Translate this page into:
Concurrent dengue infections: Epidemiology & clinical implications
For correspondence: Dr Sujatha Sunil, Vector Borne Diseases Group, International Centre for Genetic Engineering & Biotechnology, Aruna Asaf Ali Marg, New Delhi 110 067, India e-mail: sujatha@icgeb.res.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Multiple dengue virus (DENV) serotypes circulating in a geographical area most often lead to simultaneous infection of two or more serotypes in a single individual. The occurrence of such concurrent infections ranges from 2.5 to 30 per cent, reaching as high as 40-50 per cent in certain dengue hyper-endemic areas. Concurrent dengue manifests itself differently than mono-infected patients, and it becomes even more important to understand the effects of co-infecting serotypes in concurrent infections to ascertain the clinical outcomes of the disease progression and transmission. In addition, there have also been reports of concurrent DENV infections in the presence of other arboviral infections. In this review, we provide a comprehensive breakdown of concurrent dengue infections globally. Furthermore, this review also touches upon the clinical presentations during those concurrent infections categorized as mild or severe forms of disease presentation. Another aspect of this review was aimed at providing insight into the concurrent dengue incidences in the presence of other arboviruses.
Keywords
Concurrent dengue infections
dengue serotypes
epidemiology
public health
Introduction
Dengue is a mosquito-borne viral infection highly prevalent in tropical-subtropical regions of the world and is responsible for endangering more than four billion people inhabiting these regions1. As per the World Health Organisation (WHO) records, an estimated 500,000 people with the severe form of dengue infections require hospitalization annually including the paediatric group. According to the same report 2.5 per cent of hospitalized population end up dying due to complications associated with the severe form of dengue2. The studies have also shown that the chances of contracting dengue infections are highest among people of the age group 30 to 45 yr3 and the same group experiences most dengue-related deaths annually4.
The causative agent for dengue fever (DF) is dengue virus (DENV), an RNA virus from the Flavivirus genus belonging to the Flaviviridae family5. It has a positive-strand RNA genome inside a protein capsid also known as nucleocapsid which is surrounded by an envelope that gives the viral particle a roughly spherical shape5. DENV has the ability to infect a wide range of cell types including cells of the human immune system ranging from dendritic cells, monocytes, B- and T-cells, hepatocytes, endothelial cells. Considering Aedes aegypti and Ae. albopictus are natural vectors for DENV, cell lines derived from mosquito species such as C6/36 cell lines along with mammalian cell lines like BHK-21, and Vero are regularly used for propagation and studying the pathogenesis of DENV56. Evolutionary studies suggest that DENV evolved independently in nonhuman primates from ancestral sylvatic viruses and jumped from primates to humans around 500-1000 years ago in Africa or southeast Asia7.
DENV is classified into four closely related viral strains called DENV-1, DENV-2, DENV-3 and DENV-4. These four groups are termed as Siri types because of their ability to interact differently with the antibodies present in the host sera. The serotypes are named based on the chronology of the discoveries. Serotype 1 being the 1st to be discovered by Ren Kimura and Susumu Hotta in 1943 in Japan8. Each of these serotypes share approximately 65 per cent of their genomes, variations in the rest of their genome led to the presentation of different genotypes within serotypes9. In 2013, a new serotype, DENV-5 was identified from the sample of a 37 yr old patient admitted at the Sarawak State of Malaysia in the year 2007; though after that incident, it has not been further reported10. Unlike the other four serotypes, DENV-5 follows the sylvatic transmission cycle and primarily circulates among non human primates11. Reports in the past decade provided evidence for all four serotypes circulating in the same geographical as well as environmental niches, contributing equally towards the disease burden and infecting populations either as mono or concurrent in infecting states12. Simultaneous circulation of multiple serotypes within a geographical region, especially in dengue-endemic countries increase the chances of an individual getting infected with more than one serotype also known as concurrent infection, a common observation in hyperendemic regions13. This could be the result of either a single bite from a mosquito harbouring multiple serotypes or multiple bites by different mosquitoes infected with the individual serotype of the virus, considering that there has been no evidence of DENV transmission in mosquitoes and asymptomatic individuals serve as reservoirs for the virus, transmitting the virus to mono-infected mosquitoes making them concurrently infected vectors14. Some reports emphasized that the likelihood of an individual kid contracting concurrent infections is lower than being sequentially infected with different DENV serotypes which is in concordance with the laws of probability1516, although in a laboratory study, a single Ae. aegypti mosquito has been shown to transmit viruses belonging to more than one serotype during a single feeding episode17.
Concurrent infections are different from secondary infections in that there are no temporal differences in the infection conditions, i.e. multiple DENV RNA/particle are detectable in the patient sera at the time of clinical presentation while in the case of secondary infections there is only presence of antibody-positivity for either serotype during active infection of a second serotype, which makes PCR-based serotyping along with IgG/M ELISA a confirmatory tool to differentiate between concurrent and secondary dengue infections. Current diagnostic protocol is presented in diagrammatic form in Figure.
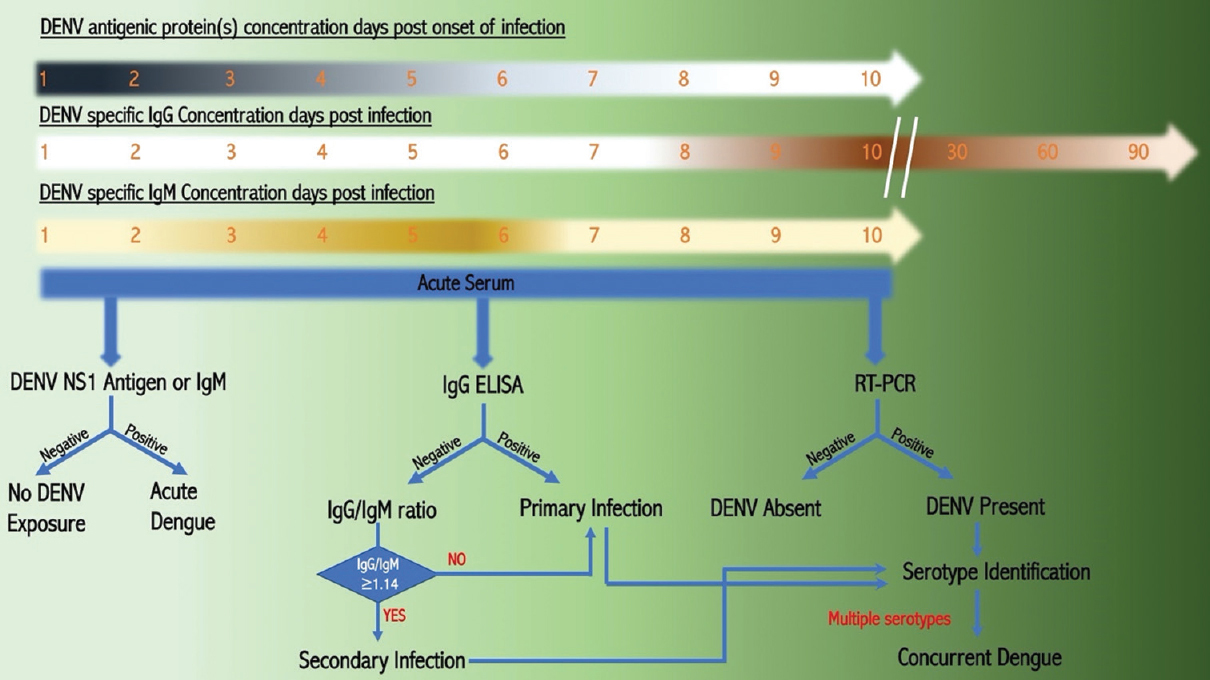
- Diagrammatic representation of diagnostic protocol for identification of dengue infection at the number of days post-infection. Increase in the concentration of DENV antigen (Viral Protein) and IgM at days post-infection.
There have been various reports from tropical and subtropical countries across the world of patients testing positive for heterosubtypic dengue infections1819202122232425262728.
In addition to the above reports, there have also been many reported incidences of concurrent dengue infections from different parts of India2930313233343536373839404142. Furthermore, disease severity in an individual has been directly linked to the circulating serotypes and to repeat exposed to different serotypes simultaneously or sequentially. The current review was aimed at providing a comprehensive overview of the epidemiology of concurrent dengue infections across the globe and also the clinical presentation of the condition. Other arboviral infections that coexist with concurrent DENV infections were also briefly broach upon.
Epidemiology of concurrent infections by dengue serotypes
Although the first dengue epidemics reported across Asia, Africa and Americas were around the same time, it was in 1789 that Benjamin Rush presented the first case report and coined the term ‘Break Bone fever’ to its symptoms of arthralgia and myalgia404344. Only in the 1960s, the serotype-specific epidemiology reports of dengue were available and individual serotypes were introduced serially. However, the epidemiology of these infections individually or concurrently have been quite varied across continents143345. In the following sections, we would like to highlight important incidences of such reports across the world.
The Americas: Although over the years there have been reports of multiple serotypes circulating in the region it was in 1982 that the first-ever confirmed case of concurrent dengue infection in the world was reported from Puerto Rico in North America14464748. Ever since there has been constant push toward investigating the serotype multiplicity in the patients presenting with dengue symptoms. The infection was caused by serotype DENV-1 and 4 in a single patient4849. Despite reports of dengue infection in parts of the rest of America, no concurrent infections have been reported7.
The 1980 data from Mexico confirms only mono infections in the south and southeastern regions of the country through DENV-1 and 2 was reported to be abundantly circulating among the population. Only in 1983 that the first-ever case of concurrent dengue infection was reported from the Central American region4850 Brazil. This was a major dengue outbreak in 1987 where over 100,000 people contracted the infection, and it was during the same outbreak that South America saw its first report of concurrent dengue infection. Looking at the serotypes of concurrent infections during the initial years, a pattern can be seen where the introduction of a new serotype brought with it a serotypic shift that resulted in concrete infection in the subsequent years4748. For example, in the case of Puerto Rico before the 1981-82 outbreak, all but DENV-4 strains were waiting in the region, but the first concurrently infected patient was found to harbor DENV-4 along with DENV-148 which was in circulation since 1977. Interestingly DENV-3 and 2 were reported to be individually circulating serotypes in the region during 1963 and 1969 outbreaks respectively47. Thereafter all four serotypes have been known to be circulating in this region47. A similar pattern could also be seen in the case of Mexico where only DENV-1 or 2 were known to be circulating as mono infections until 1982; however, during an outbreak in 1983 DENV-4 was first reported as a concurrent infection along with DENV-148, post which the DENV-1 and DENV-4 were the most frequently isolated serotypes in Mexico (47 and 37% respectively) existing as single as well as concurrent infections. Again, in 1995, there was a serotypic shift in the region, with DENV-2 being the most prevalent serotype followed by DENV-4 and 1. Simultaneously, DENV-3 serotype also began to circulate in the region. The existence of DENV-3 increased the reports of concurrence involving DENV-1, 3 and 4 to a large extent, with more than 35,000 cases being reported after 199650.
Similar to Central America, South America reported mono-infections of dengue in 1981 involving DENV-1 and 4. However, in 1987, a major outbreak affecting more than 100,000 individuals occurred in Brazil due to the introduction of DENV-2. Since 1990, many concurrent infections have been detected in several patients in Brazil5152. During 1998–2003, DENV-1 and 3 were observed in the north-eastern region of Brazil525354. During 2010 and 2011, DENV-4 re-emerged in the country, and since then, concurrent infections involving DENV-1, 3 and 4 have been reported55565758. In 2021, co-circulation of all four DENV serotype was detected in Guatemala and Mexico, while serotypes DENV 1, 2 and 3 have been co-circulating in Colombia, French Guiana, and Martinique, and in Paraguay, DENV-1, 2 and 4 have been co-circulating59.
Asia: Unlike the Americas, Asia, especially South-East Asia has remained endemic for all four serotypes of DENV since the 1960s, and their distribution patterns have hardly changed throughout the region60. In India, the first virologically confirmed case of DENV infection was reported in 1963-646061. Ever since dengue has remained endemic to India. In 2003, all DENV types were reported to be circulating in the region and Delhi became one of the worst-hit hyperendemic regions in the country3334. Since then, India has witnessed several outbreaks caused by all four serotypes, and a high percentage of concrete infections were recorded across the country. In 2006, Delhi witnessed 19 per cent patients infected with concurrent dengue, the highest ever cases in the region, with most infections involved DENV-1/3 strains. Nevertheless, other serotypic combinations such as DENV-1/4, DENV-2/3 and DENV-3/4 also reported the same here. DENV-4 was seen to be on the decline with the last report of the serotype recorded in 2009 until an outbreak in 2017 in South India where 56 (82%) samples out of 68 were found to be positive for DENV-4 and 18 (26%) were concurrently infected with at least one of the other stereotypes61. In northeast India, there were reports of the first DENV outbreak due to all DENV serotypes circulating in Manipur during 200762. The study also reported concurrent infections in the region with DENV-1/ 3, DENV-2/ 3 and DENV-1/ 4 as concurrent infections; interestingly, DENV-2 and 4 were not present alone and only cases of DENV-1 infection was reported5963. Another major outbreak reported from northeast India was in 2015 in the Pasighat region of Arunachal Pradesh, where the predominant sero type of DENV detected was DENV-1 with almost 90 per cent of 66 dengue positive cases. Five cases were of DENV-2 infection and only one case of concurrent infection with DENV-1 and 2 was identified64. In 2017–2018, during a dengue outbreak in the Theni district in Tamil Nadu, all four-dengue sero types were found circulating and cases with multiple serotype infection were also identified37. Another dengue hyper-endemic country in south Asia is Sri Lanka. This small island nation situated in the Indian ocean is also affected by all four serotypes circulating across the island65. interestingly unlike in its neighboring countries, DENV-3 has remained the pre-dominant serotype in the country and ever since Sri Lanka has seen frequent outbreaks of dengue virus infections66. A study conducted in 2011-12 spanning three different provinces showed as high as 10.3 per cent of concrete infection cases involving DENV-1 and 267.
In the case of Pakistan, dengue infections have mostly been limited to parts of the Sindh and Punjab provinces since 19946869. However, a major outbreak hit Pakistan in the year 2011 which saw 290 deaths in the city of Lahore alone7071. The report presented district-wise figures for dengue from two provinces. All four serotypes were detected in Punjab province with the presence of DENV-2 (41.64%) and DENV-3 (41.05%). The report also presents evidence of DENV-2 and 3 mixed infections in Punjab (3.81%) and 8.33 per cent mixed infections in people from the Khyber Pakhtunkhwa region in the same year. In 2013, Pakistan saw another outbreak of dengue infections with all 4 serotypes circulating amongst the population7273.
Southeast Asia presents a pattern similar to that of south Asia with respect to dengue serotype co-circulation. The first report of concurrent infection was recorded in Thailand in 1990 in two patients6774. Since then there have been several reports of concrete infections from Thailand, Malaysia, Taiwan, Vietnam, China and Indonesia747576777879. It should be noted that of all the serotypes, DENV-2 is the most predominant, with DENV-1 and 3 intermittently co-circulating and causing concurrent infections76. Among the southeast Asian countries, Malaysia reported the highest number of concurrent infections during an outbreak in 2014, with either DENV-1 or 3. Dengue has become a major public health concern in Indonesia, and the disease has spread to all 34 provinces in the country since it was first discovered in 196879. Analysis of stored DENV infected samples from the 1970s tested from different parts of the country recorded concurrent infection involving serotypes. Though concurrent infections were reported in Indonesia during 2011-12, their serotypes were not specified. More recently, concurrent infection due to DENV-2 and 3 has been reported from Jakarta80. A one-month surveillance study during the peak dengue season in 2015 conducted in Tarlac City, Philippines, identified concurrent circulation of three serotypes DENV-1, 2, and 4 in adult Ae. aegypti mosquitoes collected from the homes of suspected dengue patients and confirmed dengue patients as well81.
Other parts of the globe: Apart from Asia and the Americas, concurrent infections have been reported in other parts of the globe as well. In 1989, New Caledonia, a south Pacific island, recorded concurrent infections of DENV-1 and 3 during an epidemic82. Likewise, Yemen reported their first case of dengue in a traveller in 1984, and since 1994, this infection has become a notable disease in the country. As seen in other parts of the world, the most recent outbreak in 2012-13 revealed more than 14 per cent of the studied population tested positive for concurrent infection due to all four serotypes either as dual or in rare cases as triple infections83. Dengue was reported in Africa in the late 19th and early 20th centuries. Between 1960 and 2010, twenty laboratory-confirmed dengue outbreaks were reported in 15 African countries84. Concurrent infections were reported in a single study from Somalia during an outbreak in 199383.
The year 2019 witnessed the largest number of dengue infections worldwide and dengue cases were also recorded in Afghanistan for the first time1. However, the impact of these infections in the subsequent year, 2020, has not been well documented owing to the COVID-19 pandemic.
Imported cases of concurrent dengue infections
Travellers are more exposed to dengue infections and get infected with DENV from endemic countries. Once back in their own countries, if in the viremic phase, such people introduce new serotypes into a non-endemic country85. These cases are important as these provide the time points of the introduction of new serotypes into the country and, depending on other environmental and vector factors, may result in the permanent establishment of these serotypes in the country. Imported concurrent infections are rare and have been reported in Japan, Belgium, China and the Netherlands8586878889. In each of these cases, all the travellers were infected during their travels to dengue hyperendemic countries.
Clinical presentation in concurrent infections
One of the major hallmarks of DENV infection is the dissemblance in the clinical presentation of the disease symptoms90. WHO in its previous guidelines had classified dengue infections into three varied stages viz, dengue fever (DF), dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS)91. However, with the increase in global dengue incidences and vast spectrum of clinical presentations, it became evident that there is an overlap between DF, DHF and DSS manifestations. Based upon this observation, in 2012, WHO revised the classification of dengue infections91.
Despite the fact that all serotypes share a similar infection mechanism, the virological features have varied presentations, both within and across four serotypes. There have been reports that within a particular serotype, some genotypes are more virulent than the others and thus have a higher propensity to cause severe symptoms, resulting in a spectrum of disease severity among the population12.
In addition, it is a well accepted fact amongst the scientific fraternity that primary infections with a particular serotype provide immunity against that particular serotype only9293. The flipside to such immunity is the phenomenon called antibody-dependent enhancement or ADE in which antibodies against one serotype instead of mitigating the chances for secondary infection by another serotype aid in the viral uptake by immune cells resulting in the severe form of dengue9495. Furthermore, when an individual is infected with more than one DENV serotype, such infections become more complicated559596. There have been mixed reports on the disease severity during concurrent infections some supporting the increased severity and others reporting less severe form of dengue in multiple serotype infections. Majority of such patients have been reported to develop DF or severe dengue with mild complications and have recovered without any serious consequences9697. That being said, co-circulation of all four DENV serotypes in dengue hyperendemic areas is common and has serious ramifications towards public health considering the rise in the number of concurrent infections. Only a few studies have been conducted on the clinical implications associated with concurrent infections14555798.
Following is an overview of studies performed on concurrent dengue infection and the clinical manifestations categorized based on the severity of disease outcome and the underlying differences in the clinical presentations between the two categories.
Clinical presentation during concurrent infection – Mild: Limited studies are available that reveal concurrent dengue-infected patients recovered without relapse and presented with high fever, headache, arthralgia, myalgia, retro-orbital pain, asthenia and without any haemorrhagic manifestation308299100 (Table I). Slightly elevated transaminase levels were also reported in mild concurrently infected patients, most of whom presented with primary infection99101. On the other hand, the progression of dengue into severe forms may be driven by factors other than viral, although the recombination and emergence of DENV strains might be more virulent and aggressive in causing severe dengue.
| Symptoms | Concurrent DENV infections | References | |
|---|---|---|---|
| Mild | Severe | ||
| Fever | √ (+++) | 51,52,63 | |
| Headache | √ | √ (++) | 51,52,63 |
| Mayalgia | √ | √ (+++) | 51,52,63 |
| Arthralgia | √ | √ (+++) | 51,52,63 |
| Retro orbital pain | √ | √ (+++) | 51,52,63 |
| Rashes | √ | √ (+++) | 99 |
| Bleeding | x | √ (+++) | 46,98,103 |
| Pleural effusion | x | √ (+++) | 46,72 |
| Ascites | √ | √ (+++) | 80,99 |
| Hepatomegaly | √ | √ (+++) | 79,97 |
+, presence of symptom; −, absence of symptoms or data. DENV, dengue virus
Clinical presentation during concurrent infection – Severe: There have been lots of contrasting reports on the clinical presentations in the case of patients contacting concurrent dengue infections ranging from milder to life threatening102. Two studies from India have presented reports of patients exhibiting highly severe disease, although the reasons for such severe manifestations of the disease are unclear36103. Considering the above scenarios, it can well be hypothesized that presence of multiple serotypes in an individual most likely abates the increase in viremia in the patients, leading to increased disease severity.
The presence of significantly higher intensity of warning signs (90%) and other severe disease manifestations (15%) among patients with concurrent DENV infection were recorded in studies conducted in Malaysia, India and Brazil (Table II). The major fraction of patients suffering from dengue showed elevated creatinine levels along with pleural effusion. Furthermore, the frequency of patients with severe thrombocytopenia and lower platelet count has also been reported364976103104. Although atypical manifestations of dengue are uncommon105106, patients with concurrent dengue infection showed hepatic, pulmonary and renal complications80.
| Year | Number of CHIK patients | Genotype of CHIKV | Number of dengue patients (deaths) | Dengue serotype |
|---|---|---|---|---|
| 2006 | 67 | ECSA | 3366 (65) | DENV 3 |
| 2007 | 22 | ECSA | 548 (1) | DENV 2 |
| 2008 | 0 | ECSA | 1312 (2) | DENV 1 |
| 2009 | 17 | ECSA | 15,535 (96) | DENV 2 and 4 |
| 2010 | 120 | ECSA | 6259 (8) | DENV 1 |
| 2011 | 110 | ECSA | 1131 (8) | DENV 1 and 2 |
| 2012 | 6 | ECSA | 2093 (4) | DENV 2 |
| 2013 | 18 | ECSA | 5574 (6) | DENV 2 |
| 2014 | 8 | ECSA | 995 (3) | DENV 2 |
| 2015 | 64 | ECSA | 15,867 (60) | DENV 2 and 4 |
| 2016 | 12,221 | ECSA | 4393 (10) | DENV 3 |
CHIK, chikungunya; CHIKV, CHIK virus; ECSA, East/Central/South African
Concurrent dengue infection with other arboviruses
Considering the involvement of the same vector in the transmission of several arboviruses, there have been quite a few studies that have reported the existence of other arboviruses along with two or more dengue serotypes in the same individual at the time of presentation. Since there are similarities in the clinical characteristics between most of the arboviral infections (dengue, chikungunya, Zika, etc.), such concurrent infections have tendencies to get misdiagnosed or misinterpreted as mono-infections, especially during clinical stages. Such failures may pose a significant risk to the patient and sometimes lead the patient to life-threatening stages as has been reported in many such cases107. It becomes imperative to develop strategies such as efficient diagnostic tools for the identification of concurrent arboviral infections especially given that there have been reports of massive re-emergence of arboviral infections, hence necessitating extensive protective measures.
In the past decade, infections due to arboviruses have increased substantially, especially DENV and CHIKV in Asia-Pacific regions and the Americas108109. Furthermore, in Europe and the Western hemisphere, there has been a surge in West Nile Virus infections110. Zika virus is also re-emerging in the Americas and southeast Asia. With increased global travels, Zika virus is also spreading to other parts of the world111. Simultaneous infections of DENV along with other arboviruses usually result in severe clinical outcomes for the patients, therefore suggesting the immediate requirement for developing and implementing robust and quick diagnostic tools to counter these problems.
The role of DENV serotypes in the transmission and pathogenesis of other arboviruses presents a crucial aspect in disease epidemiology, which needs to be investigated further. As a case study, we analyzed the circulation of DENV and CHIKV in Delhi, India, during 2006-16 and have tried to correlate the outbreak of these two infections with concurrent dengue infection (Table II). Delhi has been reported to be the hyper-endemic region for DENV due to the simultaneous incidences of all four DENV serotypes112. Incidences of CHIKV infections have been reported in Delhi since 2006, both as mono-infections and co-infections along with DENV36. In 2006 there were 67 reported cases of CHIKV infections with 3366 cases of DENV-3 infections and year after that there was a switch in circulatory DENV serotype from DENV-2 to 3 and to 1 the year after, all the while CHIKV cases were on decline until 2010 when the number of cases went up to 120 (Table II). Interestingly, a year before there were reports of DENV-2 and 4 co-circulating in the region causing more than 15,000 infected cases. We also observed in 2010 the circulating strain of dengue was DENV-1. Before 2010 whenever the strains were the mono-circulating highest number of infections were reported in the case of DENV-3 that was in 2006 and the only time DENV-3 was found to be circulating in the population alone was in 2016 (Table II). In 2016, there was a surge in the number of CHIKV infections, the highest ever with 12,221 reported cases. Whenever DENV-2 was found to be co-circulating in presence of DENV-4 as reported during 2009 and 2015, unprecedented numbers of dengue cases have been reported. We also observed that in the year preceding 2016 there were reports of DENV-2 and 4 co-circulating which was also the case during 2009-2010 which showed a jump in chikungunya infections post-co-circulation of DENV-2 and 4 (Table II). The recent molecular surveillance study during 2017-18 from south Delhi comparing DENV/CHIKV co-infection and DENV mono-infection from 2008 to 2010 detected the high prevalence of CHIKV IgG antibody in 2017-18 and joint pain was particularly present in co-infection cases as compared to DENV mono-infection113. These observations prompt us to hypothesize that co-circulation of dengue serotypes may affect the occurrence of other arboviral infections, particularly CHIKV, and warrant detailed epidemiological surveillance of arboviral infections in a locality to understand the transmission dynamics of the infections (Table II).
Conclusion and future perspectives
Among the mosquito-borne arboviral infections, dengue has become a huge public health issue across the globe. Owing to the complexity of its existence as serologically distinct virus populations, concurrent dengue infections pose serious concerns. However, due to the absence of affordable diagnostic and screening assays with high sensitivity and specificity, concurrent dengue infections are poorly understood with respect to its occurrence, its clinical presentations as well as its implications. Furthermore, the impact of the presence of two or more serotypes at a given time point on other arboviral infections is poorly understood. What is the actual disease burden owing to the presence of multiple serotypes? How does this alter the complications associated with secondary dengue? Do the four serotypes replicate similarly in the vector? Does this, in turn, decide the transmission dynamics of the serotypes to the hosts? How does this change the replication of other arboviruses in the mosquito population? While much research is being pursued on dengue, concurrent dengue is one aspect of this complex disease that requires special attention and further well planned studies that answer these questions are essential.
Financial support & sponsorship: The authors thank International Centre for Genetic Engineering and Biotechnology for providing core funding for the completion of this review.
Conflicts of Interest: None.
References
- 2021. WHO dengue and severe dengue. Available from:https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- Epidemiology of dengue:Past, present and future prospects. Clin Epidemiol. 2013;5:299-309.
- [Google Scholar]
- Structure of dengue virus:Implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717-25.
- [Google Scholar]
- Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. J Virol. 2007;81:13325-34.
- [Google Scholar]
- Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74:3227-34.
- [Google Scholar]
- Molecular epidemiology of dengue viruses from complete genome sequences (Desertation). Switzerland: University of Basel; 2010.
- Global spread of dengue virus types:Mapping the 70 year history. Trends Microbiol. 2014;22:138-46.
- [Google Scholar]
- Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415.
- [Google Scholar]
- Discovery of fifth serotype of dengue virus (DENV-5):A new public health dilemma in dengue control. Med J Armed Forces India. 2015;71:67-70.
- [Google Scholar]
- Spatial-temporal co-circulation of dengue virus 1, 2, 3, and 4 associated with coinfection cases in a hyperendemic area of Brazil:A 4-week survey. Am J Trop Med Hyg. 2016;94:1080-4.
- [Google Scholar]
- Common occurrence of concurrent infections by multiple dengue virus serotypes. Am J Trop Med Hyg. 1999;61:725-30.
- [Google Scholar]
- Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015;112:14688-93.
- [Google Scholar]
- Occurrence of concurrent infections with multiple serotypes of dengue viruses during 2013-2015 in northern Kerala, India. Peer J. 2017;5:e2970.
- [Google Scholar]
- Double infection of heteroserotypes of dengue viruses in field populations of Aedes aegypti and Aedes albopictus (Diptera:Culicidae) and serological features of dengue viruses found in patients in southern Thailand. Southeast Asian J Trop Med Public Health. 2006;37:468-76.
- [Google Scholar]
- Dengue virus co-infections with multiple serotypes do not result in a different clinical outcome compared to mono-infections. Epidemiol Infect. 2020;148:e119.
- [Google Scholar]
- High dengue burden and circulation of 4 virus serotypes among children with undifferentiated fever, Kenya, 2014-2017. Emerg Infect Dis. 2020;26:2638.
- [Google Scholar]
- The clinical features of co-circulating dengue viruses and the absence of dengue hemorrhagic fever in Pakistan. Front Public Health. 2020;8:287.
- [Google Scholar]
- Occurrence of 4 dengue virus serotypes and chikungunya virus in Kilombero Valley, Tanzania, during the dengue outbreak in 2018. In:Open forum infectious diseases. US: Oxford University Press; 2021.
- Dengue virus in humans and mosquitoes and their molecular characteristics in northeastern Thailand 2016-2018. PloS One. 2021;16:e0257460.
- [Google Scholar]
- All four dengue virus serotypes co-circulate in concurrent dengue infections in a single dengue session in Chittagong, Bangladesh. Biores Commun. 2022;8:1082-48.
- [Google Scholar]
- Concurrent circulation of dengue serotype 1, 2 and 3 among acute febrile patients in Cameroon. PLoS Negl Trop Dis. 2021;15:e0009860.
- [Google Scholar]
- Dengue virus seroprevalence study in Bangphae district, Ratchaburi, Thailand:A cohort study in 2012-2015. PLoS Negl Trop Dis. 2022;16:e0010021.
- [Google Scholar]
- Circulating dengue virus serotypes and vertical transmission in Aedes larvae during outbreak and inter-outbreak seasons in a high dengue risk area of Sri Lanka. Parasit Vectors. 2021;14:1-11.
- [Google Scholar]
- Dengue virus epidemics:A recent report of 2018 from district Swat, Khyber-Pakhtunkhwa Pakistan. Int J Mosquito Res. 2021;8:105-8.
- [Google Scholar]
- Co-circulation of all four dengue virus serotypes:First report from Odisha. Indian J Med Microbiol. 2017;35:293-5.
- [Google Scholar]
- An epidemiological study of dengue and its coinfections in Delhi. Int J Infect Dis. 2018;74:41-6.
- [Google Scholar]
- Episode of coexisting infections with multiple dengue virus serotypes in central Karnataka, India. J Infect Public Health. 2013;6:302-6.
- [Google Scholar]
- Occurrence of co-infection with dengue viruses during 2014 in New Delhi, India. Epidemiol Infect. 2017;145:67-77.
- [Google Scholar]
- Circulation of dengue virus serotypes in hyperendemic region of New Delhi, India during 2011-2017. J Infect Public Health. 2020;13:1912-9.
- [Google Scholar]
- Molecular investigation of the dengue outbreak in Karnataka, South India, reveals co-circulation of all four dengue virus serotypes. Infect Genet Evol. 2021;92:104880.
- [Google Scholar]
- Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis. 2009;15:1077-80.
- [Google Scholar]
- Molecular diversity of dengue virus serotypes 1-4 during an outbreak of acute dengue virus infection in Theni, India. Indian J Med Microbiol. 2020;38:401-8.
- [Google Scholar]
- A 5-year study of dengue seropositivity among suspected cases attending a teaching hospital of North-Western region of India. J Med Virol. 2021;93:3338-43.
- [Google Scholar]
- Viral characteristics and clinical presentation in dengue co-infection –Findings from a facility based observational study in Odisha, India. J Family Med Prim Care. 2021;10:2958-63.
- [Google Scholar]
- Prevalence of dengue serotypes and its correlation with the laboratory profile at a tertiary care hospital in Northwestern India. Cureus. 2021;13:e15029.
- [Google Scholar]
- Occurrence of dengue virus infection with multiple serotypes at central Karnataka, India. J Lab Physicians 2021 doi:10.1055/s-0041-1739536
- [Google Scholar]
- Epidemiological and clinical characterization of dengue virus serotypes during 2017-2019 in southern Kerala, India. Trans R Soc Trop Med Hyg 2022 doi:10.1093/trstmh/trac001
- [Google Scholar]
- An account of the bilious remitting fever:As it appeared in Philadelphia, in the summer and autumn of the year 1780. Am J Med. 1951;11:546-50.
- [Google Scholar]
- Epidemiology of dengue:Past present and future prospects. Clin Epidemiol. 2013;5:299-309.
- [Google Scholar]
- Emergence of epidemic dengue/dengue hemorrhagic fever as a public health problem in the Americas. Infect Agents Dis. 1993;2:383-93.
- [Google Scholar]
- Lessons learned from dengue surveillance and research, Puerto Rico, 1899-2013. Emerg Infect Dis. 2019;25:1522-30.
- [Google Scholar]
- Dengue virus circulation and evolution in Mexico:A phylogenetic perspective. Arch Med Res. 2006;37:760-73.
- [Google Scholar]
- Detection of dengue fever virus serotype-4 by using one-step real-time RT-PCR in Hodeidah, Yemen. Br Microbiol Res J. 2016;14:24380.
- [Google Scholar]
- Population genetics of dengue virus and transmission of dengue fever. Salud Publica Mex. 2009;51:s403-9.
- [Google Scholar]
- Dengue in Latin America –A unique situation. Available from:https://apps.who.int/iris/bitstream/handle/10665/163727/dbv26p62.pdf;sequence=1#:~:text=It%20has%20been%20estimated%20that,increased%20rates%20of%20dengue%20transmission
- [Google Scholar]
- Molecular characterization of dengue viruses type 1 and 2 isolated from a concurrent human infection. Rev Inst Med Trop São Paulo. 2003;45:11-6.
- [Google Scholar]
- Dengue in Latin America:Systematic review of molecular epidemiological trends. PLoS Negl Trop Dis. 2017;11:e0005224.
- [Google Scholar]
- Dengue in the State of Rio de Janeiro, Brazil, 1986-1998. Mem Inst Oswaldo Cruz. 1999;94:297-304.
- [Google Scholar]
- Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerg Infect Dis. 2005;11:48-53.
- [Google Scholar]
- Concurrent infection with dengue virus type-2 and DENV-3 in a patient from Ceará Brazil. Mem Inst Oswaldo Cruz. 2006;101:925-8.
- [Google Scholar]
- Clinical and virological descriptive study in the 2011 outbreak of dengue in the Amazonas, Brazil. PLoS One. 2014;9:e100535.
- [Google Scholar]
- Phylogenetic and evolutionary analysis of dengue virus serotypes circulating at the Colombian-Venezuelan border during 2015-2016 and 2018-2019. PLoS One. 2021;16:e0252379.
- [Google Scholar]
- World Health Organization. Epidemiological update:dengue and other arboviruses. Available from:https://www.paho.org/en/documents/epidemiological-update-dengue-and-other-arboviruses-10-june-2020
- [Google Scholar]
- Emergence of dengue virus 4 as the predominant serotype during the outbreak of 2017 in South India. Indian J Med Microbiol. 2019;37:393-400.
- [Google Scholar]
- Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur, India –Association with meteorological factors. Indian J Med Res. 2012;136:649.
- [Google Scholar]
- Detection of dengue-4 virus in Pune, Western India after an absence of 30 years –Its association with two severe cases. Virol J. 2011;8:46.
- [Google Scholar]
- Molecular characterisation and phylogenetic analysis of dengue outbreak in Pasighat, Arunachal Pradesh, Northeast India. Indian J Med Microbiol. 2018;36:37-42.
- [Google Scholar]
- Epidemiology of dengue in Sri Lanka before and after the emergence of epidemic dengue hemorrhagic fever. Am J Trop Med Hyg. 2002;66:765-73.
- [Google Scholar]
- A preliminary study on clinical profiles of dengue and dengue haemorrhagic fever suspected patients from two hospitals in the Western Province of Sri Lanka. Sri Lankan J Infect Dis. 2014;4:99-107.
- [Google Scholar]
- Co-infections with multiple dengue virus serotypes in patients from 3 different Provinces of Sri Lanka, a dengue hyper endemic country. Int J Infect Dis. 2016;45:457.
- [Google Scholar]
- Evolution and heterogeneity of multiple serotypes of Dengue virus in Pakistan, 2006-2011. Virol J. 2013;10:275.
- [Google Scholar]
- Dengue haemorrhagic fever outbreak in Karachi, Pakistan, 1994. Trans R Soc Trop Med Hyg. 1995;89:619-20.
- [Google Scholar]
- A review of dengue as an emerging disease in Pakistan. Public Health. 2013;127:11-7.
- [Google Scholar]
- Dengue fever:Pakistan's worst nightmare. WHO South East Asia J Public Health. 2012;1:229.
- [Google Scholar]
- Circulating serotypes of dengue virus and their incursion into non-endemic areas of Pakistan;a serious threat. Virol J. 2016;13:144.
- [Google Scholar]
- Preliminary seroepidemiological survey of dengue infections in Pakistan, 2009-2014. Infect Dis Poverty. 2017;6:48.
- [Google Scholar]
- Applications of polymerase chain reaction for identification of dengue viruses isolated from patient sera. Microbiol Immunol. 1993;37:41-7.
- [Google Scholar]
- Early clinical and biological features of severe clinical manifestations of dengue in Vietnamese adults. J Clin Virol. 2009;45:276-80.
- [Google Scholar]
- Impact of dengue virus (DENV) co-infection on clinical manifestations, disease severity and laboratory parameters. BMC Infect Dis. 2016;16:406.
- [Google Scholar]
- Concurrent infections by two dengue virus serotypes among dengue patients in Taiwan. J Microbiol Immunol Infect. 2003;36:89-95.
- [Google Scholar]
- Detection of concurrent infection with multiple dengue virus serotypes in Thai children by ELISA and nested RT-PCR assay. Arch Virol. 2008;153:2225-32.
- [Google Scholar]
- Detection of flaviviruses by reverse transcriptase-polymerase chain reaction with the universal primer set. Microbiol Immunol. 1997;41:209-13.
- [Google Scholar]
- Concurrent infections of dengue viruses serotype 2 and 3 in patient with severe dengue from Jakarta, Indonesia. Asian Pac J Trop Med. 2016;9:134-40.
- [Google Scholar]
- Surveillance of dengue virus in individual Aedes aegypti mosquitoes collected concurrently with suspected human cases in Tarlac City, Philippines. Parasit Vectors. 2020;13:594.
- [Google Scholar]
- Demonstration of concurrent dengue 1 and dengue 3 infection in six patients by the polymerase chain reaction. J Med Virol. 1991;34:51-4.
- [Google Scholar]
- Molecular and epidemiologic analysis of dengue virus isolates from Somalia. Emerg Infect Dis. 1998;4:299-303.
- [Google Scholar]
- Dengue virus infection and associated risk factors in Africa:A systematic review and meta-analysis. Viruses. 2021;13:536.
- [Google Scholar]
- Dengue infections in travellers. Paediatr Int Child Health. 2012;32((Suppl 1)):28-32.
- [Google Scholar]
- First dengue co-infection in a Belgian traveller returning from Thailand, July 2013. J Clin Virol. 2014;61:597-9.
- [Google Scholar]
- 2021. Chikungunya and dengue—Japan:ex Cambodia (Kampong Cham). Available from: https://promedmail.org/promed-post/?id=1909917
- Dengue fever among ill-returned travellers and concurrent infection by two dengue virus serotypes. Dengue Bulletin. 2009;33:60-9.
- [Google Scholar]
- Simultaneous infection with dengue 2 and 3 viruses in a Chinese patient return from Sri Lanka. J Clin Virol. 2005;32:194-8.
- [Google Scholar]
- Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999;53:35-70.
- [Google Scholar]
- The revised WHO dengue case classification:Does the system need to be modified? Paediatr Int Child Health. 2012;32((Suppl 1)):33-8.
- [Google Scholar]
- Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921-7.
- [Google Scholar]
- Differential replication of dengue virus serotypes 2 and 3 in coinfections of C6/36 cells and Aedes aegypti mosquitoes. J Infect Dev Ctries. 2014;8:876-84.
- [Google Scholar]
- Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS One. 2016;11:0154760.
- [Google Scholar]
- Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421-67.
- [Google Scholar]
- Transmission dynamics and epidemiology of dengue:Insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999;354:757-68.
- [Google Scholar]
- Changing pattern of dengue virus serotypes in Thailand between 2004 and 2010. J Health Popul Nutr. 2012;30:366-70.
- [Google Scholar]
- Potential risk for dengue hemorrhagic fever:The isolation of serotype dengue-3 in Mexico. Emerg Infect Dis. 1996;2:133-5.
- [Google Scholar]
- Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl Trop Dis. 2011;5:e1180.
- [Google Scholar]
- Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J Exp Biol. 2010;48:849-57.
- [Google Scholar]
- Molecular surveillance of dengue in Semarang, Indonesia revealed the circulation of an old genotype of dengue virus serotype-1. PLoS Negl Trop Dis. 2013;7:2354.
- [Google Scholar]
- Dengue classification:current WHO vs. the newly suggested classification for better clinical application? J Med Assoc Thai. 2011;94((Suppl 3)):S74-84.
- [Google Scholar]
- Dengue virus and other arboviruses:A global view of risks. ISBT Sci Ser. 2012;7:274-82.
- [Google Scholar]
- Importance of case definition to monitor ongoing outbreak of chikungunya virus on a background of actively circulating dengue virus, St Martin, December 2013 to January 2014. Eurosurveillance. 2014;19:20753.
- [Google Scholar]
- Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16:1-11.
- [Google Scholar]
- Emergence of West Nile virus lineage 2 in Europe:A review on the introduction and spread of a mosquito-borne disease. Front Public Health. 2014;2:271.
- [Google Scholar]
- Molecular surveillance of Dengue Virus (DENV) and its co-infection with Chikungunya Virus (CHIKV) among febrile patients:A comparative study from South Delhi, India. J Appl Nat Sci. 2021;13:433-42.
- [Google Scholar]






