Translate this page into:
Comparison of plasma adiponectin & certain inflammatory markers in angiographically proven coronary artery disease patients with & without diabetes – A study from India
Reprint requests: Dr Vijay Viswanathan, Managing Director, M.V. Hospital for Diabetes & Prof. M. Viswanathan Diabetes Research Centre [WHO Collaborating Centre for Research, Education & Training in Diabetes] No. 4, West Mada Church Street, Royapuram, Chennai 600 013, India e-mail: drvijay@mvdiabetes.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The association between adiponectin and risk of cardiovascular disease is well known. The aim of the present study was to evaluate adiponectin and certain inflammatory markers and to determine the correlations between them in angiographically proven coronary artery disease (CAD) in subjects with and without diabetes.
Methods:
A total of 180 subjects who underwent coronary angiography for symptoms suggestive of CAD were categorised into groups based on their diabetes and/or CAD status: group1 (non-diabetic non-CAD); group2 (non-diabetic CAD); group3 (diabetic non-CAD) and group4 (diabetic CAD). Adiponectin, tumour necrosis factor α (TNF-α) and soluble form of E-selectin (sE-selectin) were estimated using quantitative sandwich enzyme immunoassay and high sensitive C-reactive protein (hsCRP) by particle enhanced immunoturbidimetric method.
Results:
Adiponectin levels were significantly lower in subjects with either diabetes or CAD and were much lower in subjects who had both. hsCRP was elevated in CAD and diabetes but did not differ significantly between groups. sE-selectin and TNF-α levels were elevated in CAD. Adiponectin negatively correlated with age, glucose, sE-selectin, total and LDL cholesterol. hsCRP correlated with BMI, sE-selectin and urea. sE-selectin correlated with BMI, triglycerides and VLDL cholesterol, whereas TNF-α correlated with fasting plasma glucose. In the logistic regression analysis, adiponectin had a significant inverse association with CAD. sE-selectin and TNF-α also showed significant independent association with CAD.
Interpretation & conclusions:
Adiponectin and other inflammatory markers such as sE-selectin and TNF-α showed a significant association with CAD. Hence, early assessment of such markers can help to identify high risk patients, and to reduce the inflammatory component of diabetes and CAD.
Keywords
Adiponectin
angiography
coronary artery disease
diabetes mellitus
India
inflammatory markers
TNF-α
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity among patients with type 2 diabetes. Also, diabetes is associated with an increased risk for cardiovascular events1. South Asians especially might have an underlying proinflammatory state that contributes to their increased risk for both type 2 diabetes and CVD2. Typical Asian Indian phenotype which includes increased insulin resistance, higher waist circumference despite lower body mass index, lower adiponectin and higher high sensitive C-reactive protein (hsCRP) makes Indians more prone to diabetes and premature coronary artery disease (CAD)3.
Secretion of various bioactive substances from adipose tissue, conceptualized as adipocytokines, has been widely recognized to play a contributory role in insulin resistance, diabetes and CVD4. In contrast to circulating inflammatory factors, adiponectin has anti-diabetic, anti-atherogenic and anti-inflammatory properties5. Lower adiponectin levels were found to be associated with obesity6, type 2 diabetes and CAD78.
Evidence suggests that arterial inflammation plays a pivotal role in the atherosclerotic process9. Elevated levels of circulating inflammatory markers like hsCRP, tumour necrosis factor alpha (TNF-α) and cellular adhesion molecules are associated with increased CVD risk10. Of these markers which are implicated in atherosclerotic process, CRP has been widely studied because of its easy measurement. Interleukin-6 (IL-6) and TNF-α are the main inducers of the secretion of CRP in the liver. TNF-α is also reported to promote inflammatory cell infiltration by upregulating leukocyte adhesion molecules on endothelial cells11.
E-selectin is a cell adhesion molecule expressed on endothelial cells at inflammation sites and plays a crucial role in monocyte trafficking. Soluble forms of E-selectin are significantly increased in patients with different inflammatory or malignant diseases and in diabetes12. An earlier report in Asian Indians showed the association of risk variables such as apolipoproteins, lipoprotein (a), oxidized low density lipoprotein (LDL) antibodies, fibrinogen and plasminogen activator inhibitor-1 with angiographically proven CAD13. There is also substantial evidence to show that low circulating adiponectin levels are associated with an increased risk of CAD, but there are limited data available from India on the association of adiponectin and inflammatory markers in angiographically proven CAD. Hence, the present study was planned to evaluate the adiponectin levels and certain inflammatory markers in angiographically proven CAD patients with and without diabetes from India. We also assessed the correlation between adiponectin concentration and inflammatory markers along with selected anthropometric, haemodynamic and biochemical parameters.
Material & Methods
The study group consisted of 180 (M:F 113:67) consecutively recruited subjects aged above 25 years who underwent coronary angiography at Apollo Hospitals, Chennai, Tamil Nadu, India for typical or atypical chest pain or symptoms suggestive of CAD from February 2009 to April 2010. Subjects with severe renal or hepatic diseases were excluded, since these conditions are known to influence the biomarker levels. All the subjects underwent catheterization procedure. Subjects without any electrocardiographic or echocardiographic evidence of myocardial infarction (MI) and with angiographically proven normal coronary arteries were considered as non-CAD subjects. Subjects with echocardiographic evidence of MI and obstructive lesions (≥50%) in any of the coronary arteries or their branches were considered as having CAD14. The severity of coronary stenosis was determined by the cardiologist using Quantitative Coronary Analysis15. Diabetes was diagnosed if the fasting plasma glucose was ≥126 mg/dl (7 mmol/l) or if there was a definite history of diabetes with records of treatment. Glucose tolerance was classified as normal if the fasting plasma glucose was <100 mg/dl (5.5 mmol/l). Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or history of antihypertensive therapy. Written informed consent was obtained from all the study subjects and the ethics committees of the Prof. M. Viswanathan Diabetes Research Centre and the Apollo Hospitals approved the study.
The study subjects were grouped as follows: group 1, non-diabetic non-CAD (non-DM/non-CAD); group 2, non-diabetic CAD (non-DM/CAD); group 3, diabetic non-CAD (DM/non-CAD) and group 4, diabetic CAD (DM/CAD). The subjects were further divided as non-CAD (group 1 and group 3) and CAD (group 2 and group 4) and non-DM (group 1 and group 2) and DM (group 3 and group 4) groups. Height and weight were measured and body mass index (BMI kg/m2) was calculated. Demographic details, clinical history, family history of diabetes and details of ischaemic heart disease, hypertension, smoking, alcohol consumption and food habits were recorded.
All biochemical investigations were done at the M.V. Hospital for Diabetes, Chennai.
HbA1c and lipids: Blood samples (9 ml) for biochemical investigations were collected from the subjects after a 12-hour overnight fast. Plasma glucose was estimated by glucose oxidase peroxidase method16. Glycosylated haemoglobin (HbA1c) was estimated by high-pressure liquid chromatography method using variant TURBO machine (BIORAD, USA). Fasting serum samples were used for estimation of total cholesterol, triglycerides, high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C)17. Ratios of total cholesterol to HDL-C and LDL-C to HDL-C were calculated. Estimation of all the lipid parameters was done using standard enzymatic procedures.
Adiponectin: Adiponectin was estimated in plasma, stored at −70°C until assayed, by using the quantitative sandwich enzyme immunoassay technique (Quantikine R&D systems, Inc, Minneapolis, USA). The lowest detection limit for adiponectin ranged from 0.079–0.891 ng/ml. The intra-assay variations were <4.7 per cent.
High sensitive C-reactive protein (hsCRP): hsCRP was estimated in serum samples using particle enhanced immunoturbidimetric method (Dia Sys Diagnostic Systems GmbH, Germany) by photometric measurement of antigen–antibody reaction of antibodies to human CRP bound to polystyrene particles with CRP present in the sample. The measuring range of hsCRP ranged between 0.05–20mg/l.
Tumour necrosis factor-alpha (TNF-α): TNF-α was estimated in serum, stored at −70°C until assayed, by the quantitative sandwich enzyme immunoassay procedure using a commercial kit supplied by R&D systems (Quantikine R&D systems, Inc, Minneapolis, USA). The lowest detection limit for TNF-α ranged from 0.5–5.5 pg/ml. The intra-assay and inter-assay coefficients of variations were <5.2 per cent and <7.4 per cent, respectively.
Soluble E-selectin (sE-selectin): Soluble form of E-selectin (sE-selectin) was estimated in plasma, stored at −70°C until assayed, by the quantitative sandwich enzyme immunoassay procedure using a commercial kit supplied by R&D systems (Quantikine R&D systems, Inc, Minneapolis, USA). The lowest detection limit for sE-selectin ranged from 0.003–0.027 ng/ml. The intra-assay and inter-assay coefficients of variations were <6.6 per cent and <8.7 per cent, respectively.
Statistical analysis: All statistical analyses were performed using SPSS 16.0 version software (SPSS Inc. Illinois, USA) and Stata (8.0 version), (Texas, USA). Data were expressed as mean ± SD or median (range). For multiple group comparisons ANOVA with Post hoc analysis using Tukey's HSD procedure was used. Non-parametric analysis of variance (Kruskal-Wallis) was applied for non-normally distributed values. Independent ‘t’ test or Mann-Whitney U tests were used for group comparison as appropriate and asymptomatic (2-tailed) P values were considered for significant differences. Proportions are reported for categorical variables and chi-square test was used to compare the significant differences between the groups. Yate's correction was done for variables (smoking, alcohol consumption, education status, family income) prior to Chi-square test. The relationship between adiponectin, inflammatory markers and other variables was tested by Pearson correlation or non-parametric Kendall's correlation test. All the groups were combined for correlation analysis. Binary logistic regression analysis was done by adjusting diabetes and MI to identify the association of the anthropometric, haemodynamic and biochemical variables with CAD. Univariate analysis was done first and the independent variables that had a P value <0.2 on univariate analysis were used for binary logistic regression analysis. A few patients underwent coronary angiography in the context of history of MI and also presence of diabetes might interfere with inflammatory marker levels. Hence, logistic regression analysis was done by adjusting DM and MI. The dependent variable was CAD. The independent variables entered were age, gender, BMI, systolic and diastolic blood pressure, smoking, adiponectin, hsCRP, sE-selectin and TNF-α. The variables were dichotomized either as Yes and No or as continuous variables. P <0.05 was considered as significant.
Results
Among the CAD subjects, 52(52%) had angina, 31(30.7%) had a history of MI, 13(13.3%) underwent coronary artery bypass graft (CABG), 1(1.3%) underwent percutaneous transluminal coronary angioplasty (PTCA), and 3(2.7%) had left ventricular dysfunction:
Table I shows the comparison of the demographic, anthropometric and clinical details of the four study groups. Age and body mass index differed significantly between the study groups. Both systolic and diastolic mean blood pressure values and presence of hypertension were similar between the groups. Positive family history of diabetes, ischaemic heart disease and hypertension were similar in the study groups. Family income showed significant difference between the groups. More than 50 per cent of the subjects had a family income of less than  10,000. Education status, rates of smoking and alcohol consumption were similar between the groups.
10,000. Education status, rates of smoking and alcohol consumption were similar between the groups.
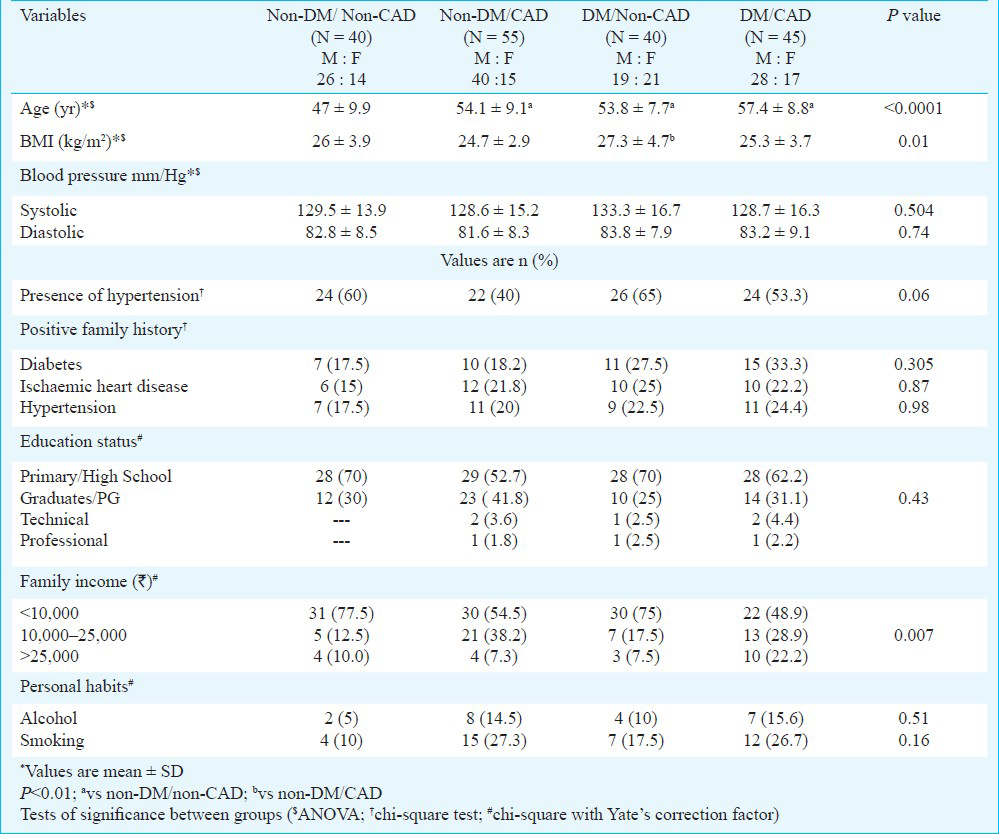
Table II shows the comparison of biochemical parameters between the different study groups. Fasting and post-prandial glucose levels and HbA1c per cent were significantly higher in the group of subjects who had presence of diabetes compared to those without diabetes. Urea levels were similar whereas creatinine levels differed between the groups with higher levels in the group of patients who had presence of CAD. Hb levels were significantly different between the groups (P=0.007). Even though the Hb levels were within the normal range, subjects with diabetes had slightly lower values compared to other groups. There was a difference in LDL-cholesterol with slightly lower levels in the group of patients with presence of either diabetes or CAD. Adiponectin levels showed significant difference between the study groups (P<0.001). Adiponectin levels were significantly higher in non-diabetic and non-CAD group, whereas the levels were lower in the group of subjects with CAD either in the presence or absence of diabetes. The values were much lower in the subjects who had presence of both diabetes and CAD. hsCRP levels were elevated in CAD and diabetes group but did not differ significantly between the study groups. Concentrations of sE-selectin were significantly higher in groups with diabetes or CAD (P<0.001). sE-selectin and TNF-α levels were elevated in all the groups irrespective of presence of either CAD or diabetes compared to non-DM non-CAD group.

The demographic, anthropometric and biochemical parameters of the subjects in the non-CAD and CAD groups are shown in Table III. Age and BMI differed significantly between these two groups. Subjects with CAD were older than those with non-CAD (P<0.001). Mean values of systolic and diastolic blood pressure were similar between the groups. Also, fasting and postprandial plasma glucose levels and HbA1c per cent values did not differ significantly between the groups. Mean urea levels also were similar, while the levels of creatinine were significantly higher (P<0.001) in the CAD group compared to the non-CAD group. The levels of all the lipid parameters were also similar between the groups. Adiponectin values were significantly lower (P<0.001) in the CAD group compared to the non-CAD group. The levels of hsCRP were elevated in CAD, but did not differ significantly between the groups, whereas sE-selectin levels were significantly higher in the CAD group (P=0.011) compared to the non-CAD group. Similarly, TNF-α levels were also significantly higher (P=0.009) in the CAD group compared to the non-CAD group.

Diabetic subjects (DM group) were older than non-diabetic subjects (non-DM) (55.7±8.5 vs 51±10.1 years) (P<0.001). Subjects in the DM group had significantly lower levels of adiponectin compared to the subjects in the non-DM group [3.6 (0.9–7.5) vs 5.5 (0.8–17.6)μg/ml] (P<0.0001), whereas hsCRP levels were similar between the groups [non-DM vs DM; 2.8 (0.1–39.8) vs 3.5 (0.2–40.5) mg/l]. The levels of sE-selectin were significantly higher in the DM group compared to the non-DM group [(34 (22–48) vs 31.6 (12–41) ng/ml)] (P<0.001). TNF-α levels were elevated in the DM group but did not show statistical significance [(24 (2.1–295) vs 26 (4.2–290) pg/ml)].
Table IV shows the correlation matrix of adiponectin, hsCRP, TNF-α and sE-selectin with anthropometric, haemodynamic and biochemical variables. Among the total subjects, adiponectin negatively correlated with age (r = −0.245, P<0.001), fasting plasma glucose (r = −0.257, P = 0.001), post-prandial plasma glucose (r = −0.184, P = 0.02), sE-selectin (r= −0.94, P<0.001), urea (r = −0.156, P=0.049), total cholesterol (r = −0.160, P = 0.043) and LDL-cholesterol (r= −0.203, P = 0.01). hsCRP showed significant correlation with BMI (r =0.153, P = 0.005), sE-selectin (r = 0.136, P= 0.013) and urea levels (r = 0.229, P=0.004). TNF-α correlated with fasting plasma glucose (r = 0.176, P = 0.028). sE-selectin correlated with BMI (r= 0.124, P=0.02), triglycerides (r = 0.206, P = 0.01) and VLDL-cholesterol (r = 0.206, P = 0.01).

The binary logistic regression analysis showed significant inverse association of adiponectin with CAD [odds ratio (OR) 0.46 (0.34–0.64) (95% confidence interval (CI), P<0.001). Inflammatory markers such as sE-selectin [OR 1.19 (1.07–1.33) (95% CI), P=0.001)] and TNF-α [OR 1.02 (1.0–1.03) (95% CI), P=0.002)] also showed significant independent association with CAD. Male sex (OR 3.86 (0.99–15.1) (95% CI), P=0.05)] just failed to reach statistical significance (Table V).

Discussion
In the present study, we investigated the levels of adiponectin and certain inflammatory markers such as hsCRP, TNF-α and sE-selectin in angiographically proven CAD, in non-diabetic and diabetic subjects in India. The current study revealed that adiponectin levels were significantly lower in subjects with CAD. Diabetic subjects irrespective of presence or absence of CAD had lower adiponectin levels compared to non-diabetic non-CAD group of subjects. sE-selectin and TNF-α levels were elevated in the subjects with either CAD or diabetes. Adiponectin showed a significant inverse association with CAD. The odds ratio was highest for sE-selectin (OR 1.19) followed by TNF-α (OR 1.02). Adiponectin negatively correlated with sE-selectin. hsCRP also showed significant correlation with sE-selectin.
Various studies have documented that adiponectin levels are decreased in type 2 diabetic patients with CAD. Hypoadiponectinaemia and increased hsCRP are seen in Asian Indians compared to Caucasians1819. Moreover, Indo-Asians were found to have lower adiponectin levels compared with Caucasians20. Hotta et al7 showed significantly lower plasma adiponectin levels in patients with type 2 diabetes and CAD, than in patients with diabetes and without CAD. Kumada et al8 demonstrated that hypoadiponectinaemia (<4μg/ml) was independently associated with the presence of CAD after adjustment for other well-known CAD risk factors in men. In our study, adiponectin negatively correlated with age, fasting and post-prandial glucose levels, sE-selectin, total cholesterol and LDL-cholesterol. This indicates that increasing age and poor glycaemic control are negatively associated with adiponectin. In contrary to the above finding, adiponectin levels were shown to correlate negatively with BMI, CRP, insulin, triglycerides and positively with HDL-cholesterol2122. Other reports from India also showed low adiponectin levels in patients with diabetes or metabolic syndrome2324. Mohan et al24 reported that lower adiponectin levels were associated with the metabolic syndrome and its components, particularly, diabetes and dyslipidaemia in Asian Indians, a high risk group for premature coronary artery disease and diabetes. Therefore, it is likely that adiponectin could be beneficial and high levels of circulating adiponectin would confer vascular protection; it inhibits TNF-α stimulated expression of adhesion molecules on endothelial cells and prevents the development of atherosclerosis. Thus, adiponectin may function as a therapeutic target for diabetic patients with and without CAD. In contrast to the above findings, adiponectin showed no significant association with later development of coronary heart disease in prospective studies2526.
In many studies hsCRP has been shown to be associated with diabetes and CAD, but there is lack of data on the association of adiponectin and hsCRP in CAD patients with and without diabetes in high risk Indian population. In the present study, hsCRP levels were elevated in CAD and diabetic subjects but no significant difference existed in hsCRP levels between the study groups. This may be accounted for by the small sample size. hsCRP did not show any significant correlation with adiponectin in this study but was correlated with sE-selectin, BMI and urea levels. In men and women who participated in the Health Professionals follow-up study and Nurses’ Health study, elevated levels of inflammatory markers, particularly CRP, indicated an increased risk of CAD. The levels of CRP remained a significant contributor to the prediction of coronary heart disease27. Another study showed that men with type 2 diabetes without CAD had CRP levels similar with non-diabetic CAD patients, whereas CRP levels of diabetic patients with CAD were higher than in non-diabetic men with CAD28. A similar observation was noted in our study.
In the binary logistic regression analysis, hsCRP did not show significant association with CAD in our study. Although hsCRP has been extensively studied, recent evidence suggests that, besides CRP, other inflammatory biomarkers may have a potential role for the prediction of risk of CAD and severity of CAD29.
Soluble form of E-selectin in plasma was found to be significantly higher in patients with coronary artery disease and impaired glucose metabolism12. The levels of inflammatory markers of endothelial damage such as sP-selectin, sE-selectin and sPECAM-1 did not differ significantly in cases as compared to controls in young cases of MI in a study30. Our study showed that sE-selectin levels were elevated in CAD and diabetic patients compared to non-diabetic non-CAD group. The odds ratio was highest for sE-selectin. sE-selectin correlated with BMI, triglycerides and VLDL-cholesterol. The inflammatory markers are significantly correlated with the body mass index which needs to be targeted to reduce inflammatory component.
The group of subjects with diabetes and CAD had significantly higher levels of TNF-α compared to other study groups. TNF-α correlated significantly with fasting plasma glucose in the present study. In another study in north Indians, TNF-α concentrations were significantly higher in patients with acute MI (86.9 ± 4.7 pg/ml) compared to controls (7.1 ± 0.67 pg/ml)31. The present study results and all the above reports on cytokines underline the role of immune processes in the pathogenesis of CAD in the Indian scenario.
Markers of systemic inflammation can predict future cardiovascular events. So identification of newer or novel risk markers facilitates early detection and thereby leads to risk reduction. These biomarkers provide independent diagnostic and prognostic value of underlying disease condition. Thus, measurement of certain inflammatory markers can help to identify high risk patients and intervention with improvement in lifestyle or therapy may reduce the inflammatory component of the disease.
One of the main limitations of the present study was the small sample size. This might have led to lack of power to detect significant associations. The small sample size could also be responsible for the fact that hsCRP did not show significant association in patients with established CAD. Another limitation was that it was not a prospective study which examined the causative association or identification of subjects with future risk of CAD. The limited information on drugs might possibly affect the reported results, especially in the case of hsCRP which can be modified by statins.
In conclusion, the current study highlighted that subjects with CAD had lower adiponectin levels. hsCRP did not show significant association with CAD. Inflammatory markers such as sE-selectin and TNF-α levels were elevated in CAD. Adiponectin levels were also decreased in subjects with diabetes as reported in the literature. These observations have implication that adiponectin and other inflammatory markers can be used to identify at risk patients and treat aggressively.
Acknowledgment
Authors acknowledge the help rendered by Ms. Priyanka in sample collection, Ms. Dhivya in biochemical investigations and Shri V. Narayan Rao in the preparation of the manuscript.
References
- Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ. 1990;301:92-5.
- [Google Scholar]
- Elevated plasma high sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88:3773-6.
- [Google Scholar]
- Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217-30.
- [Google Scholar]
- Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537-45.
- [Google Scholar]
- Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-5.
- [Google Scholar]
- Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-9.
- [Google Scholar]
- Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85-9.
- [Google Scholar]
- Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668-73.
- [Google Scholar]
- NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-3.
- [Google Scholar]
- Plasma adiponectin and E-selectin concentrations in patients with coronary heart disease and newly diagnosed disturbances of glucose metabolism. Adv Med Sci. 2006;51:94-7.
- [Google Scholar]
- Risk variables for coronary artery disease in Asian Indians. Am J Cardiol. 2001;87:267-71.
- [Google Scholar]
- Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. 1990;82:1230-42.
- [Google Scholar]
- Quantitative coronary angiography versus visual estimation from cine-film or pharmacological stress perfusion images. Eur Heart J. 1996;17:1167-74.
- [Google Scholar]
- An improved color reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142-5.
- [Google Scholar]
- Lipids, lipoproteins and apolipoproteins. In: Burtis CA, Ashwood ER, eds. Tietz textbook of clinical chemistry (3rd ed). Philadelphia: W.B. Saunders Company; 1999. p. :809-61.
- [Google Scholar]
- Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab. 2004;89:2750-5.
- [Google Scholar]
- C-reactive protein, insulin resistance, central obesity and coronary heart disease risk in Indian Asians from the United Kingdom compared with European Whites. Circulation. 2001;104:145-50.
- [Google Scholar]
- Fasting serum adiponectin concentration is reduced in Indo-Asian subjects and is related to HDL cholesterol. Diabetes Obes Metab. 2003;5:131-5.
- [Google Scholar]
- Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low density lipoprotein particle size in young healthy men. Metabolism. 2004;53:589-93.
- [Google Scholar]
- Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism. 2004;53:1454-61.
- [Google Scholar]
- Association of adipocytokines (leptin, adiponectin, TNF-alpha), insulin and proinsulin with diabetes - the Mumbai Obesity Project [MOP] J Assoc Physicians India. 2006;54:689-96.
- [Google Scholar]
- Association of low adiponectin levels with the metabolic syndrome - the Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54:476-81.
- [Google Scholar]
- Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15-6.
- [Google Scholar]
- Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677-83.
- [Google Scholar]
- Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599-610.
- [Google Scholar]
- Is serum C-reactive protein concentration correlated with HbA1c and insulin resistance in type 2 diabetic men with or without coronary heart disease? J Endocrinol Invest. 2005;28:145-50.
- [Google Scholar]
- Evaluation of markers of endothelial damage in cases of young myocardial infarction. Atherosclerosis. 2005;180:375-80.
- [Google Scholar]
- TNF-α/IL-10 ratio and C-reactive protein as markers of the inflammatory response in CAD-prone North Indian patients with acute myocardial infarction. Clin Chim Acta. 2009;408:14-8.
- [Google Scholar]






