Translate this page into:
Comparison of efficacy & safety of iron polymaltose complex & ferrous ascorbate with ferrous sulphate in pregnant women with iron-deficiency anaemia
For correspondence: Dr Proteesh Rana, C-604 Supertech Rameshwer Orkid, Kaushambi, Ghaziabad 201 010, Uttar Pradesh, India e-mail: proteesh@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Iron-deficiency anaemia (IDA) is a common nutritional deficiency among pregnant women in India. It has a significant impact on the health of the mother as well as that of the foetus. IDA generally responds well to treatment with oral iron supplementation. However, oral iron supplements are toxic to the gastrointestinal mucosa and intolerance is common, resulting in poor compliance and failure of treatment. The iron salts such as iron hydroxide polymaltose complex (IPC) and ferrous ascorbate (FeA) are claimed to have low gastrointestinal intolerance, therefore better patient compliance than the conventionally used ferrous sulphate (FS). These preparations also claim to increase haemoglobin level faster as well as improve the iron storage better than FS. This study was done to compare the efficacy and safety of FS with IPC and FeA.
Methods:
It was a randomized, parallel, open label, study among pregnant women of gestational age between 12 to 26 wk with moderate anaemia. Patients were randomly allocated to receive either FS, IPC or FeA. They were then followed up for 90 days to observe for improvement in the haemoglobin levels and other haematological parameters or any adverse drug reaction.
Results:
The haemoglobin levels were comparable in the three groups except at day 90 when FeA group had significantly higher haemoglobin level as compared to FS group (P<0.05). The overall adverse effect profiles were also comparable among the study groups except epigastric pain which was more commonly reported in the FS group.
Interpretation & conclusions:
The results of the study showed that FS, IPC and FeA have comparable efficacy and safety profile in the treatment of IDA of pregnancy.
Keywords
Anaemia
ferrous ascorbate
ferrous sulphate
iron hydroxide polymaltose
pregnancy
Iron-deficiency anaemia (IDA) is the most common nutritional deficiency in the world1. It manifests as a hypochromic microcytic anaemia with low blood indices and serum ferritin. IDA is the most common form of anaemia in the women of reproductive age group and is frequently seen among pregnant women in developing countries with a prevalence of 35-75 per cent1. In India, the prevalence of anaemia among pregnant women is about 65-75 per cent2. Anaemia has a significant impact on the health of the mother as well as that of the foetus. It leads to preterm delivery, intrauterine growth retardation, low birth weight, postpartum haemorrhage, cardiac failure and increased risk of infections3.
IDA generally responds well to treatment with iron supplementation. Iron salts such as ferrous sulphate (FS), fumarate or gluconate have been extensively used for the prevention and treatment of IDA4. However, oral iron supplements, which are usually in the form of ferrous (Fe2+) salts, are toxic to the gastrointestinal mucosa and intolerance is common, resulting in poor patient compliance and failure of treatment5. The most common iron salt used in government health facilities for oral administration is FS. It is known to produce gastrointestinal side effects (nausea, vomiting, abdominal pain, constipation and diarrhoea). Other iron salts such as iron hydroxide polymaltose complex (IPC) and ferrous ascorbate (FeA) claim to have a lower incidence of gastrointestinal adverse effects, therefore better patient compliance. These preparations are claimed to increase haemoglobin level faster as well as improve the iron storage better than the conventionally used FS67.
The present study was therefore undertaken to compare the efficacy and safety of FS with other iron preparations such as IPC and FeA, available in the market for the treatment of IDA in pregnant women.
Material & Methods
The study was a single-centre, randomized, parallel-group, open-labelled study conducted at the Antenatal Clinic, department of Obstetrics and Gynecology, Lok Nayak Hospital and department of Pharmacology, Maulana Azad Medical College, New Delhi, India between January 2014-March 2015 after obtaining approval from the Institutional Ethics Committee (IEC). Written informed consent was also obtained from all the patients after providing complete information regarding the study.
Inclusion criteria: Pregnant women attending the Antenatal clinic at the study centre of gestational age between 12 and 26 wk who had blood haemoglobin levels between 7 and 9.9 g/dl (moderate anaemia) and microscopically diagnosed microcytic hypochromic anaemia were included in this study.
Exclusion criteria: We excluded the pregnant women with haemoglobin levels below 7 g/dl; severe concurrent illness (cardiovascular, renal, hepatic or any other systemic diseases); history of chronic inflammation (rheumatoid arthritis, gout, etc.); patients with active internal bleeding like bleeding piles, peptic ulcer, oesophageal varices; family history of thalassaemia, sickle cell anaemia or malabsorption syndrome; high obstetric risk associated with hypertension, diabetes, hepatic and renal diseases; or any history of hypersensitivity to iron preparations were excluded from the study.
Outcome variables: The blood haemoglobin levels and serum ferritin levels were measured as the outcome variables in this study.
Sample size: Forty patients per group were required to detect a five per cent difference in the percentage of patients achieving normal haemoglobin level (>11 g/dl) by the 60th day of treatment at an alpha of 0.05 and power of 80 per cent. To account for patient dropout, a sample size of minimum 50 patients in each group was considered3.
Statistical analysis: Data were expressed as mean±standard deviation. Differences between the three groups, i.e. statistical significance of quantitative variables, were determined by analysis of variance and by post hoc Tukey’s HSD (honestly significant difference) test to compare between individual groups. Comparison within the group at different intervals (30th, 60th and 90th day) was done using paired t test. Qualitative data between the groups were compared using Chi-square test. P≤0.05 was considered as a level of statistical significance.
Randomization: The patients were allocated in to three groups (Group A, B and C) by generating random numbers. Group A received FS tablets (containing 60 mg elemental iron to be taken twice daily; Unicure Remedies Pvt. Ltd, Baroda). In addition, the patients received folic acid tablets (5 mg, Emcure Pharmacuticals Ltd, Pune) from hospital pharmacy. Group B received IPC capsules (Orofer; containing 100 mg elemental iron once daily and 0.55 mg folic acid to be taken once daily; Emcure pharmaceuticals). Group C received FeA tablets (Ferium XT; containing 100 mg elemental iron and 1.5 mg folic acid to be taken once daily; Emcure pharmaceuticals). Each group received the same brand of iron and folic acid throughout the study period. FS was procured from a government hospital pharmacy. IPC and FeA were obtained as a gift from Emcure pharmaceuticals. The patients were given these drugs free of cost. They were not receiving any other drugs. The therapeutic regime was decided by the obstetrician without any interference by the investigator.
Counselling of patients: All the patients were counselled about the importance of diet and taking medicines in IDA. They were informed about food rich in iron and folic acid. The study participants were advised not to take any over-the-counter drugs without prior information the investigator. They could contact the principal investigator telephonically at any time during the study period for any queries.
Laboratory investigations: The SYSMEX KX-21 (Sysmex Corporation, Kobe, Japan), a fully automated blood cell counter, was used to estimate haemoglobin level and other anaemia indices such as mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), MCH concentration (MCHC) and reticulocyte count. Haemoglobin was measured using a non-cyanide haemoglobin method. Serum ferritin estimation was also done to measure the body iron stores in the study population. It was estimated by electrochemiluminescence immunoassay method based on sandwich principle using the Ferritin kits COBAS E and Elecsys 2010 (Roche Diagnostics, USA).
Blood samples were collected for the assessment of haemoglobin and other parameters (MCV, MCH and MCHC) at baseline (day 0) and then at periodic intervals (days 30, 60 and 90). In addition, blood reticulocyte count was measured after one week of iron therapy (day 7) and serum ferritin levels were also determined at the baseline (day 0) and at the end of the study (day 90).
Follow up and compliance: After the first follow up visit on day 7, the patients were asked to follow up on days 30, 60 and 90 respectively. During each follow up visit, they were subjected to general and obstetric examination and laboratory investigations and supplied with study medication for the next 30 days. Patients were also asked to observe and report any adverse effects such as epigastric distress, abdominal pain, nausea, vomiting, diarrhoea and constipation during these visits.
Compliance was checked by verbal enquiry, verified by checking empty or used packets of the drug brought by patients and enquiry about the colour of stools. Patients were also informed and followed up telephonically regarding the date of the next visit. Few patients were, however, lost to follow up as they could not be contacted.
Results
A total of 214 patients were assessed for eligibility. Inclusion criteria were pregnant females in the second trimester of pregnancy with microcytic hypochromic anaemia and haemoglobin levels between 7 and 9.9 g/dl (moderate anaemia). Thirty seven patients were excluded because they declined to participate in the study (n=9) or did not meet the inclusion criteria (n=28) and the remaining 177 patients were randomly allocated into three groups after obtaining their informed consent. The three groups were Group A – ferrous sulphate (FS), Group B – iron hydroxide polymaltose complex (IPC) and Group C – ferrous ascorbate (FeA). A total of 150 patients (50 patients from each group) completed the study as per protocol and were analyzed for the results at the end of the study (Fig. 1).
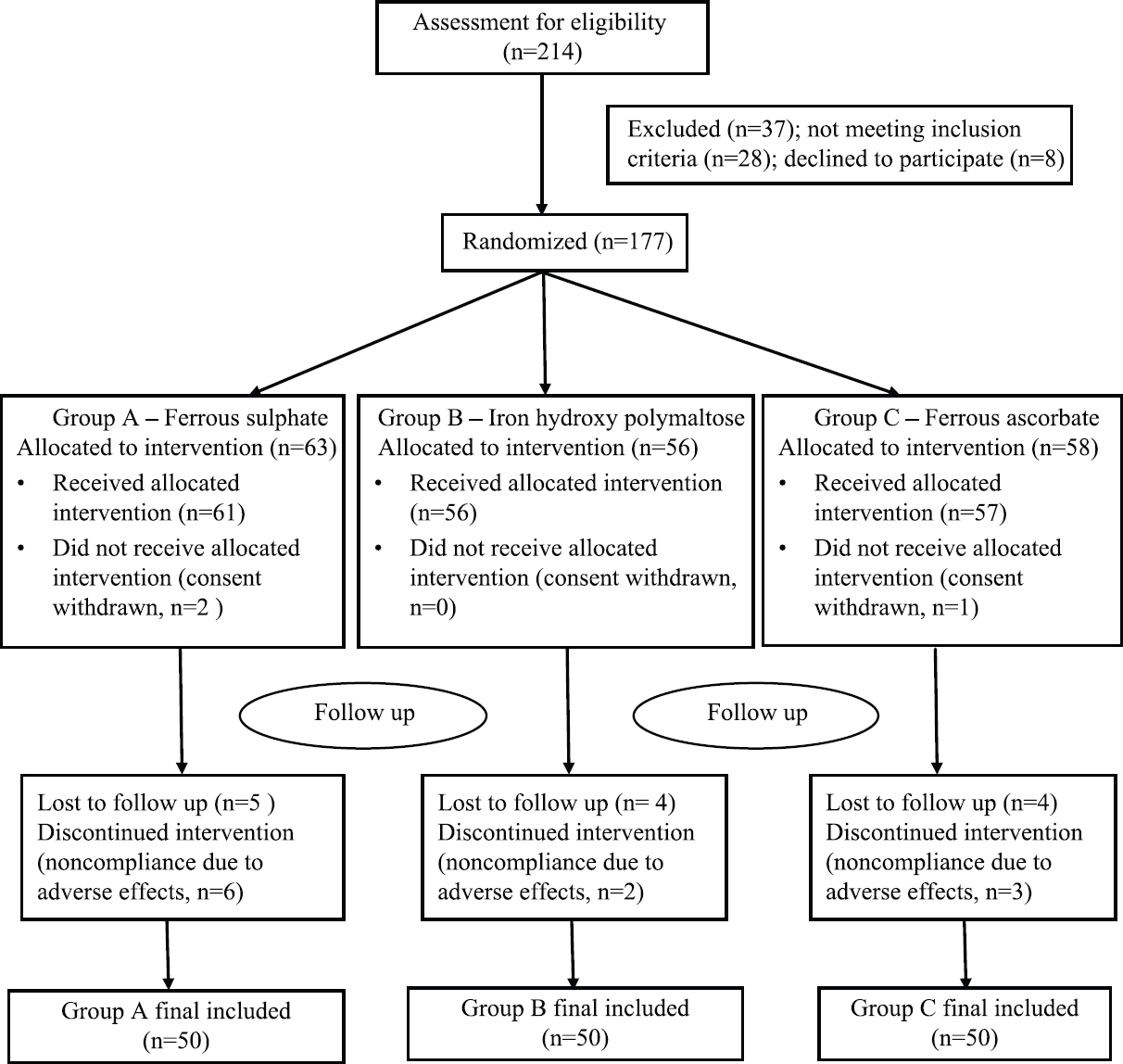
- Flow of participants through the study.
The baseline characteristics such as age, weight and gestational age and baseline haematological parameters including haemoglobin concentration, serum ferritin, MCV, MCH, MCHC and RBC count were also comparable between the three groups (Table I).
| Parameters | Group A (FS) (n=50) | Group B (IPC) (n=50) | Group C (FeA) (n=50) |
|---|---|---|---|
| Age (yr) | 23.6±1.94 | 24.04±2.11 | 23.38±1.99 |
| Gestational age (wk) | 15.9±2.10 | 15.16±1.65 | 15.48±2.05 |
| Weight (kg) | 47±4.03 | 47.32±3.51 | 47.12±4.58 |
| Primigravida (%) | 11 (22) | 10 (20) | 8 (16) |
| Multigravida (%) | 39 (78) | 40 (80) | 42 (84) |
| Haemoglobin (g/dl) | 8.56±0.57 | 8.46±0.55 | 8.61±0.56 |
| Ferritin (ug/l) | 8.84±3.25 | 8.62±2.9 | 8.7±2.43 |
| MCV (fl) | 69.55±4.8 | 69.87±4.96 | 69.71±4.44 |
| MCH (pg/cell) | 22.46±2.06 | 22.69±2.51 | 22.87±2.10 |
| MCHC (g/dl) | 29.49±1.98 | 29.29±1.74 | 29.33±1.33 |
| RBC count (million/cumm) | 4.03±0.3 | 3.97±0.2 | 3.94±0.25 |
Data presented as mean±SD. FS, ferrous sulphate; IPC, iron hydroxide polymaltose; FeA, ferrous ascorbate; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell
Response to iron therapy: The reticulocyte count was checked on the seventh day in all the patients enrolled in the study, which was above 1.5 per cent indicating good response to the iron therapy. There was a significant increase in haemoglobin levels on days 30, 60 and 90 as compared to baseline in all the three groups receiving FS, IPC and FeA (Table II). The mean rise in haemoglobin from baseline at the end of the study (day 90) was 2.43 ± 0.89 in FS group, 2.67 ± 0.76 in IPC group and 2.69 ± 0.75 in FeA group. Thus, the highest increase in haemoglobin levels was seen with FeA group as compared to the other two groups at day 90.
| Study groups | Day 0 (n=50) | Day 30 (n=50) | Day 60 (n=50) | Day 90 (n=50) |
|---|---|---|---|---|
| Group A - FS | 8.56±0.57 | 9.3±0.62 | 10.12±0.61 | 10.99±0.62 |
| Group B - IPC | 8.46±0.55 | 9.32±0.58 | 10.2±0.57 | 11.13±0.53 |
| Group C - FeA | 8.61±0.56 | 9.38±0.59 | 10.3±0.54 | 11.3±0.51* |
P*<0.05 haemoglobin levels in FeA group as compared to FS group
There was a significant difference in the haemoglobin levels between FS group and FeA group at day 90 (P<0.05). However, this difference was not found to be statistically significant between FS and IPC groups and between FeA and IPC groups at day 90 (Table II).
In case of the haematological parameters, the levels of MCV, MCH, MCHC and RBC count showed a statistically significant rise within each group at days 60 and 90 respectively in comparison to their baseline levels. However, there was no statistically significant difference in their levels between the three groups at baseline or at any subsequent point during the study (Table III).
| Tests | Group A (FS) | Group B (IPC) | Group C (FeA) |
|---|---|---|---|
| MCV (fl) | |||
| Day 0 | 69.55±4.8 | 69.874±4.96 | 69.71±4.44 |
| Day 30 | 71.25±4.95 | 71.24±4.76 | 71.7±4.56 |
| Day 60 | 73.16±5.03* | 73.16±4.96* | 73.53±4.79** |
| Day 90 | 75.42±5.62*** | 75.58±5.53*** | 76.08±5.14*** |
| MCH (pg) | |||
| Day 0 | 22.46±2.06 | 22.69±2.51 | 22.87±2.09 |
| Day 30 | 23.61±1.97* | 23.92±2.46 | 24.14±2.17* |
| Day 60 | 25.13±1.99*** | 25.11±2.40*** | 25.62±2.23*** |
| Day 90 | 26.79±2.06*** | 26.61±3.05*** | 27.04±2.44*** |
| MCHC (g/dl) | |||
| Day 0 | 29.49±1.98 | 29.29±1.74 | 29.33±1.33 |
| Day 30 | 30.49±1.77 | 30.24±1.83 | 30.66±1.51** |
| Day 60 | 31.79±1.76*** | 31.47±1.77*** | 32.24±1.75*** |
| Day 90 | 33.26±2.16*** | 33.1±2.02*** | 33.40±1.94*** |
| RBC count (million/cumm) | |||
| Day 0 | 4.03±0.3 | 3.97±0.26 | 3.94±0.25 |
| Day 30 | 4.15±0.29 | 4.09±0.27 | 4.12±0.25* |
| Day 60 | 4.27±0.26*** | 4.20±0.24*** | 4.16±0.23*** |
| Day 90 | 4.42±0.27*** | 4.36±0.25*** | 4.34±0.23*** |
| Serum ferritin | |||
| Day 0 | 8.84±3.25 | 8.62±2.9 | 8.7±2.43 |
| Day 90 | 28.59±9.64*** | 30.44±8.85*** | 31.80±6.25*** |
| Change from baseline (per cent change) | 19.74±10.17 (223) | 21.82±9.31 (253) | 23.1±6.71 (266) |
P*<0.05 versus day 0; **<0.01 versus day 0; ***<0.001 versus day 0. Data shown as mean±SD. MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell
The mean baseline levels of serum ferritin in the FS, IPC and FeA groups were 8.84, 8.62 and 8.7 ug/l, respectively. At the end of the study (day 90), the serum ferritin level rose to 28.59 ug/l in FS group, 30.44 ug/l in IPC group and 31.80 ug/l in FeA group. In all the three groups, there was a significant increase in serum ferritin levels from the baseline. This rise in ferritin levels at day 90 was comparable in all the three groups (Table III).
Safety: Adverse effects were reported in 74 out of the total 150 patients, however, no serious adverse event was seen in any patient. The number of patients with adverse effects from FS group, IPC group and FeA group was 31 (62%), 23 (46%) and 21 (42%), respectively (Fig. 2). The most common adverse effect noted within all the groups was nausea, seen in 43 out of 150 patients. Overall, nausea, epigastric pain and constipation were the three most common adverse effects followed by abdominal pain, diarrhoea and vomiting in descending order. These adverse effects were, however, observed with all the three medications administered. The adverse effects were higher in FS group; but there was no significant difference when compared with the other two groups except for epigastric pain. Epigastric pain was significantly higher in the FS group as compared to the other two groups (P<0.05). Epigastric pain was seen in 34 out of 150 patients (15 patients in FS group, 10 patients in IPC group and 9 patients in FeA group; P<0.05). The adverse effects between IPC and FeA groups were comparable (Fig. 2).
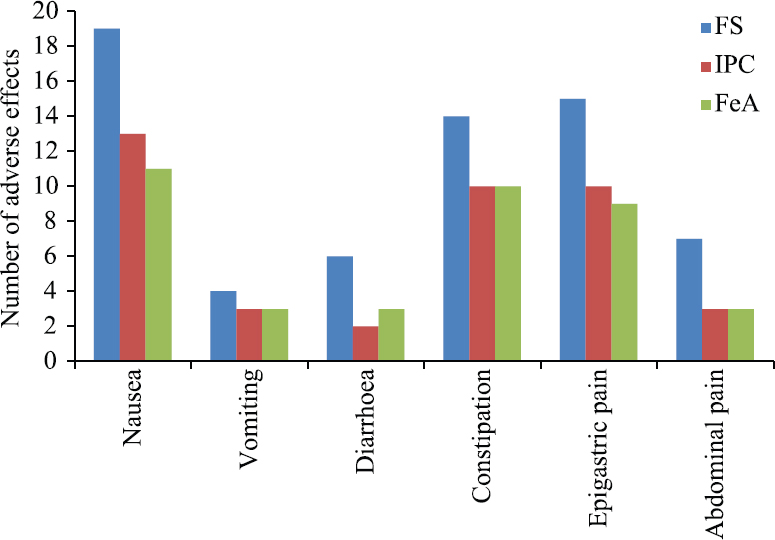
- Comparison of adverse effects between the group A-FS, group B-IPC and group C-FeA. FS, ferrous sulphate; IPC, iron hydroxide polymaltose; FeA, ferrous ascorbate.
Discussion
IDA is a common cause of morbidity and mortality in developing countries. It is the most common form of anaemia in Indian women during pregnancy and adversely affects the health6 and well-being of the mother and the foetus8. FS is recommended for the treatment for IDA by the Government of India and the World Health Organization (WHO)910 and hence is the most common iron preparation prescribed to anaemic pregnant women7. Although FS is cheap and effective, it frequently produces gastrointestinal adverse effects which affects the patients’ compliance1213. There are various iron preparations in markets which claim to have better efficacy and tolerability than FS.
In the present study, FS was compare with two such commercially available iron preparations – FeA and IPC1415. The rise in haemoglobin from baseline in the three groups was in the range of 0.74-0.77 g/dl at the end of the first month and it increased to 1.56-1.74 g/dl at the end of two months. This rise in haemoglobin was lower than that observed by Sarkate et al16, who reported a mean rise of 1.63-1.84 g/dl of haemoglobin at the end of 75 days of iron therapy. However, ferrous salts that were used in this study were ferrous fumarate and ferrous feredetate. The present study showed that there was a significant improvement in haemoglobin in all the three groups over the study period. The highest rise in haemoglobin was seen with FeA followed by IPC and the least rise amongst the three groups was seen with FS.
The level of haemoglobin at days 30 and 60 was comparable across the three groups, but at day 90, a significantly higher haemoglobin level was observed in the FeA group as compared to the FS group (P<0.05). This finding is corroborated by the study of Panchal et al15 who also observed a greater rise in haemoglobin with FeA than with FS. In the present study, there was no significant difference in the mean haemoglobin levels between IPC group and FS group even at day 90. However, Saha et al3 have reported that IPC causes a significant rise in haemoglobin and related anaemia indices as compared to FS in pregnant women with anaemia. It may be observed that as in the present study, the dose and duration of iron therapy were similar to that of the study done by Saha et al3.
The secondary end point in this study was to assess the change in serum ferritin level as a measure of body iron stores. The baseline serum ferritin levels of the study population were in the range of 8.62-8.84 ug/l denoting low iron stores. At day 90, the level of serum ferritin was highest in the FeA group followed by IPC group and least in the FS group, but this difference was not significant. Similar findings were also observed by Sarkate et al16. Other haematological parameters such as MCV, MCH, MCHC and RBC count showed comparable improvement across all groups denoting improvement in the iron status of anaemic patients. Thus, all the three iron salts were successful in improving the anaemia indices as well as in reconstituting iron stores as compared to the baseline levels.
In the management of IDA, tolerability and compliance to the therapy are as important as the bioavailability and efficacy of the iron salt used. In this study, the most common adverse effects observed were epigastric pain, nausea, vomiting, constipation, diarrhoea and abdominal pain. There were no serious adverse events and apart from the epigastric pain which was more common in the FS group, all other adverse effects were comparable in all three study groups. Previous studies have also shown that FS is associated with higher incidence of gastrointestinal adverse effects, but efforts to improve gastrointestinal tolerance using different types of iron preparations such as IPC and FeA have provided only marginal improvement171819. It has been seen that IPC, which contains iron in ferric form, causes lesser gastrointestinal adverse effects as it does not induce the generation of reactive oxygen species20. In the study done by Saha et al3 in anaemic pregnant women, lesser adverse effects and better compliance were observed in patients treated with IPC in comparison with FS. Similarly, FeA is known to produce lesser adverse effects and better compliance than FS21.
This study was limited by the fact that it was a small trial with 150 subjects and a larger sample size is needed to provide better information on the efficacy and safety of these iron preparations. Another limitation was the open-label study design which could have resulted in bias in reporting of adverse effects and a single- or double-blind study would have provided more meaningful information on the drug safety. Overall, this study shows that FS, IPC and FeA have comparable efficacy in the treatment of IDA of pregnancy, whereas both IPC and FeA had lower incidence of gastrointestinal adverse effects.
Acknowledgment:
Authors acknowledge Dr Sameer Deshmukh for his assistance with the collection of study data. Authors also acknowledge Emcure Pharmaceuticals, Pune, who gifted tablet Orofer (IPC) and Ferium XT (FeA) which was used in this study.
Conflicts of Interest: None.
References
- Iron deficiency anaemia:assessment, prevention, and control:A guide for programme managers. Available from: https://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf
- Anemia 'a silent killer'among women in India:present scenario. Eur J Zool Res. 2014;3:32-6.
- [Google Scholar]
- Comparison of efficacy, tolerability, and cost of iron polymaltose complex with ferrous sulphate in the treatment of iron deficiency anemia in pregnant women. Med Gen Med. 2007;9:1.
- [Google Scholar]
- Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Available from : https://www.who.int/nutrition/publications/micronutrients/guidelines_for_Iron_supplementation.pdf?ua=1
- Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults:a systematic review and meta-analysis. PLoS One. 2015;10:e0117383.
- [Google Scholar]
- Safety and efficacy of iron (III)-hydroxide polymaltose complex/a review of over 25 years experience. Arzneimittelforschung. 2007;57:439-52.
- [Google Scholar]
- Comparison between ferrous ascorbate and colloidal iron in the treatment of Iron deficiency anemia in children. Br J Med Med Res. 2012;2:195-205.
- [Google Scholar]
- Parks textbook of preventive and social medicine (23rd ed). Jabalpur: Banarasidas Bhanot Publishers; 2015. p. :242-4.
- National Iron-plus initiative guidelines for control of iron deficiency anaemia in India, 2013. Natl Med J India. 2014;27:27-9.
- [Google Scholar]
- Preventing and controlling iron deficiency anaemia through primary healthcare:A guide for health administrators and programme managers. Available from: https://apps.who.int/iris/handle/10665/39849
- A comparative study of the efficacy and tolerability of carbonyl iron and ferrous sulfate in iron deficiency anaemia. Int J Health Sci Res. 2012;2:46-52.
- [Google Scholar]
- Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults:A systematic review and meta-analysis. PLoS One. 2015;10:e0117383.
- [Google Scholar]
- Ferrous versus ferric oral iron formulations for the treatment of iron deficiency:A clinical overview. Sci World J. 2012;2012:846824.
- [Google Scholar]
- Comparison of efficacy, safety and cost of therapy with oral ferrous ascorbate and ferrous sulphate in patients with iron deficiency anemia. J App Pharm Sci. 2015;5:66-72.
- [Google Scholar]
- A randomised double-blind study comparing sodium feredetate with ferrous fumarate in anaemia in pregnancy. J Indian Med Assoc. 2007;105:278. 280-1, 284
- [Google Scholar]
- Iron hydroxide polymaltose-cause of persistent iron deficiency anemia at delivery. Indian J Med Sci. 2001;55:616-20.
- [Google Scholar]
- Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren:A randomized controlled trial. Nutr J. 2014;13:71.
- [Google Scholar]
- Evaluation of ferric and ferrous iron therapies in women with iron deficiency anaemia. Adv Hematol. 2014;2014:297057.
- [Google Scholar]
- Anaemia in pregnancy in developing countries. Br J Obstet Gynaecol. 1998;105:385-90.
- [Google Scholar]
- The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12-33.
- [Google Scholar]






