Co., Ann Arbor, Michigan.
Statistical analysis: SPSS version 13 (SPSS Software Inc., Chicago, IL, USA) was used for statistical analysis. All the data were presented as mean±SD. Normality of data was tested by Kolmogorov–Smirnov test. The level of significance between the groups was tested using one-way ANOVA, and Tukey Krammer post hoc test was used for inter-group comparison. The independent contribution of various factors to HOMA-IR (in pre-obese and obese groups) and BRS (in IR and NIR groups) was assessed by multiple linear regression analysis.
Results
There was no significant difference in age (mean age 28.50 yr) and FBG between the groups (Tables I and II). Further, the BMI was not significantly different between the IR and NIR groups in both the pre-obese and obese participants (Table I). Insulin levels and HOMA-IR was found to be significantly higher, in IR participants compared to NIR participants in both pre-obese and obese groups and IR obese group compared to IR pre-obese group (Table II). The basal CV parameters such as BHR and RPP were found to be significantly increased, and BRS was decreased in IR group compared to NIR group in both pre-obese and obese participants (Table I).
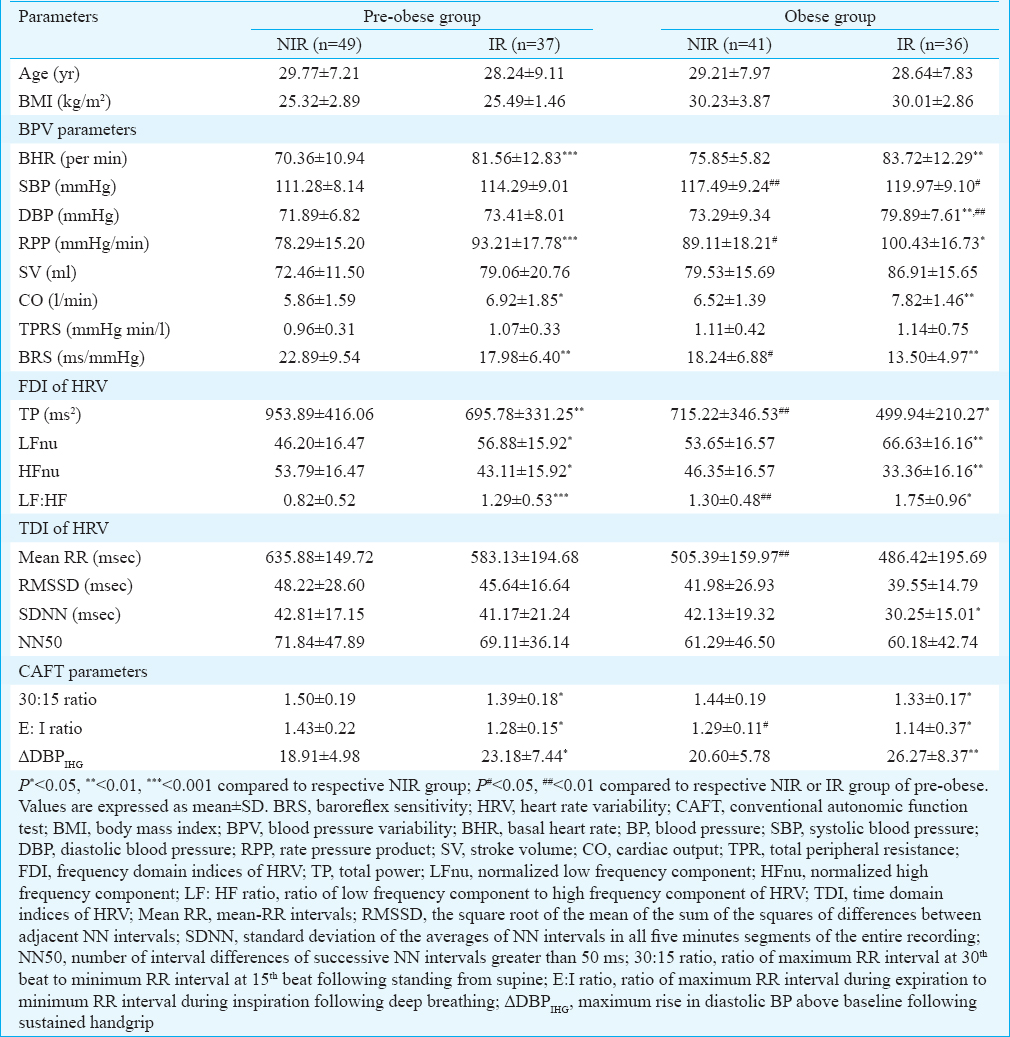

Among the FDI of HRV, TP and HFnu were significantly reduced, and LFnu and LF-HF ratio were significantly increased in IR group compared to NIR group in both pre-obese and obese participants (Table I). TP decreased and LF-HF ratio increased significantly in NIR obese group compared to NIR pre-obese participants (Table II). The 30:15 ratio and E:I ratio were significantly decreased and ΔDBPIHG (P>0.01) was significantly increased in IR individuals compared to NIR in both the pre-obese and obese groups (Table I). All lipid parameters (except HDL, which was decreased) and lipid risk factors were significantly increased in IR group compared to the NIR group in both pre-obese and obese individuals, but there was no difference in these parameters between the IR obese group and IR pre-obese group (Table II).
Inflammatory markers such as hs-CRP, TNF-α, IL-6, IFN-g and neopterin were significantly increased in IR group compared to NIR group in both pre-obese and obese individuals. Furthermore, IL-6 and IFN-g significantly increased in IR obese compared to IR pre-obese individuals, and the TNF-α and IL-6, were found to be significantly increased in NIR obese participants compared to the NIR pre-obese participants (Table II). Among the adipokines, leptin (P>0.001) was found to be significantly increased, and adiponectin significantly decreased in IR group compared to NIR group in both pre-obese and obese participants, and in NIR obese compared to NIR pre-obese (Table II). TBARS and TAO were significantly increased in obese group individuals compared to pre-obese group (in both IR and NIR groups) (Table II).
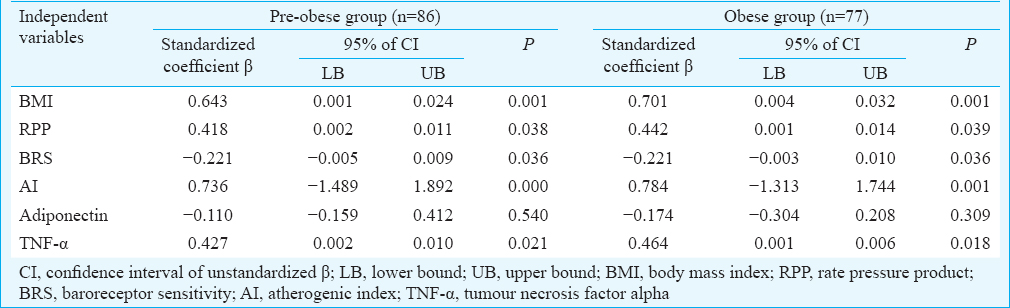
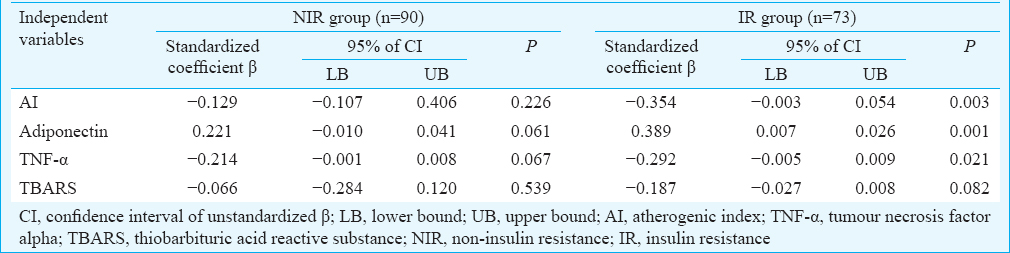
Multiple regression analysis revealed the significant individual contribution of BMI, RPP, BRS, AI and TNF-α to HOMA-IR in both pre-obese and obese group (Table III). Further, individual contribution of AI [β - 0.354, confidence interval (CI) −0.003–0.054, P=0.003], adiponectin (β 0.389, CI 0.007–0.026, P=0.001) and TNF-α (β −0.292, CI −0.005–0.009, P=0.021) to BRS was revealed by regression analysis in IR group, but not in NIR group (Table IV).
Discussion
In this study, significantly decreased BRS, was found in the IR group compared to the NIR groups (in both pre-obese and obese subjects) representing increased CV risks in individuals with IR. This was further supported by the increased resting heart rate (HR), RPP, cardiac output and decreased TP in IR group compared to the NIR groups (in both pre-obese and obese subjects), as these parameters have been reported to be associated with increased CV morbidities242529. However, there was no significant difference in the BRS, resting HR, RPP and cardiac output between IR pre-obese and IR obese groups suggesting that the IR appeared early in these individuals and predisposed them to future CV morbidities irrespective of their adiposity status.
There was a significant increase in lipid profile parameters (expect HDL) and the lipid risk factors in IR group compared to the NIR groups (in both pre-obese and obese). AI was used in the regression model, and had an independent contribution to BRS in IR individuals, suggesting that dyslipidaemia could be the likely contributor to CV risks in these individuals.
The increased LF-HF ratio in resting supine condition represents increased sympathetic and decreased parasympathetic activity, and is considered as a sensitive marker of SVI2530. In the present study, LF-HF ratio was significantly increased in IR pre-obese and IR obese individuals, representing considerable SVI. Increased sympathetic activity in the pre-obese and obese individuals was further demonstrated by the increase in LFnu, as increased LFnu reflects increased cardiac sympathetic drive2530. Decreased parasympathetic activity was further confirmed by the decrease in HFnu and the TDI (Mean RR, RMSSD, SDNN and NN50) of HRV, as a reduction in these HRV indices reflects decreased vagal modulation of cardiac drive2530. These findings were in conformity with the findings of our previous study27 that SVI in pre-obese and obese prehypertensives was linked to the sympathetic activation and vagal withdrawal. In IR individuals TDI (Mean RR, RMSSD and NN50) and BHR did not show a significant difference compared to NIR in both pre-obese and obese groups, indicating significant reduction in the cardiac vagal modulation in individuals with IR. BRS has been reported to be an index of SVI in various CV disease states12. Therefore, the SVI observed in individuals with IR might be linked to decreased BRS.
The decrease in E/I ratio and 30:15 ratio in IR pre-obese and IR obese individuals compared to their NIR counterparts demonstrated decreased vagal reactivity, as E/I and 30:15 ratios represent parasympathetic reactivity27. Further, a significant increase in DBP in response to isometric handgrip (ΔDBPIHG) in IR pre-obese and IR obese individuals reflected heightened sympathetic reactivity. There was no significant difference in the autonomic activity and reactivity between the IR pre-obese and IR obese individuals. These observations suggested that alteration in the sympathetic activity and reactivity and parasympathetic activity and reactivity might be influenced by their IR status rather than the level of BMI.
The findings of the present study demonstrated that IR might play a central role in the pathophysiology associated with obesity and its related co-morbidities, independent of their adiposity status. Further, south Asians were reported to have increased IR and all-cause mortality compared to their western counterparts6. Thus, the status of IR, which occurs much earlier than the other metabolic abnormalities, could be used for identifying individuals at increased risk for future cardiac morbidities, especially among the Indian population. In the present study, inflammatory markers such as hs-CRP, TNF-α and IFN-g were significantly associated with BRS in individuals with IR and further, TNF-α had an independent contribution to BRS. Thus, findings of the present study suggested that the retrograde inflammation primarily mediated by TNF-α could be the potential link between increased CV risk (decreased BRS) in these IR individuals.
Adiponectin, an anti-inflammatory cardio-protective adipocytokine has been well known for its anti-atheroscelortic and insulin-sensitizing properties3. Significantly decreased circulatory adiponectin in those with IR (pre-obese and obese) indicated the vulnerability of these individuals to increased risk of CV disease. This was further supported by the increased serum neopterin in IR pre-obese and IR obese individuals, which has been reported to be closely associated with adverse CV events4. Hypoadiponectinaemia plays a crucial role in the pathophysiology of IR3. Thus, the independent contribution of hypoadiponectinaemia to decreased BRS in individuals with IR might predispose them to CV related morbidities compared to their NIR counterparts.
The major limitation of the present study was that plasma non-epinephrine or its metabolites in serum were not estimated to support SVI. Further, the influence of visceral fat on IR might have provided additional information.
In conclusion, decreased TP and BRS, and increased resting HR and RPP in both IR pre-obese and obese groups suggested that the cardiometabolic risk profile was comparable between the IR, pre-obese and obese individuals. Pro-inflammatory state, dyslipidaemia and hypoadiponectinaemia might contribute to CV risks in those with IR. IR could possibly be the major contributor for increased CV risks in this population and could be used for identifying individuals at increased risk for future cardiac morbidities. However, the findings of the present study should be further validated in a larger population to assess whether these cardiometabolic risk profile observed in the present study are individual specific or adiposity specific.
Financial support & sponsorship: Authors acknowledge Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, for providing financial assistance in the form of an intramural PhD research grant.
Conflicts of Interest: None.
References
- Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-81.
- [Google Scholar]
- Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham heart study. Circulation. 1983;67:968-77.
- [Google Scholar]
- Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100:1654-8.
- [Google Scholar]
- American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young), American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An American Heart Association Scientific Statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448-53.
- [Google Scholar]
- Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism. 2014;63:226-32.
- [Google Scholar]
- Leptin and heart sympathetic activity in normotensive obese and non-obese subjects. Ital Heart J. 2004;5:29-35.
- [Google Scholar]
- Association of sympathovagal imbalance with obesity indices, and abnormal metabolic biomarkers and cardiovascular parameters. Obes Res Clin Pract. 2015;9:55-66.
- [Google Scholar]
- Obesity and heart rate variability in men with myocardial infarction. Cardiol J. 2008;15:43-9.
- [Google Scholar]
- Reduced heart rate variability correlates with insulin resistance but not with measures of obesity in population undergoing laparoscopic roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:237-41.
- [Google Scholar]
- Improvement of heart rate variability after decreased insulin resistance after sleeve gastrectomy for morbidly obesity patients. Surg Obes Relat Dis. 2015;11:557-63.
- [Google Scholar]
- Sudden death after myocardial infarction. Prediction based on the baroreceptor reflex. Arch Mal Coeur Vaiss. 1990;83:1521-7.
- [Google Scholar]
- Predictive value of cardiac autonomic indexes and MIBG washout in ICD recipients with mild to moderate heart failure. Ann Nucl Med. 2009;23:677-84.
- [Google Scholar]
- Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J Hypertens. 2006;24:143-50.
- [Google Scholar]
- Insulin resistance and hypersecretion in obesity. European Group for the study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166-73.
- [Google Scholar]
- Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501-8.
- [Google Scholar]
- Differences in cardiometabolic risk between insulin-sensitive and insulin-resistant overweight and obese children. Child Obes. 2015;11:289-96.
- [Google Scholar]
- Cardiac vagal tone, exercise performance and the effect of respiratory training. Eur J Appl Physiol. 2005;94:681-9.
- [Google Scholar]
- Effect of yogic bellows on cardiovascular autonomic reactivity. J Cardiovasc Dis Res. 2011;2:223-7.
- [Google Scholar]
- Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15-26.
- [Google Scholar]
- World Health Organization, International Association for the Study of Obesity, International Obesity Task Force, International Diabetes Institute. The Asia-Pacific perspective: Redefining obesity and its treatment. Melbourne: Health Communications Australia; 2000.
- Fifteen years experience with finger arterial pressure monitoring: Assessment of the technology. Cardiovasc Res. 1998;38:605-16.
- [Google Scholar]
- Heart rate and the rate-pressure product as determinants of cardiovascular risk in patients with hypertension. Am J Hypertens. 1999;12:50S-5S.
- [Google Scholar]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-65.
- [Google Scholar]
- Assessment of sympathovagal imbalance by spectral analysis of heart rate variability in prehypertensive and hypertensive patients in Indian population. Clin Exp Hypertens. 2011;33:478-83.
- [Google Scholar]
- Association of sympathovagal imbalance with cardiovascular risks in young prehypertensives. Am J Cardiol. 2013;112:1757-62.
- [Google Scholar]
- Elevated resting heart rate, physical fitness and all-cause mortality: A 16-year follow-up in the Copenhagen MALE study. Heart. 2013;99:882-7.
- [Google Scholar]
- Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225-36.
- [Google Scholar]






