Translate this page into:
Complete response of metastatic gastric cancer to chemoimmunotherapy
*For correspondence: kina@hospy.or.jp
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 64 yr old man with a complaint of abdominal pain, presented to the department of Medical Oncology, Nagoya Memorial Hospital, Nagoya, Japan, in August 2010. Computed tomography demonstrated multiple liver tumours (Fig. 1). Advanced gastric cancer type 2 was diagnosed using gastrointestinal videoendoscopy (Fig. 2). Triple therapy with S-1, paclitaxel and cisplatin in combination with lentinan, a representative mushroom β-glucan, was initiated. Re-evaluation after six cycles showed complete disappearance of the primary gastric lesion in April 2011 and only S-1 was continued in combination with lentinan. Complete disappearance of liver metastases and primary gastric lesions was observed in April 2013 (Figs. 3 and 4). Chemoimmunotherapy was stopped due to grade 3 skin-related adverse effects. During the follow up period of 33 months no evidence of recurrence was seen.
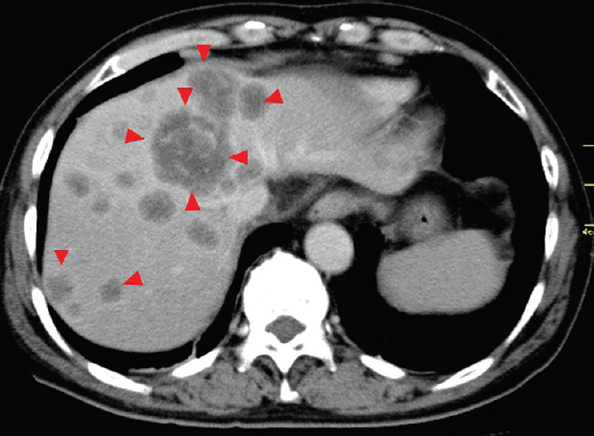
- Computed tomography scan showing multiple liver tumours in both lobes before the initiation of chemoimmunotherapy (arrow heads).
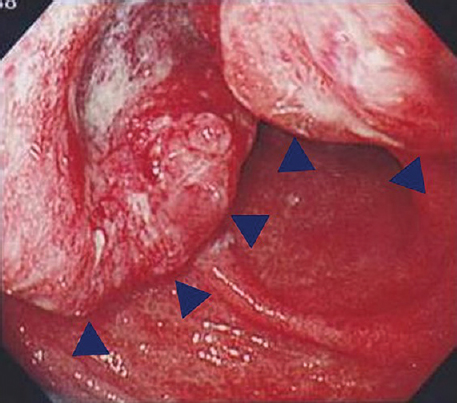
- Gastrointestinal videoendoscopy showing round wall with central ulceration in the antrum of the stomach (arrow heads) on admission. The histological diagnosis of biopsied samples was well-differentiated adenocarcinoma.
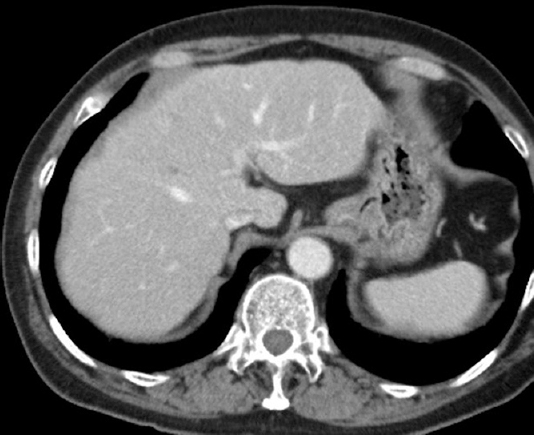
- Computed tomography scan demonstrating complete disappearance of metastatic liver tumours after chemoimmunotherapy.
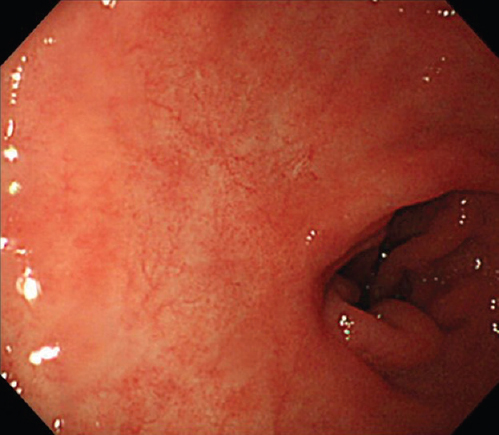
- Gastrointestinal videoendoscopy showing complete resolution of the primary gastric lesions after chemoimmunotherapy.





