Translate this page into:
Comparison of laboratory-developed test & validated assay of programmed death ligand-1 immunohistochemistry in non-small-cell lung carcinoma
For correspondence: Dr Deepali Jain, Department of Pathology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: deepalijain76@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Inhibitors of immune checkpoint regulators, programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), improve outcome in advanced non-small-cell lung carcinoma (NSCLC). Tumours expressing PD-L1 protein are more likely to benefit from this targeted therapy. Multiple concurrent clinical trials evaluating different anti-PD-1/PD-L1 therapies have validated five different immunohistochemistry (IHC) assays using varied antibody clones and staining conditions. This study was aimed at identification of a single harmonized PD-L1 assay for tumour tissue conservation and cost-effectiveness in patients with NSCLC.
Methods:
The performance of low-cost, manual, laboratory-developed technique (LDT) PD-L1 IHC assay using the easily available SP142 clone was compared with trial validated Ventana SP263 IHC performed on automated Ventana staining platform on tumour sections of NSCLCs.
Results:
Eighty cases of NSCLC were included. SP263 and SP142 stained both tumour cells and immune cells. The concordance rate of tumour cell staining was about 76 per cent, with SP263 detecting more tumour cells in 16 per cent of cases. The concordance rate of immune cell staining was only 61 per cent, with SP142 detecting more immune cells in 24 per cent of cases. The sensitivity, specificity, positive and negative predictive values of manual SP142 LDT assay against gold standard SP263 Ventana assay were 70, 94, 86 and 86 per cent, respectively, at positivity thresholds of ≥1 per cent tumour cell staining.
Interpretation & conclusions:
The study findings suggested that LDT using SP142 clone showed only moderate concordance with SP263 Ventana assay, and the two assays were not interchangeable. More such validation studies need to be done to generate information that can complement patient therapy in cases of NSCLC.
Keywords
Immunohistochemistry
non-small-cell lung carcinoma
programmed cell death ligand-1
resection
SP142
SP263
Recent advances in cancer immunotherapy for advanced non-small-cell lung carcinoma (NSCLC) led to development of treatments targeting immune check point interactions such as programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1)1. PD-1 inhibitor nivolumab and PD-L1 inhibitor atezolizumab are approved for second-line treatment in NSCLC patients with unresectable, locally advanced or metastatic lung disease, PD-L1 inhibitor durvalumab is approved as maintenance therapy for stage IIIB lung cancer and PD-1 inhibitor pembrolizumab is approved for first-line treatment of patients with metastatic NSCLC2. Other PD-L1 inhibitors such as avelumab are still under clinical trials2. PD-L1 protein detection by immunohistochemistry (IHC) is a valuable biomarker for the selection of patients who are more likely to benefit from anti-PD-1/PD-L1 therapies3. However, multiple concurrent clinical trials evaluating different anti-PD-1/PD-L1 therapies have validated five IHC assays using different antibody clones, laboratory techniques and expression thresholds for immunopositivity23. These include two Ventana antibodies (SP263 and SP142) and three Dako antibodies (28-8, 22C3 and 73-10), recommended to be performed only on automated Ventana Benchmark and Dako Link 48 platforms, respectively23.
The Ventana PD-L1 (SP263 clone) assay utilizes a rabbit monoclonal anti-PDL1 antibody against an intracellular domain localized to an epitope corresponding to amino acids 284-2904. This assay was part of the clinical trials that demonstrated the survival benefit of PD-L1 inhibitor durvalumab in patients with NSCLC and urothelial carcinomas5. This assay has been approved by the U.S. Food and Drug Administration (FDA) as a complementary diagnostic assay for treatment selection with durvalumab in urothelial carcinomas2. It has also been marked by European Conformity (CE) as a companion and complementary diagnostic test for pembrolizumab and nivolumab, respectively, in NSCLC patients2.
The Ventana PD-L1 (SP142 clone) assay utilizes a rabbit monoclonal anti-PD-L1 antibody against the intracellular domain of the PD-L1 protein ligand6. This antibody assay was utilized in the OAK and POPLAR trials that evaluated the therapeutic role of PD-L1 inhibitor atezolizumab in advanced NSCLC78. Based on the treatment benefit observed in those with PD-L1 immunopositive NSCLC, this assay has been approved (FDA and CE) as a complementary diagnostic test to select patients with advanced NSCLC for atezolizumab therapy2.
The availability of five different anti-PD-1/PD-L1 drugs, each with its own clinical trial-validated IHC assay, is posing considerable challenges to oncologists and pathologists. These assays utilize antibody clones against different epitopes of PD-L1 and different amplification systems, and hence lack uniformity in performance. It is necessary to harmonize PD-L1 detection using a common assay to improve cost-effectiveness and simplify implementation of predictive PD-L1 testing in all centres2. This study was conducted to assess the performance of our low-cost, manual, laboratory-developed technique (LDT) PD-L1 assay using SP142 clone in comparison to the trial-validated Ventana SP263 PD-L1 assay using the SP263 clone.
Material & Methods
The study was of retrospective design and conducted at the department of Pathology, All India Institute of Medical Sciences, New Delhi, India, over a span of seven years (2009-2015). The study was approved by the Institutional Ethics Committee (IEC/NP-223/5.6.2015). All cases of NSCLC diagnosed on resection specimens during the study period were retrieved from the departmental archives. As per the standard procedure in our laboratory, all resection specimens were received in 10 per cent neutral-buffered formalin and were fixed overnight for a minimum duration of 24 h. The paraffin-embedded sections cut at 3-5 μ thickness were used for haematoxylin and eosin (H and E) staining. Tissue blocks were archived and stored in a cool dry place.
Histopathological assessment: All H and E-stained slides of the patients were reviewed for the reconfirmation of NSCLC diagnosis, subclassification and pathological staging (tumour node metastasis; TNM) according to the WHO classification of lung tumours9. One tissue block was selected from each patient for IHC. The presence of tumour-infiltrating immune cells was noted on H and E-stained sections and graded as the following: 0 - none; 1 - focal, perivascular; 2 - moderate, prominent extension of inflammation away from perivascular regions and reaching tumour, and 3 - severe, obscuring tumour stromal interface with inflammatory cells permeating and inserting between individual tumour cells10.
Immunohistochemistry for PD-L1: One representative section was selected in each tumour comprising at least 100 viable tumour cells with associated stroma. Four serial sections of 3-4 μ thickness were cut on fresh polylysine-coated slides: one stained with H and E for histopathology, second and third for anti-PD-L1 IHC staining and fourth for negative reagent IHC control.
IHC using SP142 anti-PD-L1 clone (Spring Bioscience, USA) was performed by manual LDT. Sections were deparaffinized, rehydrated and washed with Tris chloride buffer (pH 7.5). For antigen retrieval, optimum staining results were obtained with citrate buffer at pH 6. Sections were rehydrated and antigen retrieval was done by boiling in citric acid buffer (pH 6) for 30 min. After antigen retrieval using citrate buffer, endogenous peroxidase activity was blocked using hydrogen peroxide. Following endogenous peroxidase blocking, incubation with the primary SP142 antibody (dilution 1:100 v/v) was done overnight at 4°C. Bound antibodies were detected with UltraTek HRP Anti-Polyvalent Staining System (ScyTek, Logan, USA) using 3,3’-diaminobenzidine as the chromogen along with mild counterstaining with haematoxylin. Section of tonsil was used as positive control showing moderate intensity staining in the lymphocytes and macrophages of germinal centre, diffuse staining of reticulated crypt epithelial cells, and no staining of interfollicular regions or of the superficial squamous epithelium.
IHC using SP263 anti-PD-L1 clone (Ventana Medical Systems, Inc., USA) was performed on VENTANA Benchmark XT platform optimized with the OptiView DAB IHC Detection kit (Ventana Medical Systems Inc., USA) at the department of Pathology, Dr. Ram Manohar Lohia Hospital, Lucknow, India. Sections of placenta were included as positive controls and showed moderate to high intensity, and uniform membranous, with/without cytoplasmic staining of trophoblastic cells. Negative reagent control served as negative control.
Immunohistochemical scoring: Each tumour section was scanned at high magnification. The percentage of viable tumour cells showing membranous with/without cytoplasmic staining of any intensity as a proportion of all tumour cells in the entire section was noted as tumour proportion score. Tumour proportion score ≥1 per cent was considered positive5111213. Staining, either membranous or cytoplasmic of any intensity in immune cells, was noted separately as a proportion of tumour and tumour-associated stroma showing PD-L1-positive immune cells813. The immune cell immunopositivity was further graded as <1, 1-5, >5-10 and >10 per cent. Scoring was done independently by two pathologists, and all conflicting reviews were resolved by consensus through multi-head microscope review.
Statistical analysis: A Bland-Altman graph was plotted on Microsoft Excel to demonstrate the agreement between the two immunohistochemical assays using average of tumour cell positivity and error. Fisher's exact test was performed to analyze categorical data using STATA v.13 (StataCorp, Texas, USA).
Results
A total of 80 cases of NSCLC were included in the study. The median age of the patients at diagnosis was 58 yr, ranging from 29 to 78 yr. There was a male preponderance (male:female ratio, 4:1). Smoking status was available in 62 patients; 47 (75.8%) were smokers and 15 were non-smokers. The surgical procedures included lobectomy (51/80), bilobectomy (5/80), pneumonectomy (22/80), wedge resection (1/80) and axillary lymph node wedge biopsy (1/80). According to the TNM staging, the patients corresponded to pathological stage I (29/80), stage II (33/80), stage IIIA (17/80) and stage IV (1/80). Tumour histopathology was squamous cell carcinoma (SCC) (42/80), adenocarcinoma (ADC) (31/80), adenosquamous carcinoma (AdSq) (1/80), large-cell carcinoma (LCC) (2/80) and sarcomatoid carcinoma (4/80). The SCCs showed keratinizing (23/42) and non-keratinizing histology (19/42). The ADCs included two mucinous ADCs, one foetal ADC and 28 non-mucinous ADC. The latter showed lepidic-predominant (4/28), acinar-predominant (7/28), papillary-predominant (5/28) and solid-predominant (12/28) architectural patterns. Tumour-infiltrating immune cells were seen in 75 of 80 cases, ranging from mild (38/75), moderate (28/75) to severe (9/75) in density.
Comparison of PD-L1 staining between commercial SP263 assay and laboratory-developed technique assay using SP142: Tumour cell staining (>1%) for SP263 was seen in 33.8 per cent (27/80) of cases. Among the 75 cases with immune infiltrates, variable degree of immune cell staining (>1%) was noted in 20 per cent (15/75), of which 73 per cent (11/15) cases showed isolated immune cell staining. The remaining cases were negative for SP263. Tumour cell staining (>1%) for SP142 was noted in 27.5 per cent (22/80) of cases. Among the 75 cases with immune infiltrates, variable degree of immune cell staining (>1%) was noted in 29.3 per cent (22/75), of which 88.4 per cent (13/22) cases showed isolated immune cell staining only. The remaining cases were negative for SP142. Tabulating the tumour cell positivity using different thresholds as per ‘Cologne score’14, the concordance rate between SP142 and SP263 was 76.3 per cent, with SP263 detecting more tumour cells in 16.3 per cent (13/80) of cases and SP142 detecting more tumour cells in 7.5 per cent (6/80) of cases (Fig. 1A). The Bland-Altman plot showed that the differences in the proportion of tumour cell staining between SP142 and SP263 fell largely within the lines of agreement in majority of cases with occasional outliers. The calculated bias between SP142 and SP263 was −1.6 (Fig. 1B). Tabulation of the proportion of immune cells stained using different threshold intervals showed that the concordance rate between SP142 and SP263 was 61.3 per cent, with SP142 detecting more immune cells in 24 per cent (18/75) of cases and SP263 staining more immune cells in 14.6 per cent (11/75) of cases (Fig. 1C). The Bland-Altman plot showed that there was a wider variation in immune cell staining between SP142 and SP263 with bias of 2.62 (Fig. 1D).
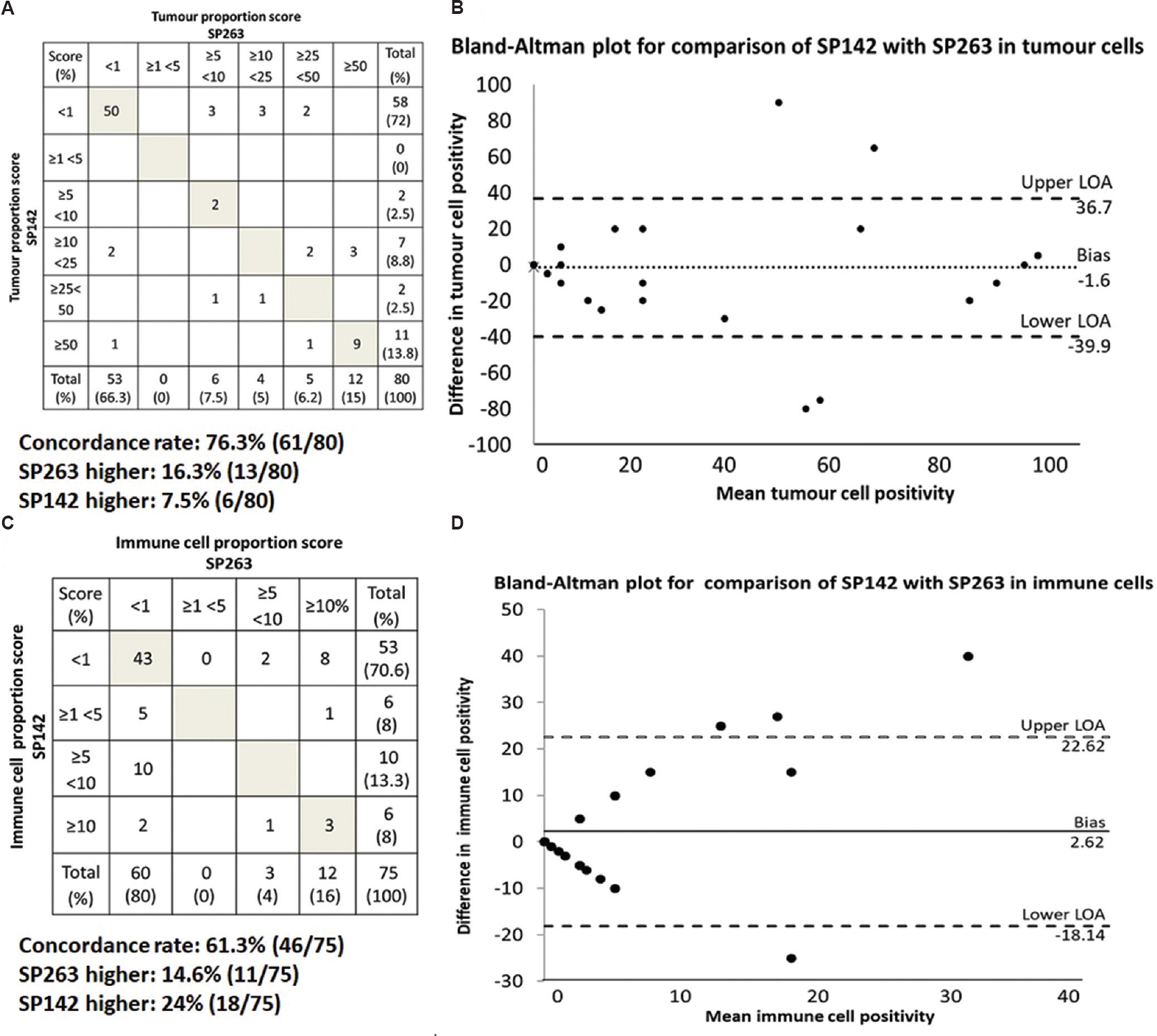
- (A) Tabulation of the correlation between anti-PD-L1 staining using SP142 and SP263 cones in tumour cells based on different cut-offs. Shaded boxes indicate concordant proportions of tumour cells stained. Boxes above the shaded boxes indicate the number of cases where SP263 stained tumour cells in higher cut-offs while boxes below the shaded boxes indicate the number of cases where SP142 stained tumour cells in higher cut-offs. (B) Bland-Altman plot for comparison of individual tumour cell proportions stained by SP142 with SP263. Upper and lower LOAs indicate the lines of agreement. (C) Tabulation of the correlation between anti-PD-L1 staining using SP142 and SP263 clones in immune cells based on different cut-offs. (D) Bland-Altman plot for comparison of individual immune cell proportions stained by SP142 with SP263.
Using SP263 as gold standard and ≥1 per cent tumour cell staining as positive, SP142 showed sensitivity of 70 per cent (19/27), specificity of 94 per cent (50/53), positive predictive value (PPV) of 86 per cent (19/22) and negative predictive value (NPV) of 86 per cent (50/58). At positivity threshold of ≥50 per cent tumour cell staining, SP142 staining showed sensitivity of 75 per cent (9/12), specificity of 97 per cent (66/68), PPV of 81 per cent (9/11) and NPV of 97 per cent (66/69) (Fig. 1A). Within tumour subsets, SP263 stained more tumour cells in squamous cell carcinomas (mean 18.5 vs. 12.5 tumour cells in SP142), resulting in a 14 per cent increase in the proportion of PD-L1-positive SCCs by SP263 staining (Table). On the other hand, SP142 stained more tumour cells in sarcomatoid carcinomas (mean 46.2 vs. 28.8 tumour cells in SP263), resulting in a 50 per cent increase in the proportion of PD-L1-positive sarcomatoid carcinomas by SP142 staining (Table). Within adenocarcinomas, the difference in the proportion of PD-L1-positive cases between the two antibodies was <1 per cent. None of these differences reached significance. Figure 2 shows the staining patterns of SP142 as compared to that of SP263.
| PD-L1 expression thresholds for positivity (n) | PD-L1 antibodies | |||
|---|---|---|---|---|
| SP263 | SP142 | |||
| Positive TC proportion (mean±SD) | TC ≥1% n (%) | Positive TC proportion (mean±SD) | TC ≥1% n (%) | |
| All cases (80) | 15.3±30 | 27 | 13.7±29 | 22 |
| Adenocarcinoma (31) | 10.6±27 | 7 (22.5) | 12.5±31 | 6 (19.3) |
| SCC (42) | 18.5±31 | 18 (42.9) | 12.5±26 | 12 (28.6) |
| AdSq (1) | 0 | 0 (0) | 0 | 0 (0) |
| Large cell carcinoma (2) | 0 | 0 (0) | 0 | 0 (0) |
| Sarcomatoid carcinoma (4) | 28.8±36 | 2 (50) | 46.2 (34) | 4 (100) |
TC, tumour cell; SD, standard deviation; SCC, squamous cell carcinoma; AdSq, adenosquamous carcinoma

- Comparison of staining patterns of SP142 and SP263. (A) Haematoxylin and eosin-stained section of solid variant, adenocarcinoma, showing strong and diffuse membranous staining for SP263 (B) and SP142 (C). (D) Non-keratinizing squamous cell carcinoma showing similar staining of tumour cells with SP263 (E) and SP142 (F). (G) A discrepant case of non-keratinizing squamous cell carcinoma showing staining of tumour cells at stromal interface with SP263 (H), but completely absent staining with SP142 (I). (J) A sarcomatoid carcinoma showing lack of staining with SP263 (K) but diffuse membranous and cytoplasmic staining of tumour cells with SP142 (L). Depth of each panel was 100 μm.
Discussion
Inhibition of PD-1 and its ligand PD-L1 has emerged as an important therapeutic modality in a number of malignancies including NSCLC1. During inflammatory states, healthy cells express PD-L1 that binds to PD-1 on cytotoxic T-lymphocytes, leading to inhibition of cell-mediated cytotoxicity and immune escape15. Tumour cells exploit similar mechanisms to escape from immune recognition and anti-tumour surveillance16. Expression of PD-L1 in tumour cells has been observed in many neoplasms including melanomas and urothelial carcinomas, with increased expression levels found to be associated with worse survival1617. Anti-PD-L1 or anti-PD-1 antibodies interfere with the interaction between PD-L1 on tumour cells and PD-1 on immune cells, thereby reactivating T-cell-mediated anti-tumour immune responses. Multiple clinical trials using these inhibitors in NSCLC have shown promising results7812. It has been found that tumours expressing PD-L1 respond better to these drugs, resulting in the evaluation of PD-L1 immunoassays as predictive markers for anti-PD-L1/PD-1 therapies3. Considering that all validated PD-L1 assays are performed only on dedicated automated platforms (Vantana or Dako), laboratories without these platforms are at a disadvantage. Hence, comparison and standardization of manual LDT assays become important for wide implementation of PD-L1 testing2.
In our laboratory, the SP142 clone has been in use in a manual LDT assay for research purposes13. The SP263 clone has been the most concordant on LDT platforms in previous harmonization studies18. By comparing the results of the SP142 manual LDT protocol, and the SP263 Ventana assay performed on the Ventana BenchMark automated platform on the step sections of same tumour block, significant differences were found in the proportion of tumour cells and immune cells stained by these two antibody clones. SP142 clone detected marginally less tumour cells in 16 per cent of cases, and more immune cells in 24 per cent of cases, as compared to SP263 clone. No significant differences in staining intensity of tumour cells or immune cells was observed. Comparing staining results across different tumour cell proportions, only a moderate level of concordance (76%) was achieved. Scheel et al14 compared SP142 (Ventana) with SP263 (Ventana), 22C3 (Dako) and 22-8 (Dako). The study reported that while 28-8 and 22C3 were comparable in terms of staining intensity and proportion scores, SP142 and SP263 detected less and more tumour cells, respectively, with increased staining intensities. On direct comparison between SP142 and SP263, they observed a low concordance (41%) in tumour cell staining, with SP263 detecting higher number of tumour cells in nearly 59 per cent of cases14. Three other studies have also found that SP142 (Ventana) was an outlier and detected less number of tumour cells and immune cells as compared to the other four antibodies192021. In the study by Brunnstorm et al21, SP142 (Ventana) picked up only half of NSCLCs that were detected to be PD-L1 positive by assays using the other four antibodies. On the contrary, Parra et al22 noted that SP142 (Ventana) staining was comparable to that of 28.8 and 22C3, while SP263 picked up more tumour cells. These conflicting findings are not surprising as one-on-one comparison of staining properties among these antibody clones on the same tumour is unlikely to be similar as these antibodies are directed towards different binding domains of PD-L1 and are performed under different staining conditions.
The major limitation of our study was that one-on-one comparison of SP142 LDT with SP142 Ventana assay (Ventana staining platform) could not be performed. Our study supported previous findings that the SP142 clone, irrespective of manufacturing company, appeared to be an outlier and was unlikely to be successfully harmonized with other PD-L1 clones142122.
Another common dilemma faced by researchers attempting harmonization of PDL1 assays is in determination of appropriate cut-offs for positivity when interchanging clones. A cut-off of tumour cell staining ≥25 per cent has been determined for SP263 assay in durvalumab treatment selection and ≥1 per cent tumour cell/immune cell staining for SP142 Ventana assay in atezolizumab treatment selection23242526. Different cut-offs determine positivity for 22C3, 22-8 and 73-10 in treatment selection for the respective drugs3. In the phase I results of the Blueprint PDL1 IHC Assay Comparison Project, although 22-8, 22C3 and SP263 clones showed good agreement, PD-L1 expression status of nearly one-third of cases were misclassified when they used the clone-specific scoring systems20. Thus, the positivity threshold for a predictive PD-L1 assay would ideally depend on the drug that is to be administered. For instance, based on the high agreement of SP263 with 28-8 and 22C3 assays, the former has been approved as a predictive marker for treatment response to pembrolizumab (validated using 28-8 assay) and nivolumab (validated using 22C3 clone) in non-squamous NSCLC, using the cut-offs validated for the 28-8 and 22C3 PDL1 assays, respectively314202728. As the harmonization attempt between our manual SP142 LDT with SP263 Ventana assay produced suboptimal results in our study, it was examined whether manual LDT assay could serve as an economical screening tool to select cases for the more expensive SP263 Ventana assay. At the clinically relevant cut-offs of ≥1 and ≥50 per cent tumour cell staining (SP263 Ventana assay) that determines whether a patient with advanced NSCLC will receive pembrolizumab as first-line or second line-therapy, the observed sensitivity and NPV of our manual SP142 LDT was insufficient for screening, missing out nearly one-fourth of pembrolizumab eligible patients.
Unlike tumour cell staining, interpretation of IC staining showed increased variability among different clones. Further, even for specific PD-L1 clones, studies have found poor inter-observer concordance (19% for SP142)219. PD-L1 expression in immune cell expression has been postulated to be an indicator of pre-existing suppressed immunity in a treatment-naïve patient, while tumour cell expression of PD-L1 is said to be an adaptive response mediated by interferon for immune escape, occurring post-treatment in responders29. Although immune cell staining occurs in all clones, the predictive and prognostic value of immune cell staining has been validated only for SP142 clone, wherein immune cell staining ≥1 per cent by SP142 Ventana assay is predictive of treatment response to atezolizumab, independent of the degree of tumour cell staining78101829. In a previous study in our laboratory using the same SP142 clone, it was shown that PD-L1 expression in immune cells was an independent marker for better prognosis in NSCLC patients, although the predictive value could not be determined due to lack of treatment data13. In this study, SP142 clone picked up more number of immune cells as compared to SP263; however, further understanding of this finding was limited due to the lack of treatment response data.
The proportion of PD-L1-positive NSCLC ranged from 27.5 (SP142) to 33.8 per cent (SP263) in our study, comparable to previous described frequencies132229. PD-L1 immunopositivity with both SP263 and SP142 was highest among squamous cell carcinomas, followed by adenocarcinomas, as previously observed13. Unlike adenocarcinomas that showed comparable proportions of tumour cell staining with both clones, SP263 stained more tumour cells in squamous cell carcinomas while SP142 stained more tumour cells of sarcomatoid carcinomas.
In conclusion, our study findings suggested that the manual LDT assay using SP142 clone showed only moderate concordance with the SP263 Ventana assay and lacked sufficient sensitivity as a screening tool. The development of an economical LDT assay using the SP263 clone would be beneficial in centres with limited resources. While our study attempted to address an important issue i.e., utility of an economical laboratory-developed assay as an alternative to expensive automated commercial predictive IHC assay on a large number of cases, the lack of clinical follow up particularly treatment response data was a limiting factor. In the era of molecular medicine, such validation of LDT assays by comparison with trial validated assays or using clinical evidence in the form of treatment response data may be attempted, so that patient therapy remains uncompromised and affordable.
Financial support & sponsorship: The study was partially supported by intramural funding from the Research Section, All India Institute of Medical Sciences, New Delhi (Grant no. A-132).
Conflicts of Interest: None.
References
- A mini-review for cancer immunotherapy: Molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int J Mol Sci. 2016;17 pii. E1151
- [Google Scholar]
- Programmed death-ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867-76.
- [Google Scholar]
- Immunohistochemistry for PD-L1. In: Tsao MS, Kerr KM, Dacic S, Yatabe Y, Hirsch FR, eds. IASLC Altas of PD-L1 immunohistochemistry testing in lung cancer (1st ed). North Fort Myers: IASLC Publication; 2017. p. :35-48.
- [Google Scholar]
- PD-L1 expression in melanoma: A quantitative immunohistochemical antibody comparison. Clin Cancer Res. 2017;23:4938-44.
- [Google Scholar]
- Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11:95.
- [Google Scholar]
- PD-L1 SP142 assay. In: Tsao MS, Kerr KM, Dacic S, Yatabe Y, Hirsch FR, eds. IASLC Altas of PD-L1 immunohisto-chemistry testing in lung cancer (1st ed). North Forst Myers: IASLC Publication; 2017. p. :63-72.
- [Google Scholar]
- Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-65.
- [Google Scholar]
- Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-46.
- [Google Scholar]
- Adenocarcincoma. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, eds. WHO classification of tumors of the lung, pleura, thymus and heart (4th ed). Lyon: IARC; 2015. p. :26-37.
- [Google Scholar]
- Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052-62.
- [Google Scholar]
- Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257-65.
- [Google Scholar]
- Clinicopathologic correlation of programmed death ligand-1 expression in non-small cell lung carcinomas: A report from India. Ann Diagn Pathol. 2017;31:56-61.
- [Google Scholar]
- Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016;29:1165-72.
- [Google Scholar]
- PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-71.
- [Google Scholar]
- Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965-70.
- [Google Scholar]
- PD-L1 expression as a predictive biomarker in cancerimmunotherapy. Mol Cancer Ther. 2015;14:847-56.
- [Google Scholar]
- PL04a.04: Multicentric French harmonization study for PD-L1 IHC testing in NSCLC. J Thorac Oncol. 2017;12(Suppl 1):S11-2.
- [Google Scholar]
- A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051-8.
- [Google Scholar]
- PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208-22.
- [Google Scholar]
- PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: Inter-pathologist variability is higher than assay variability. Mod Pathol. 2017;30:1411-21.
- [Google Scholar]
- Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1 (PD-L1) on non-small cell lung carcinoma. Appl Immunohistochem Mol Morphol. 2018;26:83-93.
- [Google Scholar]
- Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65.
- [Google Scholar]
- Safety, activity and immune correlates of anti-PD-L1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
- [Google Scholar]
- Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH) J Clin Oncol. 2017;35:2781-9.
- [Google Scholar]
- Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33(Suppl 15):8028.
- [Google Scholar]
- Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res. 2017;23:3585-91.
- [Google Scholar]
- A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 2017;3:256-9.
- [Google Scholar]
- Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-7.
- [Google Scholar]






