Translate this page into:
Comparison of intranasal midazolam-fentanyl with dexmedetomidine–fentanyl as pre-medication in the paediatric age group
For correspondence: Dr Tanveer Singh Kundra, Department of Anaesthesiology & ICU, Government Medical College, Patiala 147 001, Punjab, India e-mail: tvskundra@yahoo.co.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Intranasal midazolam-fentanyl is commonly used as pre-medication in paediatric patients, but there is a risk of respiratory depression with this combination. Dexmedetomidine is a drug that preserves respiratory function. The objective of this study was to compare the efficacy of intranasal midazolam-fentanyl and dexmedetomidine-fentanyl in paediatric patients undergoing elective surgeries.
Methods:
Hundred children in the age group of 3-8 yr of American Society of Anaesthesiologists physical status grade 1 were randomized into two groups- group A received intranasal midazolam (0.2 mg/kg)-fentanyl (2 µg/kg) and group B received intranasal dexmedetomidine (1 µg/kg)-fentanyl (2 µg/kg) 20 min before induction of general anaesthesia. Heart rate and SpO2 were monitored. Sedation score, parental separation and response to intravenous cannulation were seen after 20 min. Children were monitored for 2 h for post-operative analgesia by Oucher’s Facial Pain Scale.
Results:
Sedation scores were satisfactory in both groups, although children in group A were more sedated than in group B. Parental separation and response to intravenous cannulation were comparable in both the groups. The two groups were also haemodynamically comparable intraoperatively. Post-operative heart rate was also comparable at all-time intervals in both the groups except for heart rate at 100 and 120 min which were more in group A. Group A experienced more post-operative pain as assessed by Oucher’s Facial Pain Scale as compared to group B. Children receiving intranasal dexmedetomidine-fentanyl had better post-operative analgesia as compared to those who received intranasal midazolam-fentanyl.
Interpretation & conclusions:
Both intranasal midazolam with fentanyl and intranasal dexmedetomidine with fentanyl provided satisfactory sedation. Both groups were comparable in separation reaction and response to intravenous cannulation with better post-operative analgesia in children receiving intranasal dexmedetomidine-fentanyl.
Keywords
Dexmedetomidine
fentanyl
intranasal
midazolam
paediatric
pre-medication
Anxiety and fear before surgery is commonly seen in children. It is mainly due to fear of surgery, operation theatre, encountering strange new faces and forced parental separation1. This anxiety causes psychological stress in tender minds of children and can lead to negative post-operative behavioural changes in the child. Therefore, this stress and anxiety need to be reduced before surgery.
The aim of the anaesthesiologist should be to bring the child in the operation theatre calm and quiet. This can be achieved by administering pre-medication to the child to allay fear and anxiety. Intranasal route is most commonly chosen in paediatric patients because intranasal route is atraumatic and absorption by this route occurs directly into central circulation, bypassing the enterohepatic circulation2.
Midazolam is a benzodiazepine drug that causes sedation, anxiolysis, amnesia and hypnosis by its action on gamma-aminobutyric acid (GABA) receptors3. Fentanyl, a synthetic opioid, binds mainly to mu-opioid receptors and produces analgesia4. However, this combination may be associated with respiratory depression. There is a need to find a drug or a drug combination that is as effective and at the same time is associated with minimal respiratory depression. Thus lacunae in this area necessitated the present study to find out a better drug or drug combination.
Dexmedetomidine, a selective alpha-2 adrenoceptor agonist produces sedation, analgesia, anxiolysis with preservation of respiratory function5. Thus, this drug may be useful as pre-medication in paediatric patients undergoing elective surgeries.
The objective of the present study was to compare the efficacy of midazolam-fentanyl and dexmedetomidine-fentanyl in children for pre-medication through the intranasal route.
Material & Methods
The study was conducted in the department of Anaesthesiology, Government Medical College Patiala, Punjab from January 2018 to July 2019. After obtaining approval from the Institutional Ethical Committee, informed written consent was obtained from the parents of all children prior to commencement of the study.
Sample size calculation: The two independent groups to be compared were of equal size ‘n’ and drawn from populations of equal and known variances. The sample size was calculated using the formula:
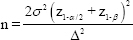
where, α=0.05; z(1−α/2)=1.95996; β=0.10; Power=1−β=0.90; z(1−β)=1.28155; σ=5.02; Δ=2.89; n=48 per group. Alpha (α) is the level of significance; beta (β) is the type II error whose complement to 1 is the power; sigma (σ) is the common standard deviation, whereas ‘σ2’ is its square, the common variance; delta (∆) is the hypothesized difference between the two groups, whereas ‘∆2’ is its square. ‘z(1−α/2)’ and ‘z(1−β)’ are the respective tail areas under the standard normal curve (we assumed that the two means followed the normal distribution, at least approximately).
Inclusion criteria: The present study was conducted prospectively in a double-blinded manner and included 100 children in the age group of 3-8 yr of either sex as per the American Society of Anaesthesiologists (ASA) physical status class I, scheduled to undergo elective surgery under general anaesthesia lasting 30-120 min.
Exclusion criteria: Children with nasal atresia, history of nasal bleed or nasal discharge, with upper respiratory tract infection, or hypersensitive to benzodiazepines, fentanyl or dexmedetomidine were excluded from the study.
Sampling and randomization: The children were randomly allocated into two groups of 50 each, through a computer-generated random table. Group A received intranasal midazolam (0.2 mg/kg)-fentanyl (2 µg/kg) and group B received intranasal dexmedetomidine (1 µg/kg)-fentanyl (2 µg/kg).
The anaesthetist administering the allocated drug was given the drug in an unlabelled syringe by the principal investigator and since both drug combinations were colourless, the administering anaesthetist was not aware which drug was being administered.
The same preparation of midazolam which is used intravenously and available commercially in the concentration of 5 mg/ml was used and the dose was calculated as per the bodyweight of the child. The total volume was diluted to 4 ml. Similarly, the same dexmedetomidine preparation available for intravenous use (concentration of 100 µg/ml) was used, with the final volume being 4 ml.
The doses used in the present study were as per the usual doses used in previous studies6,7. The dose range of intranasal midazolam is typically 0.2-0.3 mg/kg, while dexmedetomidine has been used in doses ranging between 1 and 3 µg/kg in various studies. Hence, the authors decided to use 0.2 mg/kg midazolam and 1 µg/kg dexmedetomidine. Ideally, a dose equivalence study should have been conducted earlier, which can be regarded as a limitation of the present study.
While intranasal midazolam and intranasal fentanyl are FDA approved, intranasal dexmedetomidine is not yet FDA approved for intranasal use. However, in India, as per the Drugs Controller General of India notification, a drug that is approved for the use by some other route can be used in an alternate route by taking Institutional Ethical Committee approval8. Hence, the authors decided to go ahead with the study.
Pre-anaesthetic checkup was done on every patient. As per the institutional protocol, children were allowed to take clear fluids 4 h before surgery. On the day of the surgery, children along with one parent were taken to the pre-operative room. No pre-medication was given in the ward. The parent was informed about the advantages of intranasal anti-anxiety pre-medication. Written informed consent was obtained from those willing to participate in the study. Baseline heart rate and SpO2 were recorded. The calculated dose for each patient was administered 20 min before induction of anaesthesia. The investigator who further assessed and managed the patient was different from the one who administered the pre-medication. Neither the participants nor the observers (who collected the data) knew which drug was being used. The dose was divided equally in each nostril, with the child in his/her mother’s lap, with the help of a syringe. Children were constantly observed for any side effects, which included odd behaviour or unexplained distress, nausea, vomiting, excessive sedation to the limit of non-arousal, itching or excessive salivation, bradycardia below 60 beats/min, desaturation below 95 per cent. Before induction in the operation theatre, each patient was observed for the degree of sedation recorded on the five-point sedation scale, separation reaction and response to venipuncture.
Level of sedation: Sedation scale was adopted from Wilton et al9 who performed a composite evaluation based on sedation, anxiolysis and co-operation leading to the determination of sedation score. Children with scores of three, four or five were considered to have good/excellent sedation while scores of one or two were considered unsatisfactory/fair sedation.
Separation reaction: This was also graded based on the grading reported by Wilton et al9. Children with scores of three or four were considered an acceptable separation while scores of one or two were considered an unsatisfactory/difficult separation from the parent.
Response to intravenous cannulation: This was graded similar to Gharde et al10. Scores of three or four were considered satisfactory and one or two were unsatisfactory.
General anaesthesia was standardized for all 100 patients. As per the institutional protocol, intravenous glycopyrrolate (0.004 mg/kg) was administered as an antisialogogue and induction was done with propofol, O2 and N2O. Intubation was facilitated by suxamethonium (2 mg/kg). All patients were maintained on oxygen, nitrous oxide, isoflurane and vecuronium. Patient’s HR and SpO2 were monitored throughout the surgery. At the end of the surgery, paracetamol (15 mg/kg) was given and reversal was done with glycopyrrolate (0.01 mg/kg) and neostigmine (0.05 mg/kg). All patients were extubated when fully awake. All patients were monitored for at least 2 h in the recovery room for post-operative analgesia by the anaesthetist posted there using Oucher’s facial pain scale.
Post-operative analgesia - Oucher’s facial pain scale:
Scores from 0 to 100: The severity of the pain and cardiorespiratory parameters were recorded at 20, 40, 60, 80, 100 and 120 min. Oucher’s facial pain scale of more than 30 was considered significant and the patient was given analgesia as per the institutional protocol. All patients were observed for adverse reactions, if any, especially odd behaviour or unexplained distress, nausea, vomiting, sedation, itching or excessive salivation.
Statistical analysis: Descriptive statistics were done for all data and were reported in terms of mean and percentages. Appropriate statistical tests of comparison were applied. The continuous independent variables were analyzed with Mann-Whitney U test and t test and Wilcoxon test. The categorical variables were analyzed with the help of the Chi-square test. The data were analyzed using the Statistical Package for the Social Sciences (SPSS) software version 22 (SPSS Inc. Chicago, IL, USA).
Results
The demographics of the patients and the time of surgery were comparable in the two groups. There was no significant difference among the age (5.450±1.765 yr in group A vs. 5.810±1.687 yr in group B; P=0.723), sex (P= 0.834), weight (17.502±5.131 kg in group A vs. 19.240±6.261 kg in group B; P=0.132), duration of surgery (68.600±26.265 min in group A vs. 75.200±27.198 min in group B; P=0.274) and ASA physical status of the patients. The pre-operative heart rate and SpO2 were comparable in both the groups at baseline, five, 10, 15 and 20 min.
The mean sedation score in children receiving intranasal midazolam–fentanyl was 3.68±0.81 as compared to 3.10±0.58 in children receiving intranasal dexmedetomidine–fentanyl, 20 min after the administration of pre-medication. The mean sedation scores in both the groups were found to be satisfactory, but group A produced superior sedation as compared to group B (P<0.001).
The number of children with unsatisfactory sedation scores (scores 1 and 2) were four (8%) in group A as compared to six (12%) in group B. The number of children with satisfactory sedation scores (3, 4 and 5) were 46 (92%) in group A as compared to 44 (88%) in group B. The number of children with satisfactory scores were comparable in both the groups (P>0.05).
The mean separation score in our study, in children receiving intranasal midazolam–fentanyl was 3.22±0.70 and in children receiving intranasal dexmedetomidine–fentanyl, it was 3.10±0.61. The mean separation scores in both groups were found to be satisfactory. They were comparable in both groups (P>0.05).
The number of children with unsatisfactory scores of parental separation (scores 1 and 2) were 6 (12%) in group A and 7 (14%) in group B and those with satisfactory scores (scores of 3 and 4) were 44 (88%) in group A and 43 (86%) in group B. The number of children with satisfactory scores were comparable in both the groups without any significant difference (P>0.05).
The mean scores of response to intravenous cannulation in our study in group A were 3.22±0.76, and in group B, it was 3.20±0.57 and were found to be satisfactory. These were comparable in both the groups, and there was no significant difference between the two groups (P>0.05).
The number of children with unsatisfactory scores of response to intravenous cannulation (Score 1 & 2) were eight (16%) in group A and four (8%) in group B and those with satisfactory scores (score of 3 & 4) were 42 (84%) in group A and 46 (92%) in group B. The number of children with satisfactory scores were comparable in both the groups without any significant difference (P>0.05).
The intraoperative SpO2 and heart rate were comparable in both groups with no significant difference. The difference in the post-operative heart rate of two groups was found to be significant 100 and 120 min after the surgery, whereas post-operative SpO2 and respiratory rate were comparable in both the groups.
The mean Oucher’s facial pain scores in both the groups showed significant difference at all time intervals as children who received intranasal midazolam–fentanyl had higher Oucher scores post-operatively, at all-time intervals as compared to children receiving intranasal dexmedetomidine–fentanyl (Table).
| Time (min) | Groups | 0-30 | 40-70 | 70-100 | χ2 | P |
|---|---|---|---|---|---|---|
| 20 | Group A | 41 | 9 | 0 | 9.89 | 0.002 |
| Group B | 50 | 0 | 0 | |||
| 40 | Group A | 21 | 29 | 0 | 40.85 | <0.001 |
| Group B | 50 | 0 | 0 | |||
| 60 | Group A | 8 | 42 | 0 | 68.58 | <0.001 |
| Group B | 49 | 1 | 0 | |||
| 80 | Group A | 3 | 47 | 0 | 77.44 | <0.001 |
| Group B | 47 | 3 | 0 | |||
| 100 | Group A | 1 | 49 | 0 | 62.88 | <0.001 |
| Group B | 40 | 10 | 0 | |||
| 120 | Group A | 0 | 47 | 3 | 36.45 | <0.001 |
| Group B | 26 | 24 | 0 |
Discussion
In the present study, the authors observed superior sedation scores with intranasal midazolam–fentanyl (group A) as compared to intranasal dexmedetomidine–fentanyl (group B) after 20 min, although the sedation scores were satisfactory in both the groups. The authors also observed that the mean scores of parental separation reaction and response to intravenous cannulation were satisfactory and comparable in the two groups. The two groups were comparable with regard to haemodynamics (HR and SpO2) pre- and intra-operatively. Post-operatively, heart rate was also comparable at all-time intervals in both the groups except for the heart rate at 100 and 120 min which were more in group A. Children receiving intranasal dexmedetomidine-fentanyl had better post-operative analgesia compared to those who received intranasal midazolam-fentanyl as demonstrated by lower Oucher’s facial pain scores.
In the present study, the mean sedation score in children receiving intranasal midazolam-fentanyl was 3.68±0.81 as compared to 3.10±0.58 in children receiving intranasal dexmedetomidine-fentanyl (P<0.001), 20 min after the administration of pre-medication. The number of children with unsatisfactory sedation scores (score 1 and 2) were four (8%) in group A as compared to six (12%) in group B (P=0.505).
The results of the present study correspond to the results of a study conducted by Schmidt et al11 where the authors evaluated the effects of pre-anaesthetic midazolam, clonidine or dexmedetomidine on post-operative pain and anxiety in children. They found satisfactory sedation scores with both intranasal dexmedetomidine (1 µg/kg) and oral midazolam (0.5 mg/kg).
However, Kumar et al12 in their study found that children who received 1 µg/kg intranasal dexmedetomidine were more satisfactorily sedated at the time of separation and induction of anaesthesia as compared to children who received 0.5 mg/kg oral midazolam. However, the authors were comparing the drugs through a different route of administration as compared to the present study.
The mean separation scores in both groups were found to be satisfactory and comparable in the two groups (P>0.05). Similar results were observed by Yuen et al13, who found no significant difference in the behaviour scores at separation from parents and at the time of induction in their three groups: oral midazolam (0.5 mg), intranasal dexmedetomidine (0.5 µg/kg) and intranasal dexmedetomidine (1 µg/kg).
However, slightly differing result was observed by Chatrath et al14 with regard to parental separation where they observed that intranasal dexmedetomidine provided better parental separation score than intranasal midazolam. However, the pre-medication drugs were administered 50 min prior to surgery and the short duration of action of a single dose of midazolam could have led to this different result. Furthermore, the number of patients recruited in their study (n=25) was less than that in the present study (n=50).
The mean scores of response to intravenous cannulation were comparable in both the groups in the present study. Tawfic15 compared midazolam plus fentanyl lozenges with midazolam syrup alone and found the combination to be superior in reducing apprehension at intravenous cannulation. Thus, the two drugs may have an additive effect on each other as far as reducing apprehension is concerned. Again Chatrath et al14 found a significant difference between midazolam and dexmedetomidine with regard to apprehension at intravenous cannulation, which could again be attributable to the short duration of action of a single dose of midazolam and to a different sample size.
The mean Oucher’s facial pain scores in both the groups showed significant difference as children who received intranasal midazolam-fentanyl had higher Oucher scores post-operatively, i.e. they experienced more pain, at all time intervals as compared to children receiving intranasal dexmedetomidine-fentanyl. This could be due to the inherent analgesic property of dexmedetomidine. Dexmedetomidine is a selective alpha-2 agonist. Alpha-2 adrenergic receptors act on the locus ceruleus area, inhibiting nociceptive neurotransmission through the posterior horn of the spinal cord. Alpha-2 adrenergic receptors also act on the presynaptic membrane, inhibiting the release of norepinephrine, which in turn induces hyperpolarization and inhibits the pain signals to the brain16. Moreover, dexmedetomidine promotes the release of acetylcholine from spinal interneurons; the resulting increased synthesis and release of nitric oxide could be involved in the regulation of analgesia16. Similar results were observed by Schmidt et al11 who concluded that dexmedetomidine (1 µg/kg) was related to lower pain scores as compared to midazolam (0.5 mg/kg). In another study conducted by Dewhirst et al17, the authors concluded that pain scores were comparable in intranasal dexmedetomidine and intranasal fentanyl groups but scores were higher in children receiving oral midazolam with intranasal dexmedetomidine as compared to children who received oral midazolam with intranasal fentanyl. A direct comparison between the pain scores was not observed between intranasal midazolam and dexmedetomidine in their study.
No major side effect in the form of odd behaviour or unexplained distress, nausea, vomiting, excessive sedation to the limit of non-arousal, itching or excessive salivation, bradycardia below 60 beats/min and desaturation below 95 per cent was observed in either group.
One of the limitations of the present study was that the doses used were as per the usual doses in previous studies. Ideally, a dose equivalence study should have been conducted earlier. Another limitation was the intranasal usage of injectable preparations of all drugs. However, this was the best available to us in our limited resources and these preparations have been used intranasally in previous studies as well6,7. Time of onset and peak sedation were not studied.
In conclusion, intranasal midazolam in a dose of 0.2 mg/kg with 2 µg/kg of fentanyl and intranasal dexmedetomidine in a dose of 1 µg/kg along with 2 µg/kg of fentanyl provided acceptable levels of sedation, parental separation reaction and response to intravenous cannulation when given 20 min before induction of anaesthesia without affecting the haemodynamics of the patient. However, intranasal dexmedetomidine-fentanyl provides better post-operative analgesia with lower Oucher’s facial pain scores as compared to intranasal midazolam–fentanyl. The higher level of sedation with intranasal midazolam–fentanyl combination calls for more vigilance and monitoring in the pre-operative room in patients receiving this combination. Further studies should be planned taking into consideration the time of onset, peak sedation and parental satisfaction.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Visiting the operating theatre before surgery did not reduce the anxiety in children and their attendant parent. J Pediatr Nurs. 2018;38:e24-9.
- [Google Scholar]
- Intranasal medications in pediatric emergency medicine. Pediatr Emerg Care. 2014;30:496-501.
- [Google Scholar]
- Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-23.
- [Google Scholar]
- Intranasal drug delivery: An efficient and non-invasive route for systemic administration: Focus on opioids. Pharmacol Ther. 2012;134:366-79.
- [Google Scholar]
- Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128-33.
- [Google Scholar]
- Nasal and buccal treatment of midazolam in epileptic seizures in pediatrics. Clinical Medicine Insights: Pediatrics. 2012;6:51-60.
- [Google Scholar]
- Systematic review and meta-analysis found that intranasal dexmedetomidine was a safe and effective sedative drug during paediatric procedural sedation. Acta Paediatrica. 2020;109:2008-16.
- [Google Scholar]
- Regulatory requirements for clinical trials in India: What academicians need to know. Indian J Anaesth. 2017;61:192-9.
- [Google Scholar]
- Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972-5.
- [Google Scholar]
- Evaluation of efficacy of intranasal midazolam, ketamine and their mixture as premedication and its relation with bispectral index in children with tetralogy of fallot undergoing intracardiac repair. Ann Card Anaesth. 2006;9:25-30.
- [Google Scholar]
- Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667-74.
- [Google Scholar]
- Efficacy of intranasal dexmedetomidine versus oral midazolam for paediatric premedication. Indian J Anaesth. 2017;61:125-30.
- [Google Scholar]
- A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: A double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715-21.
- [Google Scholar]
- Intranasal fentanyl, midazolam and dexmedetomidine as premedication in pediatric patients. Anesth Essays Res. 2018;12:748-53.
- [Google Scholar]
- Oral preanesthetic medication in children: A comparison of midazolam syrup versus midazolam syrup plus fentanyl lozenges. Alex J Anaesth Intensive Care. 2006;9:13-22.
- [Google Scholar]
- Dexmedetomidine in perioperative acute pain management: A non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899-904.
- [Google Scholar]
- Pain management following myringotomy and tube placement: Intranasal dexmedetomidine versus intranasal fentanyl. Int J Pediatr Otorhinolaryngol. 2014;78:1090-4.
- [Google Scholar]






