Translate this page into:
Contribution of acrB upregulation & OmpC/Ompk36 loss over the presence of blaNDM towards carbapenem resistance development among pathogenic Escherichia coli & Klebsiella spp.
For correspondence: Dr Anusri Tripathi, Department of Biochemistry & Medical Biotechnology, Calcutta School of Tropical Medicine, 108, C.R. Avenue, Kolkata 700 073, West Bengal, India e-mail: anusri.stm@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The global spread of carbapenem-resistant Enterobacteriaceae (CRE) is an emerging clinical problem. Hence, in this study, the plausible role of extended-spectrum beta-lactamases (ESBLs)/carbapenemases, OmpC/Ompk36, acrB and their combinations was explored among CRE.
Methods:
The minimum inhibitory concentration (MIC) of meropenem, enzyme-phenotypes (ESBLs/IR and metallo-beta-lactamase (MBL)/non-MBL carbapenemase), genotypes (blaTEM, blaSHV and blaCTX-M; blaNDM and blaVIM; blaKPC and blaOXA-48-like variants), acrB and outer membrane protein (OMP) expressions were analyzed with a total of 101 non-duplicate clinical isolates, obtained from various samples of patients visiting two tertiary care units of Eastern India during May 2013 - October 2016. This included Escherichia coli (n=36) and Klebsiella pneumoniae (n=65), categorized into two groups, namely Group I (resistant to all carbapenems; n=93; E. coli=34 and Klebsiella spp.=59) and Group II (non-resistant to all the carbapenems; n=8; E. coli=2 and Klebsiella spp.=6).
Results:
Though 88.17 per cent of Group I isolates exhibited ESBL property, the presence of carbapenemase activity (70.96%) and that of blaNDM gene (42/66: 63.63%) indicated their contributions towards the emergence of CRE. Further, porin loss and/or efflux pump activation among ESBL/carbapenemase-producing isolates heightened the MIC of meropenem from 64 to 256 mg/l (range exhibited by only ESBL/carbapenemase-producing isolates) to >256 mg/l.
Interpretation & conclusions:
These findings implied the major contribution of porin loss and/or efflux pump activation over the presence of ESBLs/carbapenemases in imparting carbapenem resistance in pathogenic bacteria.
Keywords
acrB, blaNDM
carbapenem-resistant Enterobacteriaceae
epidemiology
extended-spectrum beta-lactamase
OmpC/Ompk36
The global emergence of antibiotic resistance, which results in tremendous morbidity and mortality worldwide, has been considered to be one of the greatest threats in international public health12. Among all the commercially available antibiotics, resistance towards several carbapenems, considered to be the 'drugs of last resort', is supposed to be clinically most important because it delimits the treatment options against bacterial infections23. Although fewer medicines such as aztreonam, tigecycline, colistin and fosfomycin retain in vitro activity against carbapenem-resistant Enterobacteriaceae (CRE), their usages are restricted by either their side effect profile or uncertainty of in vivo efficacy3. From 2008 onwards, resistance towards several widely used carbapenems, namely imipenem, meropenem and ertapenem, had increased significantly in India4. While, only 11-22 per cent of enterobacterial isolates were found to be resistant towards at least one of these three carbapenems during 2007-2008, such resistance has been found to rise to at least 37.9 per cent in recent years45.
Combined expression of extended-spectrum beta-lactamase (ESBL)-type enzymes with either loss/reduced expression of major outer membrane proteins (OMPs) (OmpF/Ompk35 of MW 36kDa and OmpC/Ompk36 of MW 38kDa in Escherichia coli/Klebsiella spp., respectively) or overexpression of efflux pumps (AcrAB-TolC) as well as expression of metallo (NDM; VIM)/non-metallo-beta-lactamase (KPC; OXA)-type carbapenemases alone was known to be responsible for carbapenem resistance development among pathogenic Enterobacteriaceae467. However, the nature of contribution of these mechanisms towards carbapenem resistance development, whether additive, augmentative or diminutive, has not yet been well documented. Although the association of expression of porins and efflux pumps with ESBL is well known for carbapenem resistance development, but their relation with carbapenemases has not been studied before. Besides, reports on contribution of all these mechanisms for such resistance development among isolates of Indian origin are limited. Thus, the present cross-sectional study was undertaken to address all the aforesaid lacunae for better understanding the increasing emergence of CRE in India.
Material & Methods
Enterobacterial samples were obtained from urine, blood, sputum, body fluid, wound and pus of different non-duplicate and unrelated patients visiting the outpatient department of Seth Sukhlal Karnani Memorial Hospital-Institute of Post Graduate Medical Education and Research (SSKM-IPGMER) and Calcutta School of Tropical Medicine (CSTM, Kolkata, India), from May 2013 to October 2016. Samples were subjected to Gram staining and streaked on nutrient agar, blood agar and MacConkey's agar media and incubated overnight at 37°C. Identification of E. coli and Klebsiella spp. isolates was done by growing isolates on organism-specific HiCrome™ selective agar (HiMedia Laboratories Pvt. Ltd., India) as well as by standard biochemical methods8. The study was approved by the Institutional Ethical Committee of CSTM, Kolkata (Ref. No. CREC-STM/260 dated 9/1/2013).
Antimicrobial susceptibility assay for the categorization of isolates: Antimicrobial susceptibility of the isolates was determined by Kirby-Bauer standard disc diffusion method according to the guidelines of Clinical and Laboratory Standards Institute (CLSI), 20139, for the following antimicrobial agents (μg/disc): ceftazidime (30), cefotaxime (30), cefpodoxime (10), imipenem (10), meropenem (10) and ertapenem (10) (HiMedia Laboratories Pvt. Ltd., Mumbai). Isolates resistant and non-resistant to all carbapenems were categorized as Group I (resistant to all carbapenems) and Group II (non-resistant to all the carbapenems), respectively. The minimum inhibitory concentration (MIC) value (mg/l) of meropenem (AstraZeneca, UK) against Groups I and II isolates was determined using microdilution method and interpreted according to the guidelines of CLSI9.
Phenotypic characterization of Group I and Group II isolates: Screening of ESBLs was done according to the guidelines of CLSI9. Isolates exhibiting an increase of >5 mm in inhibition zone of the combined ceftazidime/cefotaxime (30 μg)-clavulanic acid (10 μg) disc, compared to ceftazidime/cefotaxime (30 μg) alone, were categorized as ESBL positive. Carbapenemase activity was assayed among these isolates following the protocol described by Bernabeu et al10. Briefly, the isolates were sonicated and the supernatant was used to determine meropenem-hydrolyzing activity spectrophotometrically. Percentage activity was calculated by the following formula:

Detection of metallo-β-lactamases (MBLs) among isolates with carbapenemase activity was evaluated by double-disc synergy test (DDST)4, where imipenem (10 μg) and imipenem-ethylenediaminetetraacetic acid (10/750 μg) combination disc (HiMedia Laboratories Pvt. Ltd., Mumbai) were placed 40-50 mm apart over the lawn of bacteria, spread aseptically over Mueller-Hinton agar and incubated overnight at 37°C4. Isolates exhibiting an increase of >5 mm in the inhibition zone of the combination disc, compared to imipenem alone, were categorized as MBL positive.
Genomic DNA isolation, PCR detection of ESBLs and carbapenemases: Plasmid and chromosomal DNA was extracted from Groups I and II isolates by alkaline lysis and Hancock's method, respectively1112. PCR amplification was carried out for detection of the following ESBL, MBL and non-MBL genes - ESBL: blaTEM, blaSHV and blaCTX-M; MBL: blaNDM and blaVIM and non-MBL: blaKPC and blaOXA-48-like variants using DNA and gene-specific primers, designed through Primer3 server, web version 4.0.0 (http://bioinfo.ut.ee/primer3/) (Table I). Based on PCR data, Group I isolates were further genotypically categorized into four subgroups, namely ESBL only (Ia), carbapenemase only (Ib), both ESBL and carbapenemase-producing (Ic) and none present (Id).
| Primer | Forward and Reverse Primer Sequences | Amplicon size (bp) |
|---|---|---|
| Primer sets used in PCR | ||
| blaTEM type | 5’- ATGAGTATTCAACATTTTCGTC-3’ | 860 |
| blaSHV type | 5’- TTACCAATGCTTAATCAGTGAG-3 | 863 |
| blaCTX_M type | 5’- GCGTTATWTTCGCCTGTG-3’ | 820 |
| 5’- GCTTTTAKYGTTGCCAGT-3’ | ||
| 5’- GYCAGTTCACGCTGATGG-3’ | ||
| 5’- CGCCGACGCTAATACATC-3’ | ||
| blaNDM type | 5’- AAGCTGAGCACCGCATTA- 3’ | 758 |
| 5’- CGGGCCGTATGAGTGATT-3’ | ||
| blaVIM type | 5’- GTCTATTTGACCGCGTCT-3’ | 778 |
| 5’- CTCAACGACTGAGCGATT-3’ | ||
| blaOXA type | 5’- TGCGTGTATTAGCCTTATCG-3’ | 773 |
| 5’- GAGCACTTCTTTTGTGATGG-3’ | ||
| blaKPC type | 5’- CTGTATCGCCGTCTAGTTC-3’ | 824 |
| 5’- GCTGTRCTTGTCATCCTT-3’ | ||
| Primer sets used in qRT-PCR | ||
| E. coli AcrB | 5’- GAGAAATCATCCAGCAGCT- 3’ | 156 |
| 5’- CTGTGAACCGAACAACTGA- 3’ | ||
| E. coli rpoB | 5’- GAAGGCACCGTAAAAGACA- 3’ | 174 |
| 5’- ACCCGAAGAGTGGGTTTTA- 3’ | ||
| Klebsiella AcrB | 5’- GTTAATGACGCCGACAAC- 3’ | 125 |
| 5’- TACGCTGACCTTGCAATC- 3’ | ||
| Klebsiella rpoB | 5’- GTTGACTACATGGACGTATCC- 3’ | 175 |
| 5’- AACAGCACGTTCCATACC- 3’ |
Analysis of expression of acrB efflux pump gene: Group I and II isolates were grown in Müller-Hinton Broth at 37°C with continuous orbital shaking and harvested at mid-log phase by centrifugation 12,300 × g 10 min. Bacterial total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed to cDNA by first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc., MA, USA) following manufacturer's standard protocols. Expression of acrB was normalized against that of housekeeping gene, RNA polymerase B (rpoB), used as internal control13. The level of acrB expression among isolates of different subgroups was determined by calculating 2−ΔΔCT and compared to that of Group II isolates, using DataAssist™ software v2.1 (Thermo Fisher Scientific Inc., USA).
Analysis of bacterial outer membrane protein (OMP) expression: Group I and group II isolates were grown in Müller-Hinton Broth at 37°C with continuous orbital shaking and harvested at mid-log phase by centrifugation at 12,300 × g 10 min. Bacterial OMPs were isolated and purified following the protocols described earlier14. OMPs were quantified in Lowry's method15. About 100 μg of OMPs/well were separated by 15 per cent sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining using 0.25 per cent Coomassie brilliant blue. Five microliters of PageRuler pre-stained protein ladder (10-170 kDa range) (Thermo Fisher Scientific Inc., USA) was also subjected to SDS-PAGE for determining the molecular weight of OMPs. De-stained gels were photographed through Gel documentation system (UVI-tech Ltd., Cambridge, UK), and OMP bands were subjected to densitometric scanning through Image Processing and Analysis in Java (ImageJ, NCBI, USA). OMP expression of Group I isolates was compared with that of Group II isolates and was expressed in terms of percentage reduction in expression. Inter-subgroup comparison was also done with the expression data normalized against Group II isolates.
Statistical analysis: MIC values were represented in range and the distribution pattern was depicted by bar diagrams. The mode values of MIC with frequencies were calculated for both E. coli and Klebsiella spp. of Groups I and II isolates. Carbapenemase activity, level of expression of acrB and OMPs among Group I isolates were represented in percentage activity, fold changes and percentage reductions normalized against the values of Group II isolates, respectively. First, Shapiro-Wilk normality test was performed to evaluate the distribution of data16. Data showing P>0.05 were considered to follow normal distribution. One-way ANOVA followed by Tukey's honestly significant difference (HSD) post hoc analysis test was performed to compare MIC values, percentage carbapenemase activity and expression level of acrB and OMP among various phenotypic-genotypic subgroups of Group I isolates17. The statistical analyses were performed through SPSS software v17.0 (IBM Inc., NY, USA). Overall contribution of all the strategies, namely expression of beta-lactamases (B: ESBL; C: carbapenemases), efflux pump's upregulation (E) and porin loss (P) and their combinations in carbapenem resistance development was represented through proportionate Venn diagram by eulerAPE v3.0.018.
Results
From May 2013 to October 2016, a total of 101 pathogenic E. coli and Klebsiella spp. were isolated from urine (71/101: 70.29%), wound pus (14/101: 13.86%), sputum (8/101: 7.92%), body fluids (5/101: 4.95%) and blood (3/101: 2.97%) of different non-duplicate unrelated patients (female: male: 1.24:1). Standard biochemical tests and overnight culture of the collected bacteria on organism-specific selective media identified 35.64 per cent (36/101) and 64.36 per cent (65/101) bacteria to be E. coli and Klebsiella spp., respectively. All the data generated from the above study settings followed normal distribution pattern as analyzed by Shapiro-Wilk normality test.
Antimicrobial susceptibility assay and selection of Group I and Group II isolates: Antibiograms of E. coli (n=36) and Klebsiella spp. (n=65) identified 93 (Group I: n=93; E. coli=34 and Klebsiella spp.=59) and eight (Group II: n=8; E. coli=2 and Klebsiella spp.=6) isolates as resistant and non-resistant towards all carbapenems, respectively, which were considered for further analyses. All the 93 Group I isolates demonstrated MIC values of meropenem in the range of 64 to >1024 mg/l, with central tendency (mode) of >1024 mg/l and having frequency of 0.706 and 0.644 for E. coli and Klebsiella spp., respectively. On the other hand, carbapenem non-resistant Group II isolates revealed MIC of meropenem in the range of 1-4 mg/l with mode value of 2 mg/l, having frequency of 1.0 and 0.667 for E. coli and Klebsiella spp., respectively.
Phenotypic screening of ESBLs and MBLs: Phenotypic screening identified 25.81 per cent (n=24; E. coli=7 and Klebsiella spp.=17) of Group I isolates to exhibit only ESBL property; 8.60 per cent (n=8; E. coli=4 and Klebsiella spp.=4) to demonstrate only carbapenemase activity and 62.36 per cent (n=58; E. coli=22 and Klebsiella spp.=36) to exhibit both ESBL and carbapenemase activities (Table II). However, 3.22 per cent (n=3; E. coli=1 and Klebsiella spp.=2) exhibited neither ESBL nor carbapenemase activity. Among the carbapenemase-producing isolates (n=66), 93.94 per cent of isolates (n=62; E. coli=25 and Klebsiella spp.=37) demonstrated to have MBL activity by DDST.
| Organism (n) | Phenotype | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|
| ESBL genes (%) | Non-MBL genes (%) | MBL genes (%) | ||||||
| blaTEM | blaSHV | blaCTX_M | blaOXA-48-like | blaKPC | blaNDM | blaVIM | ||
| Escherichia coli (n=7) | ESBL only | 1 (14.3) | - | - | - | - | - | - |
| Klebsiella spp. (n=17) | 8 (47.1) | 2 (11.8) | 5 (29.4) | - | - | - | - | |
| E. coli (n=4) | Carbapenemase only | - | - | - | 1 (25.0) | - | 1 (25.0) | - |
| Klebsiella spp. (n=4) | 1 (25.0) | - | - | - | - | 1 (25.0) | ||
| E. coli (n=22) | Both ESBL and carbapenemase producing | 5 (22.7) | - | 2 (9.1) | 3 (13.6) | 4 (18.2) | 17 (77.3) | 3 (13.6) |
| Klebsiella spp. (n=36) | 3 (8.3) | 2 (5.6) | 2 (5.6) | 7 (19.4) | 3 (8.3) | 23 (63.9) | 3 (8.3) | |
| E. coli (n=1) | No | - | - | - | - | - | - | - |
| Klebsiella spp. (n=2) | beta-lactamase activity | 1 (50.0) | - | - | - | - | - | - |
| Total (n=93) | 19 (20.4) | 4 (4.3) | 9 (9.7) | 11 (11.8) | 7 (7.5) | 42 (45.2) | 6 (6.4) | |
Percentage of individual beta-lactamase genes was calculated using the numbers in parentheses mentioned besides the name of organisms. ESBL, extended spectrum beta lactamase; MBL, metallo beta lactamase
PCR detection of ESBL and carbapenemase genes: PCR screening revealed 64.52 per cent (n=60; E. coli: 20/34 and Klebsiella spp.: 40/59) of Group I isolates to harbour at least one ESBL or carbapenemase gene. Among the ESBL and MBL genes, blaTEM (20.40%, n=19; E. coli: 6/34 and Klebsiella spp.: 13/59) and blaNDM (45.20%, n=42; E. coli: 18/26 and Klebsiella spp.: 24/40) were found to be the most prevalent ones among Group I isolates (Table II). These genes were mostly found among ESBL only and both ESBL- and carbapenemase-producing phenotypes. Distribution of beta-lactamase genes among different phenotypic groups is elaborated in Table II. Overall co-existence of multiple carbapenemase and/or ESBL genes was found among 24.73 per cent of group I isolates (n=23; E. coli: 10/34 and Klebsiella spp.: 13/59), of which 69.56 per cent (16/23) were found to harbour MBL gene(s) along with ESBL/non-MBL gene(s).
Expression analysis of acrB efflux pump gene: Expression analysis of acrB demonstrated that while 76.47 per cent (26/34) of Group I E. coli exhibited 34.2+5.11-fold higher expression of this gene than that of Group II isolates, 66.1 per cent (39/59) of Group I Klebsiella spp. demonstrated 10.65+3.05-fold higher expression of acrB compared to the respective Group II isolates. Overexpression level of acrB was found to be significantly associated with ESBL and carbapenemase production among both E. coli and Klebsiella spp. (one-way ANOVA: P<0.05; post hoc Tukey's HSD test:P<0.05 for both ESBL- and carbapenemase-producing isolates) (Table III). Isolates producing both ESBL and carbapenemase exhibited the highest level of acrB expression, while isolates having no such activity demonstrated minimal acrB overexpression when compared to the respective Group II isolates.
| Phenotypes | Organism (n) | Fold increase in acrB expression (mean±SE) Normalized against Group II isolates | Per cent reduction in Omp-C/-k36 expression (mean±SE) normalized against Group II isolates |
|---|---|---|---|
| ESBL only | Escherichia coli (7) | 24.56±9.48 | 54.46±6.25 |
| Klebsiella spp. (17) | 18.60±7.30 | 27.40±1.52 | |
| Overall (24) | 20.34±7.89* | 35.29±3.65* | |
| Carbapenemase only | E. coli (4) | 35.45±1.76 | 47.34±2.37 |
| Klebsiella spp. (4) | 11.92±0.84 | 0.682±0.26 | |
| Overall (8) | 23.68±1.56* | 24.01±1.65* | |
| Both ESBL and carbapenemase producing | E. coli (22) | 38.45±3.79 | 57.34±4.59 |
| Klebsiella spp. (36) | 18.78±3.28 | 37.03±1.86 | |
| Overall (58) | 26.24±3.35*,† | 44.73±2.73*,† | |
| No beta-lactamase activity | E. coli (1) | 0.26 | 35.44 |
| Klebsiella spp. (2) | 3.81±0.79 | 16.49±0.68 | |
| Overall (3) | 2.63±0.65 | 22.81±0.53 |
One-way ANOVA: *P<0.05; †P<0.05 through Tukey’s HSD post-hoc test. Three, one and two E. coli isolates from ESBL only, carbapenemase only and both ESBL- and carbapenemase-producing phenotypic classes exhibited complete loss of porin respectively. Similarly, among Klebsiella spp., five, one, three and one isolates from ESBL only, carbapenemase only, both ESBL- and carbapenemase-producing and no beta-lactamase activity-showing phenotypic classes showed complete loss of porin, respectively. HSD, honestly significant difference; SE, standard error
Expression analysis of bacterial OMPs: Comparative analysis of OMP expression between Groups I and II isolates revealed 61.76 per cent (21/34) of Group I E. coli to have 45.36 per cent of reduced OmpC expression, whereas 69.49 per cent (41/59) of Group I Klebsiella spp. exhibited approximately 30.1 per cent of diminished Ompk36 expression (Fig. 1). Complete loss of OmpC/Ompk36 was documented in 17.65 per cent (6/34) of E. coli and 30.51 per cent (18/59) of Klebsiella spp., respectively. Loss of OmpC/Ompk36 was found to be significantly associated with both ESBL and carbapenemase production among Group I E. coli and Klebsiella spp. isolates (one-way ANOVA:P<0.05; post hoc Tukey's HSD test: P<0.05 for both ESBL- and carbapenemase-producing isolates) (Table III).
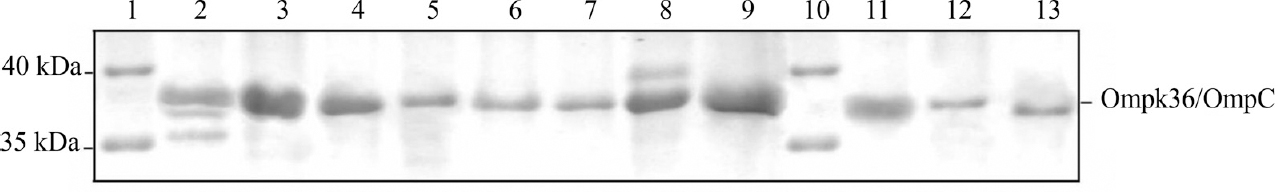
- Representative photograph of sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of extracted outer membrane proteins (OMPs) exhibiting reduced expression of Ompk36/OmpC among Group I compared to Group II isolates. Lanes 1 and 10: Pre-stained protein marker, lanes 2-4: Ompk36 of carbapenem non-resistant (Group II) Klebsiella spp., lanes 5-7: Ompk36 of carbapenem-resistant (Group I) Klebsiella spp., lanes 8-9: OmpC of carbapenem non-resistant (Group II) E. coli and lanes 11-13: OmpC of carbapenem-resistant (Group I) E. coli.
Genotypic categorization of Group I isolates and their association with MIC, carbapenemase activity and acrB and OMP expression: Among the Group I E. coli, one, 13, six and 14 isolates were categorized under Ia (ESBL gene carrying), Ib (carbapenemase gene carrying), Ic (both ESBL and carbapenemase carrying) and Id (none present) subgroups, respectively. Similarly, nine, 27, four and 19 Group I Klebsiella spp. isolates were classified under these subgroups.
Although MIC value distribution of meropenem was found to be almost consistent among the various subgroups, carbapenemase activity was found to be significantly high among Ib, Ic and Id subgroups of both organismswhen compared with that of Ia isolates (one-way ANOVA: P<0.05; post hoc Tukey's HSD test: P<0.01 and P<0.05 for Ib and Ic, respectively) (Table IV). Moreover, single and multiple gene harbouring isolates among Klebsiella spp. of subgroup Ib revealed marked difference in their meropenem-hydrolyzing proficiency (P<0.01).
| Sub groups | Organism (n) | Number of genes present per isolate (n) | MICmrp (mg/l) (frequency) (%) | Per cent carbapenemase activity (mean±SE) Normalized against Group II isolates | Fold increase in acrB expression (mean±SE) Normalized against Group II isolates | Per cent reduction in Omp-C/-k36 expression (mean±SE) Normalized against Group II isolates | ||
|---|---|---|---|---|---|---|---|---|
| Individual subgroup data | Comparison between Ia+Ib+Ic and Id | Individual subgroup data | Comparison between Ia+Ib+Ic and Id | |||||
| Ia | Escherichia coli (1) | Single (1) | 512 (100.0) | 0.91 | 43.38 | M=41.14±10.13 | 47.05 | M=45.79±4.40** |
| Multiple (0) | - | - | - | O=10.78±3.50 | - | O=13.98±3.73 | ||
| Klebsiella spp. (9) | Single (5) | 512 (60.0) | 0.0±0.93 | 12.40±7.11 | 20.32±1.54 [1] | |||
| Multiple (4) | >1024 (75.0) | 3.45±1.21 | 6.02±2.11 | 10.3±2.6 | ||||
| Overall (10) | >1024 (50.0) | 1.47±0.85 | 12.97±4.39 | 18.98±4.39 [1] | ||||
| Ib | E. coli (13) | Single (9) | >1024 (88.9) | 21.79±1.88 | 31.99±11.22 | 48.24±5.61 | ||
| Multiple (4) | >1024 (50.0) | 17.74±3.20 | 52.29±15.94 | 49.86±4.74s [1] | ||||
| Klebsiella spp. (27) | Single (22) | >1024 (72.7) | 7.48±1.98 | 13.93±3.78 | 18.53±5.30 [11] | |||
| Multiple (5) | >1024 (60.0) | 23.93±0.24** | 3.45±0.81* | 1.18±0.93** | ||||
| Overall (40) | >1024 (65.0) | 13.78±1.86*,† | 20.52±6.30 | 26.18±4.39[12] | ||||
| Ic | Escherichia coli (6) | Multiple (6) | >1024 (50.0) | 16.69±0.38 | 47.07±6.30 | 39.21±3.09 [1] | ||
| Klebsiella spp. (4) | Multiple (4) | >1024 (50.0) | 8.78±0.52 | 5.41±2.27 | 0.77±0.97 | |||
| Overall (10) | >1024 (50.0) | 13.53±0.44*,† | 30.41±4.69 | 23.83±2.24 [1] | ||||
| Id | Escherichia coli (14) | NA | ≥1024 (85.7) | 9.41±0.33 | 23.31±5.01 | 37.11±1.99 [4] | ||
| Klebsiella spp. (19) | NA | ≥1024 (68.4) | 8.64±0.29 | 12.46±3.40 | N=23.31±5.01 | 33.22±0.65 [11] | N=37.11±1.99 | |
| Overall (33) | ≥1024 (75.75) | 8.97±0.31* | 17.06±4.08 | P=12.46±3.40 | 34.87±1.22 [15] | P=33.22±0.65 | ||
P*<0.05, **<0.01 (one-way ANOVA); †P<0.05 (Tukey’s HSD post-hoc test); Ia, Ib, Ic and Id represented ESBL only, carbapenemase only, both ESBL- and carbapenemase-producing and none present isolates, respectively. Number of isolates showing complete loss of OMPs was mentioned in square brackets. M=Average expression of acrB/OmpC among (Ia+Ib+Ic) E. coli; N=Average expression of acrB/OmpC among Id E. coli; O=Average expression of acrB/Ompk36 among (Ia+Ib+Ic) Klebsiella spp. and P=Average expression of acrB/Ompk36 among Id Klebsiella spp. One-way ANOVA and Tukey’s HSD post-hoc analysis test was conducted: (i) to compare MIC values, carbapenemase activities and changes in the expression levels of acrB, OmpC/Ompk36 among various subgroups of Group I isolates (Ia-Id), (ii) to detect meropenem-hydrolyzing proficiency and changes in expression levels of acrB, OmpC/Ompk36 among single and multiple resistant gene harbouring E. coli and Klebsiella spp. isolates of each Ia/Ib subgroup, (iii) to identify the role of acrB upregulation and OMP loss in carbapenem resistance among beta-lactamase-producing and non-beta-lactamase-producing E. coli (M vs. N) and Klebsiella spp. (O vs. P) isolates. NA, not applicable; HSD, honest significant difference; SE, standard error; OMPs, outer membrane proteins
Inter subgroup comparison revealed significant overexpression of acrB among beta-lactamase-producing E. coli of subgroups Ia, Ib and Ic with respect to Id (no beta-lactamase gene present) (P<0.05) (Table IV). However, this trend was not followed among Klebsiella spp., where acrB gene was found to be slightly underexpressed among these subgroups.
Comparable expression of OmpC was documented among all the subgroups of E. coli, whereas significant loss/reduced expression of Ompk36 was found among Id Klebsiella spp. (no beta-lactamase gene present) when compared to beta-lactamase-producing Klebsiella spp. of subgroups Ia, Ib and Ic (P<0.01) (Table IV).
Role of different strategies in carbapenem resistance emergence: Although the presence of only ESBL/carbapenemase genes (B/C), efflux pump upregulation (E), porin loss (P) or their combinations, namely B/C-E, B/C-P, PE and B/C-PE exhibited differential contribution in carbapenem resistance emergence among E. coli and Klebsiella spp., PE (10/34: 29.41%), B/C-PE (11/34: 32.35%) of E. coli and B/C-E (18/59: 30.51%), B/C-P (13/59: 22.03%) and PE (13/59: 22.03%) of Klebsiella spp. were found to be the most predominant strategies (Fig. 2A). Most of these isolates following these predominant strategic combinations exhibited very high level of meropenem resistance (MIC >1024 mg/l), whereas isolates with only B/C strategy demonstrated low level of meropenem resistance (MIC: 64-256 mg/l) indicating the major role of P, E and their combined strategies to increase meropenem resistance (one-way ANOVA: P<0.01; post hoc Tukey's HSD test: P<0.01 for all the six strategies including P, E and their combinations with respect to B/C alone) (Fig. 2B). Further, E. coli of subgroups Ib/Ic following PE strategy always had higher percentage of OmpC reduction when compared to those following P strategy within the same subgroups - indicating the positive role of acrB in augmenting OmpC reduction (one-way ANOVA: P<0.01 for Ib and Ic) (Table V). Unlike this mechanism, Klebsiella spp. of subgroups Ia following PE strategy had higher level of acrB upregulation when compared to those following E strategy within the same subgroups - indicating the positive role of Ompk36 in augmenting acrB upregulation (one-way ANOVA: P<0.05). These two findings implicated mutual complementation of P and E among Ia, Ib and Ic isolates following PE strategy.
![(A) Venn diagram showing the prevalence of various strategies in carbapenem resistance emergence. Numerical after abbreviations for individual strategies indicate the number of isolates exhibiting that strategy. (B) Contribution of various strategies in carbapenem resistance emergence [as indicated by the minimum inhibitory concentration (MIC) value ranges]. *indicates significant changes in (MIC) values when compared to B/C strategy (P<0.01 for one-way ANOVA with post hoc Tukey' honestly significant difference test P<0.01). B/C, ESBL/ Carbapenemases; P, porin loss; E, efflux pump's upregulation; CP, carbapenemase expressing isolates with porin loss; CE, carbapenemase expressing isolates with efflux pump's upregulation; PE, both porin loss and efflux pump's upregulation; CPE, carbapenemase expressing isolates with both porin loss and efflux pump's upregulation.](/content/175/2019/149/4/img/IJMR-149-528-g003.png)
- (A) Venn diagram showing the prevalence of various strategies in carbapenem resistance emergence. Numerical after abbreviations for individual strategies indicate the number of isolates exhibiting that strategy. (B) Contribution of various strategies in carbapenem resistance emergence [as indicated by the minimum inhibitory concentration (MIC) value ranges]. *indicates significant changes in (MIC) values when compared to B/C strategy (P<0.01 for one-way ANOVA with post hoc Tukey' honestly significant difference test P<0.01). B/C, ESBL/ Carbapenemases; P, porin loss; E, efflux pump's upregulation; CP, carbapenemase expressing isolates with porin loss; CE, carbapenemase expressing isolates with efflux pump's upregulation; PE, both porin loss and efflux pump's upregulation; CPE, carbapenemase expressing isolates with both porin loss and efflux pump's upregulation.
| Organisms | Strategies | Ia (ESBL expressing) | Ib (carbapenemase expressing) | Ic (both ESBL and carbapenemase expressing) | Id (none expressing) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SE | |||||||||
| Fold increase in acrB expression | Per cent reduction in Omp-C/-k36 expression | Fold increase in acrB expression | Per cent reduction in Omp-C/-k36 expression | Fold increase in acrB expression | Per cent reduction in Omp-C/-k36 expression | Fold increase in acrB expression | Per cent reduction in Omp-C/-k36 expression | ||
| Escherichia coli | E | None | None | 48.87±13.91 | - | None | None | 10.69±5.15 | - |
| P | None | None | - | 11.34±0.9 (1) | - | 11.86±2.09 (1) | - | 42.00±1.72 (1) | |
| PE | 43.38 | 47.05 | 36.34±12.27 | 54.55±5.03** | 47.07±7.72 | 45.03±4.74** | 26.11±6.52* | 44.77±2.89 (3) | |
| B/C only | None | None | - | - | - | - | NA | NA | |
| Klebsiella spp. | E | 5.85±1.09 | - | 7.45±3.65 | - | 5.71±3.17 | - | 8.03±2.56 | - |
| P | - | 18.67 | - | 22.06±0.92 (5) | None | None | - | 33.29±1.62 (1) | |
| PE | 15.76±1.87* | 4.94 (1) | 13.22±7.90 | 5.75 (6) | None | None | 17.46±6.48 | 0.0 (10) | |
| B/C only | - | - | - | - | None | None | NA | NA | |
P*<0.05, **<0.01 (one-way ANOVA) Number of isolates showing complete loss of OMPs was mentioned in brackets. None, no representative; NA, not applicable; ESBL, extended spectrum beta lactamase; E, efflux pump's upregulation; P, porin loss; PE, both porin loss and efflux pump's upregulation; B/C, ESBL/Carbapenemases
Discussion
In this study, majority of the CREs were isolated from urinary infections, treatment of which renders a serious challenge to clinicians. As most of the CREs isolated from urine exhibited resistance to aminoglycosides, fluoroquinolones and carbapenems - commonly used to treat urologic infections, there is an increased risk of receiving inappropriate empiric treatment, thereby resulting in higher rate of morbidity/morality associated with CRE infections1920. Although treatment options are limited, old antibiotics - temocillin, fosfomycin, pivmecillinam, ceftolozane-tazobactum and combination therapy - were often recommended as available line of treatment against such infections20.
Similar to a previous study by Dhara and Tripathi21 in the current study phenotypic screening identified ESBL property among majority of the isolates. The presence of carbapenemase activity and blaNDM indicated their contribution towards the emergence of carbapenem resistance among these bacteria. However, percentage prevalence of carbapenemase/MBL/blaNDM gene was comparable among Group I E. coli and Klebsiella spp. isolates, and the co-existence of ESBL/MBL/non-MBL genes was identified among isolates of both the genera. Similar observation has earlier been reported222324. Besides, rare co-existence of blaOXA-48-like variants and blaNDM genes was noted among carbapenem-resistant E. coli isolates, which was consistent with the earlier findings25.
Although previous studies reported the additive role of ESBL production, decreased OMP expression and an active efflux pump system towards carbapenem resistance development, association of carbapenemases with OMP loss and acrB upregulation was not explored before262728. In addition to exploring the well-known association between ESBL production and acrB upregulation/porin loss, a significant association of carbapenemase production with acrB upregulation was observed in the current study among CREs. Although similar significant association between ampC production and efflux pump upregulation was reported among Pseudomonas aeruginosa previously29, there is no report of such association among CREs. Significant association between ESBL/carbapenemase production and OmpC/Ompk36 reduction was also found in the present study, which was consistent with the results of Wozniak et al30. The current study demonstrated the positive role of acrB in augmenting OmpC reduction among carbapenem-resistant E. coli and that of Ompk36 in facilitating acrB upregulation among carbapenem-resistant Klebsiella spp. The overall study indicated greater contribution of Ompk36 loss over acrB expression, particularly among Klebsiella spp. Similar association between the presence of IMP-4 MBL production and loss of Ompk36 was reported in carbapenem-resistant Klebsiella oxytoca isolate ZC101 of Chinese origin31. In the present study, isolates having no ESBL and carbapenemase activities demonstrated minimal acrB overexpression and OmpC/Ompk36 loss. Thus, ESBL or carbapenemase production only in combination with acrB overexpression and porin downregulation could have played a major role in imparting carbapenem resistance among these bacteria.
Though difference in carbapenemase activity was noted among inter subgroups (Ia-Id), but mode MIC value against meropenem remained almost consistent all throughout indicating the secondary role of carbapenemases and the contribution of other mechanisms in imparting carbapenem resistance among these subgroups. Though similar findings were reported earlier among pathogenic Pseudomonas aeruginosa32, such observations were not reported for CRE. This was also evident on comparing the role of different strategies on MIC values of meropenem. In case of both E. coli and Klebsiella spp., presence of only ESBL/carbapenemases imparted MIC of meropenem within only 64-256 mg/l range, whereas the presence of ESBL/carbapenemases along with porin loss and/or efflux pump activation heightened the MIC value above 256 mg/l. All these findings indicated the contribution of efflux pump activation and porin loss over the presence of ESBLs/carbapenemases towards imparting carbapenem resistance among pathogenic enterobacterial isolates. Since the drugs targeting these mechanisms are limited in commercial markets, targeted mechanisms towards porin loss and efflux pump activation must be developed to address the carbapenem resistance issue of pathogenic bacteria.
Acknowledgment
The authors acknowledge the suggestions of Dr Syamsundar Mandal, Statistical Officer, Department of Epidemiology and Biostatistics, Chittaranjan National Cancer Research Institute, Kolkata.
Financial support & sponsorship: The first author (AP) acknowledges the financial support in the form of Junior Research Fellowship from the Indian Council of Medical Research, New Delhi [no. 3/1/3/WL/JRF-2011/HRD-129 (41937)].
Conflicts of Interest: None.
References
- Trends and patterns in antibiotic prescribing among out-of-hours primary care providers in England, 2010-14. J Antimicrob Chemother. 2017;72:3490-5.
- [Google Scholar]
- Carbapenem- resistant non-glucose-fermenting Gram-negative Bacilli: The missing piece to the puzzle. J Clin Microbiol. 2016;54:1700-10.
- [Google Scholar]
- New Delhi metallo-β-lactamase-1 in Enterobacteriaceae: Emerging resistance. CMAJ. 2011;183:59-64.
- [Google Scholar]
- A five-year experience of carbapenem resistance in Enterobacteriaceae causing neonatal septicaemia: Predominance of NDM-1. PLoS One. 2014;9:e112101.
- [Google Scholar]
- Study on MICs of tigecyclinein clinical isolates of carbapenem resistant Enterobacteriaceae (CRE) at a tertiary care centre in North India. J Clin Diagn Res. 2017;11:DC18-21.
- [Google Scholar]
- Emergence of Carbapenem-non-susceptible extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tübingen, Germany. J Med Microbiol. 2009;58:912-22.
- [Google Scholar]
- Combined porin loss and extended spectrum Beta-Lactamase production is associated with an increasing Imipenem minimal inhibitory concentration in clinical Klebsiella pneumoniae strains. Curr Microbiol. 2009;58:366-70.
- [Google Scholar]
- Enterobacteriaceae: Introduction and identification. In: Murray PR, Baron EJ, Pfaller MA, Jorgensen JH, Yolken RH, eds. Manual of clinical microbiology (8th ed). Washington, DC: ASM Press; 2003. p. :636-53.
- [Google Scholar]
- Performance standards for antimicrobial susceptibility testing. In: CLSI approved standard M100-S23 23rd informational supplement. Wayne: CLSI; 2013.
- [Google Scholar]
- Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn Microbiol Infect Dis. 2012;74:88-90.
- [Google Scholar]
- Molecular cloning: A laboratory manual (3rd ed). New York, USA: Cold Spring Harbor Laboratory, Cold Spring Harbor; 2001.
- Preparation of chromatin from animal tissues and cultured cells. Methods Cell Biol. 1978;17:27-50.
- [Google Scholar]
- Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob Agents Chemother. 2006;50:2833-5.
- [Google Scholar]
- A highly carbapenem-resistant Pseudomonas aeruginosa isolate with a novel blaVIM-4/blaP1b integron overexpresses two efflux pumps and lacks OprD. J Antimicrob Chemother. 2007;60:132-5.
- [Google Scholar]
- Chronic periodontitis and RANKL/OPG ratio in peri-implant mucosae inflammation. Braz Dent J. 2018;29:14-22.
- [Google Scholar]
- Different phenotypes of polycystic ovary syndrome in Turkish women: Clinical and endocrine characteristics. Gynecol Endocrinol. 2013;29:931-5.
- [Google Scholar]
- EulerAPE: Drawing area-proportional 3-venn diagrams using ellipses. PLoS One. 2014;9:e101717.
- [Google Scholar]
- Trends in antibiotic resistance in urologic practice. Eur Urol Focus. 2016;2:363-73.
- [Google Scholar]
- Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis. 2017;17:279.
- [Google Scholar]
- Genetic and structural insights into plasmid-mediated extended-spectrum β-lactamase activity of CTX-M and SHV variants among Pathogenic Enterobacteriaceae infecting Indian patients. Int J Antimicrob Agents. 2014;43:518-26.
- [Google Scholar]
- Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: Report from the SENTRY antimicrobial surveillance program, 2006-2007. Antimicrob Agents Chemother. 2011;55:1274-8.
- [Google Scholar]
- Coexistence of plasmid-mediated KPC-2 and IMP-4 carbapenemases in isolates of Klebsiella pneumoniae from China. J Antimicrob Chemother. 2011;66:2670-1.
- [Google Scholar]
- First description of bla(NDM-1), bla(OXA-48), bla(OXA-181) producing Enterobacteriaceae strains in Romania. Int J Med Microbiol. 2013;303:697-700.
- [Google Scholar]
- Emergence of Escherichia coli, co-producing NDM-1 and OXA-48 carbapenemases, in urinary isolates, at a tertiary care centre at central India. J Clin Diagn Res. 2014;8:DC01-4.
- [Google Scholar]
- Multidrug efflux pumps over-expression and its association with porin down-regulation and β-lactamase production among nosocomial P. aeruginosa isolates from university of Malaya medical center, Malaysia. Int J Chem Environ Biol Sci. 2015;3:125-35.
- [Google Scholar]
- A snapshot of co-resistance to carbapenems and tigecycline in clinical isolates of Enterobacter cloacae. Microb Drug Resist. 2017;23:1-7.
- [Google Scholar]
- Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese University Hospital. J Clin Microbiol. 2012;50:223-6.
- [Google Scholar]
- Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: Prevalence and impact on resistance in a Spanish multicenter study. Antimicrob Agents Chemother. 2011;55:1906-11.
- [Google Scholar]
- Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J Med Microbiol. 2012;61:1270-9.
- [Google Scholar]
- Combination of IMP-4 metallo- beta-lactamase production and porin deficiency causes carbapenem resistance in a Klebsiella oxytoca clinical isolate. Diagn Microbiol Infect Dis. 2009;65:163-7.
- [Google Scholar]
- Efflux pumps expression and its association with porin down-regulation and beta-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiol. 2010;10:217-24.
- [Google Scholar]






