Translate this page into:
Comparative analysis of the genomes of Shigella dysenteriae type 2 & type 7 isolates
Reprint requests: Dr Rupak K. Bhadra, Infectious Diseases & Immunology Division, CSIR-Indian Institute of Chemical Biology 4, Raja S.C. Mullick Road, Kolkata 700 032, India e-mail: rupakbhadra@iicb.res.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The four species of the genus Shigella, namely, S. dysenteriae, S. flexneri, S. boydii and S. sonnei cause a wide spectrum of illness from watery diarrhoea to severe dysentery. Genomes of these four species show great diversity. In this study, NotI, XbaI or I-CeuI restriction enzyme digested genomes of two Shigella dysenteriae isolates belonging to the serotypes 2 and 7 were extensively analyzed to find their relatedness, if any, with the whole genome sequenced strains of S. dysenteriae type 1 and S. flexneri type 2a.
Methods:
Pulsed-field gel electrophoresis (PFGE) technique was used to determine the diversity of Shigella genomes by rapid construction of physical maps. DNA end labelling, Southern hybridization and PCR techniques were also applied for mapping purposes.
Results:
The intron-coded enzyme I-CeuI cuts the bacterial genome specifically at its rrn operon. PFGE of I-CeuI digested S. dysenteriae genomes were found to carry seven rrn operons. However, I-CeuI profiles showed distinct restriction fragment polymorphism (RFLP) between the isolates as well as with the whole genome sequenced isolates. Further studies revealed that the genome sizes and I-CeuI linkage maps of the S. dysenteriae type 7 and type 2 isolates were similar to that of S. dysenteriae type 1 and S. flexneri type 2a genomes, respectively.
Interpretation & conclusions:
Our findings indicate that the type 7 and type 1 isolates of S. dysenteriae were probably evolved from a same precursor, while the type 2 and S. flexneri type 2a were probably evolved and diversified from a common progenitor.
Keywords
PFGE
rrn operon
RFLP
Shigella dysenteriae
S. flexneri
Shigellosis or bacillary dysentery is a severe diarrhoeal disease caused by the Gram-negative bacteria Shigella spp1–3. The genus Shigella is comprised of four species, namely, S. flexneri, S. dysenteriae, S. boydii and S. sonnei, and each of these species is further classified into 6, 15, 18 and 1 serogroups45, respectively, based on the ‘O’ antigen component of the lipopolysaccharide moieties present on the outer membrane of the cell wall. Among the four species, S. dysenteriae and S. flexneri are the major pathogen in developing countries6. It is believed that the manifestation of S. dysenteriae type 1 infection is more severe because of its exclusive property to produce shiga toxin, a potent enterotoxin1. The Shigella organisms invade and multiply within colonic epithelial cells causing mucosal ulcers but the organism rarely invades into the blood stream1. Shigella entry into susceptible host cells depends upon the coordinated actions of numerous genes that are activated in response to environmental cues78. Another important feature of Shigella spp. is that its virulence is absolutely dependent upon a large plasmid, which carries several genes needed for invasion of host epithelial cells8. However, chromosomal genes present in the ‘pathogenicity islands’ also participate in virulence processes directly or contribute to survival in the environment encountered during infection9.
Although various aspects of S. dysenteriae type 1 and S. flexneri type 2a including whole genome sequences are known, but very little information is currently available on S. dysenteriae non-type 1 strains. In India, it was observed that dominant serotypes of Shigella spp. changed over time10. In Bangladesh, a significant number of Shigella strains isolated between 1999 and 2002 from hospitalized diarrhoeal patients could not be serotyped using the existing serotyping scheme6. These isolates exhibited very similar biochemical traits of Shigella and were S. dysenteriae type 2 isolates.
In this study, pulsed-field gel electrophoresis (PFGE)-based macrorestriction mapping (MRM) of two non-type 1 S. dysenteriae isolates, one belonging to type 2 and the other type 7 was performed to get insight about their genomic structures and compare with the genomes of well-studied S. dysenteriae type 1 and S. flexneri type 2a isolates. This technique has now been commonly used for demonstrating intra-species genome size variation1112. Because of high conservation of intron coded I-CeuI megarestriction sites in the bacterial 23S rRNA genes so far examined and conservation of the number and locations of rrn genes, the I-CeuI MRM provides an excellent tool for rapid examination of genomes of related species of bacteria. In this study, we have also compared the I-CeuI profiles of S. dysenteriae type 2 and type 7 isolates with reported whole genome sequenced S. dysenteriae type 1 and S. flexneri type 2a isolates. Further, we have constructed the I-CeuI linkage maps of the genomes of type 2 (F23659) and type 7 (SH89) isolates.
Material & Methods
The study was conducted in the laboratory of Infectious Diseases and Immunology Division, Indian Institute of Chemical Biology, Kolkata, India, during 2004 and 2009.
Bacterial strains, plasmids and growth conditions: Shigella isolates obtained from stool samples of dysentery patients between 1999 and 2000 were included in this study. Two S. dysenteriae type 1 isolates, (283 and SH6), one each of S. dysenteriae type 2 (F23659), type 7(SH89) and S. flexneri type 2a (SH1) were also included in this study. The plasmids pKP31 (carries dnaK gene of Escherichia coli13), pOF12 (carries groEL genes of E. coli14), pKK3535 (carries rrn operon of E. coli15) and pALS10 (carries relA gene of E. coli16) were used for gene probing. Before preparation of Shigella genomic DNA, the serotypes of the strains were reconfirmed using the commercially available antisera kit (Denka Seiken, Tokyo, Japan). Shigella cells were grown in a gyratory shaker (200 rpm) at 37°C in Luria broth (LB; Difco, Detroit, USA) or in LB containing agar (1.5%) and were maintained at -70°C in 20 per cent glycerol stocks.
Preparation of high-molecular-weight genomic DNA and enzyme digestion: Intact genomic DNA of bacterial cells in agarose blocks was prepared essentially as described previously17. Agarose blocks were stored in a solution containing 0.5 M EDTA (pH 9.0), 1 per cent sarkosyl (Sigma-Aldrich, USA), and 1 mg/ml of proteinase K (Sigma-Aldrich) at 4°C for future use. Prior to restriction enzyme digestion, the blocks were washed with 1 mM phenylmethylsulphonyl fluoride (Sigma-Aldrich) in TE buffer [10 mM Tris-HCl, 1 mM ethylenediamine tetraacetic acid (EDTA), pH 8.0]. Finally, blocks were washed four times with TE buffer and used directly for restriction digestion. All restriction enzymes used in this study were obtained from New England Biolabs, USA and digestion of DNA was done essentially as directed by the manufacturer.
Pulsed-field gel electrophoresis (PFGE): PFGE was carried out in a Pulsaphor Plus System with a hexagonal electrode array (Pharmacia, UK) essentially as described previously17. Gels were usually run for 24 h at 5-10 V/cm and various pulse times. As molecular mass markers phage lambda multimeric DNA and yeast chromosomal DNA (New England Biolabs) were used.
End labelling: End labelling of DNA fragments following enzyme digestion was done by incubating the agarose slices in a buffer containing Klenow enzyme and [α-32P]dCTP, and the preparations were subjected to PFGE followed by drying of the gel and autoradiography17.
Molecular biological techniques and PCR: Standard molecular biological methods18 were followed throughout the study unless otherwise mentioned. Bacterial chromosomal DNA was prepared by cetyl trimethyl ammonium bromide (CTAB, Sigma-Aldrich)/NaCl method as described by Ausubel et al19. For hybridization experiment, 0.7 kb DNA fragment of the ompR gene was PCR amplified using 10 pmoles each of primers [ompR-F (5′-CTTTAGAGCCGTCCGGTACA-3′) and ompR-R (5′-CAAGATTCTGGTGGTCGATG-3′)]20 and genomic DNA of the S. dysenteriae type 2 isolates F23659 as template and used as a probe. PCR amplification conditions were as follows: initial denaturation at 94°C for 3 min followed by 30 cycles for amplification, each cycle comprises denaturation at 94°C for 1 min followed by annealing for 1 min at 55°C and extension at 72°C for 1 min. Final extension of DNA was carried out at 72°C for 7 min. PCR amplification was carried out by Taq DNA polymerase (Invitrogen, USA). PCR assays were performed using the GeneAmp PCR system (Model 9700; Applied Biosystems, USA). PCR amplified DNA was purified by electroelution method18. For labelling of DNA, about 50 ng of DNA was labelled with [α-32P]dCTP (Amersham Bioscience, UK) by the random-priming method using NEBlot kit (New England Biolabs). For Southern blot hybridization, restriction enzyme digested bacterial genomic DNA was separated by electrophoresis, transferred to the Hybond-N+ membrane (Amersham Biosciences) and hybridized with a labelled DNA probe at 60°C as described before2122. The membranes were washed under stringent conditions21, dried, and exposed to Kodak X-OMAT AR5 films.
Results
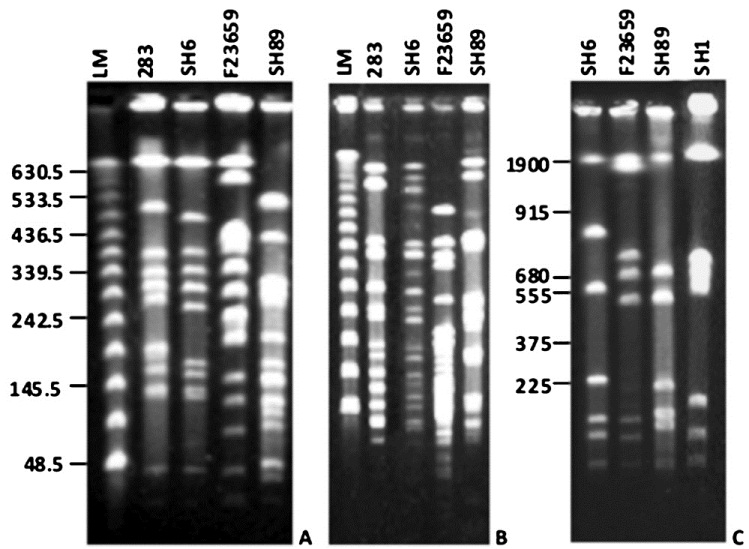
Comparison of NotI, XbaI and I-CeuI genomic profiles of S. dysenteriae isolates: It was previously reported that NotI digestion of genomes of Shigella spp., produces small number of large fragments that could be separated by PFGE202324. The enzyme NotI was also used earlier to construct the physical map of S. flexneri type 2a strain YSH600 by Okada et al20. Therefore, this enzyme was selected in this study to examine the extent of RFLP in the genomes of S. dysenteriae serotypes. Comparative analysis of macrorestriction patterns generated with NotI revealed distinct RFLP among the S. dysenteriae isolates (Fig. 1). The number of resolvable NotI fragments obtained from S. dysenteriae type 2 or 7 genome was found to be higher compared to that of S. dysenteriae type 1 (Fig. 1A). Talukder et al showed that digestion with the NotI enzyme of the genome of S. dysenteriae type 1 could generate about 12 fragments with top NotI fragment of about 1300 kb in size24. Similar NotI profile of the S. dysenteriae type 1 was obtained here. However, pulse conditions used here did not allow separation of that size of DNA fragment. Therefore, the top NotI fragment of S. dysenteriae type 1 failed to resolve properly and it appeared just above the largest lambda concatamer separated at about 630.5 kb region as shown in Fig. 1A. Hgher pulse conditions were used to separate the large NotI fragments of S. dysenteriae type 1 and non-type 1 (type 2 and 7) isolates and in that case the top NotI fragments were easily separated with their expected molecular sizes (data not shown). Similarly, PFGE analysis of the XbaI digested chromosomal DNA of the S. dysenteriae isolates revealed distinct RFLPs (Fig. 1B). Only seven fragments were generated from the genomes of Shigella spp. when the intron coded enzyme I-CeuI was used. Since, I-CeuI cuts specifically at 23S rrn gene of prokaryotes, the number of fragments generated from a circular genome indicates the number of rrn operons present. Thus, like other Shigella strains the type 2 and 7 strains also possess 7 rrn operons. Like NotI and XbaI, distinct RFLP with respect to I-CeuI was also observed among the S. dysenteriae isolates (Fig. 1C). The copy number of the rrn operons was determined by end-labelling assay, which also confirmed presence of 7 rrn operons in Shigella strains (data not shown). The major variations detected among I-CeuI fragments 2, 3 and 4 suggested genomic rearrangements in S. dysenteriae isolates examined. The sizes of I-CeuI fragments obtained from the genomes of S. dysenteriae type 2 and 7 isolates were determined and the data gave an estimate of genome size of each serotype. S. dysenteriae type 1 strain SH6 was used as control. The genome sizes of S. dysenteriae non-type 1 isolates examined in this study were ranged from 4.29 to 4.75 Mb (Table I). It should be noted that the genome size of S. dysenteriae type 2 (4.75 Mb) was found to be much higher than either type 1 SH6 (4.26 Mb) or type 7 (4.29 Mb). Since, I-CeuI was used to construct the macrorestriction maps of S. dysenteriae isolates, the restriction fragments were named on the basis of the enzyme used (C for I-CeuI) and was numbered on the basis of size in descending order.
-
A. PFGE profiles of NotI-digested chromosomal DNAs of different Shigella isolates. Enzyme digested genomic DNAs were separated by PFGE with pulse times ramping from 5 to 50 sec for 24 h using 10V/cm at 4°C. Shigella isolates used were 283 (S. dysenteriae type 1), SH6 (S. dysenteriae type 1), F23659 (S. dysenteriae type 2) and SH89 (S. dysenteriae type 7) as indicated above each lane. LM is the lambda concatameric DNA used as molecular size markers and their sizes (in kb) are shown in the left margin. B. PFGE separation of XbaI-digested genomic DNAs. Lanes and pulse conditions used are as indicated in panel ‘A’. C. PFGE patterns of I-CeuI-digested chromosomal DNAs. Enzyme-digested DNAs were separated by PFGE with pulse time ramping from 20 to 150 sec for 24 h using 10V/cm at 4°C. Shigella isolates used were SH6, F23659, SH89 and SH1 (S. flexneri type 2a) as indicated above each lane. YM denotes yeast intact chromosomes used as molecular size markers. Numbers in the left margin denotes sizes (in kb) of the yeast chromosomes.
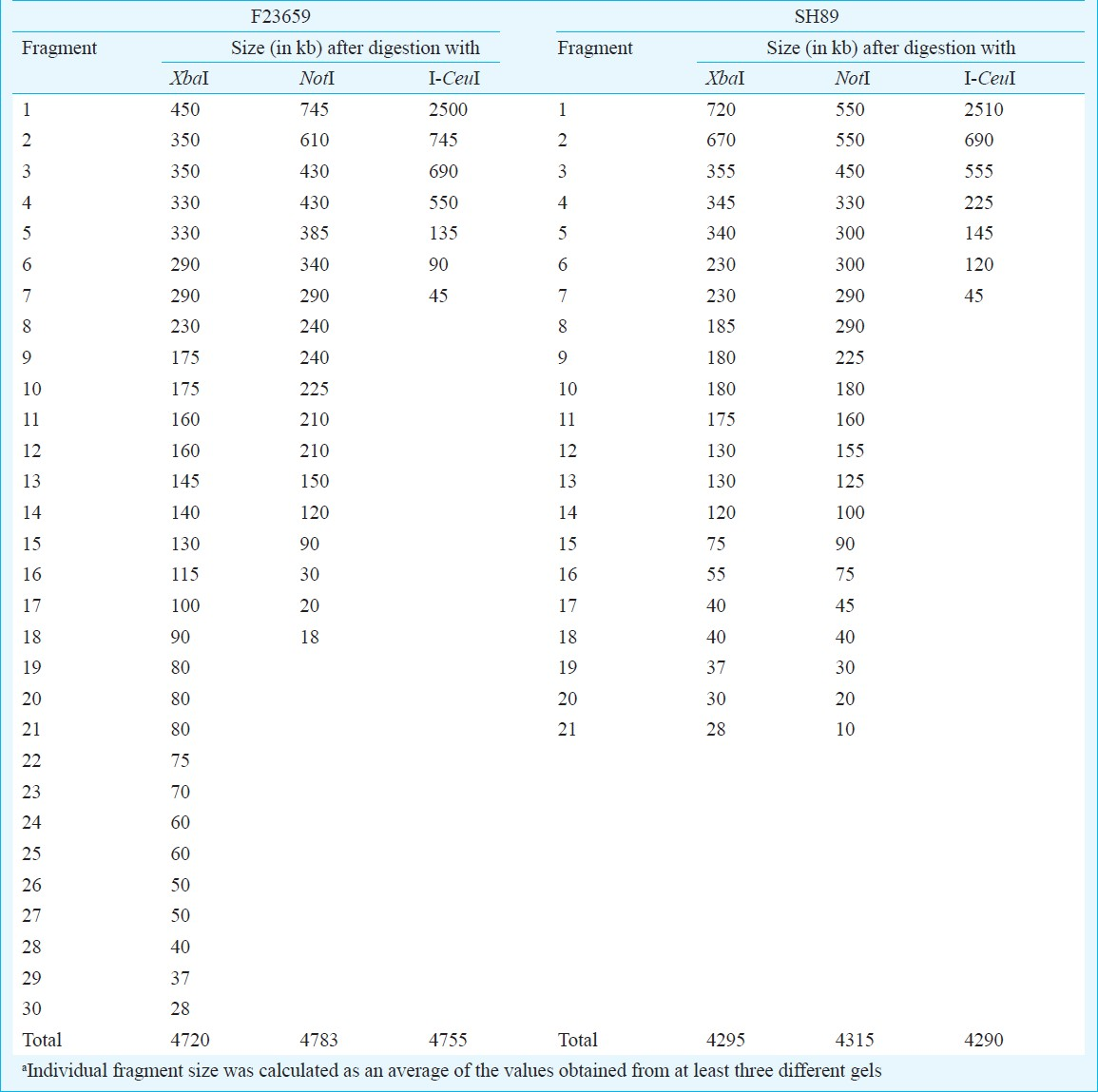
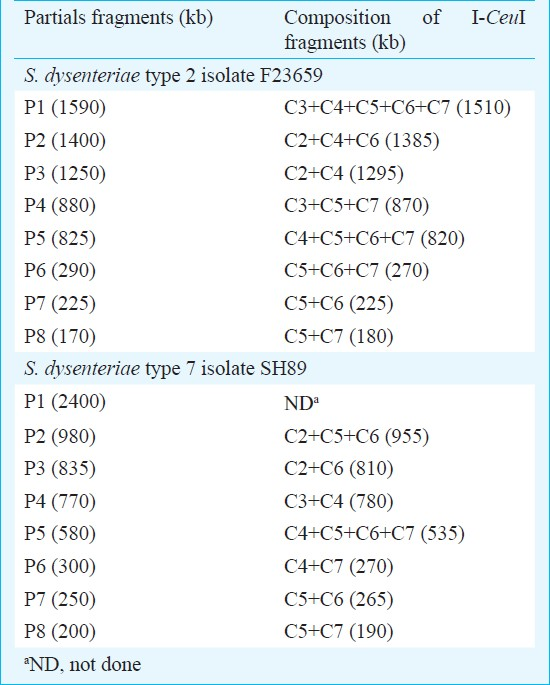
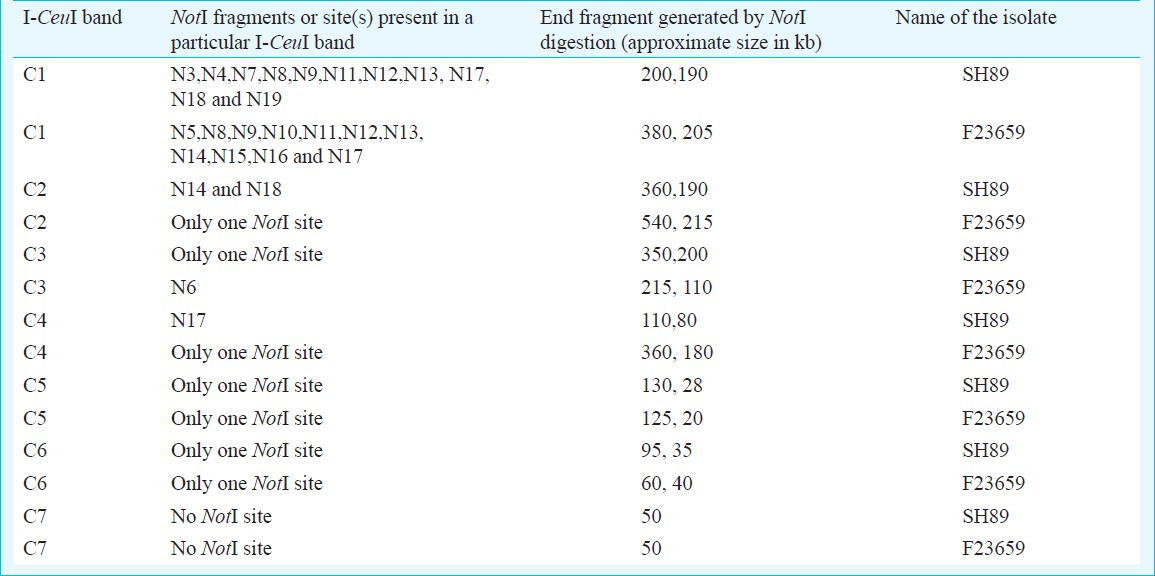
Construction of I-CeuI macrorestriction maps of S. dysenteriae type 2 and 7 isolates: Similarity in restriction digestion profiles and genome sizes between the S. dysenteriae type 2 and S. flexneri type 2a isolates or between S. dysenteriae type 7 and S. dysenteriae type 1 isolates prompted us to determine the linkage maps of non-type 1 strains for further confirmation. The I-CeuI physical maps of S. dysenteriae type 2 (F23659) and type 7 (SH89) were constructed primarily from analysis of partial fragments generated by the enzyme. The partial fragments (designated as P1, P2, etc.) obtained from each isolate using the enzyme I-CeuI and the possible linkages are described in Table II. However, linkage could not be established only for the P1 fragment (2400 kb) of the isolate SH89 (Table II) and this could be the C1 fragment of SH89. To confirm the linkages of I-CeuI fragments, individual PFGE separated fragments were excised from an agarose gel; each of these was digested separately with NotI, end-labelled and subjected again to PFGE and autoradiography. Intact fragments as well as end fragments generated by the digestion of each I-CeuI fragment by NotI are given in Table III. The end fragments were generated due to overlapping NotI fragment on an I-CeuI junction. This approach allowed in determining the linkages between I-CeuI fragments, identification of I-CeuI fragments carrying a single or no NotI site and clubbing of NotI fragments that are linked and overlapping with a particular I-CeuI fragment. For example, the gel-excised I-CeuI fragment C1 (2510 kb) of S. dysenteriae type 7 isolate SH89 (Table III) digested with NotI gave eleven fragments (N3, N4, N7, N8, N9, N11, N12, N13, N17, N18 and N19) plus two end fragments (200 and 190 kb) as detected in the autoradiogram (data not shown). Similarly, C2 contains only two NotI fragments (N14 and N18) and two end-fragments (360 and 190 kb). C1-C2 linkage was confirmed by adding the end-fragments 190 and 360 kb, which corresponded to the size of N1 (550 kb). Absence of N1 fragment in a NotI/I-CeuI double digests indicated that the N1 fragment contains I-CeuI site(s) (data not shown). Southern hybridization of NotI digested genomic DNA of isolate SH89 using rrn probe15 also proved the presence of I-CeuI sites in N1, N2, N5, N6, N12 and N17 fragments (Fig. 2). With similar analysis, the linkages between the fragments C1-C2, C1-C3, C2-C6, C3-C4, C4-C7, C5-C6 and C5-C7 of isolate SH89 were determined. Using the same approach, the linkages among the I-CeuI fragments of the isolate F23659 were established, which links C1-C2, C1-C3, C2-C4, C3-C7, C4-C6, C5-C6 and C5-C7 (Table III). The I-CeuI linkage maps were thus constructed for F23659 and SH89 isolates (Fig. 4).
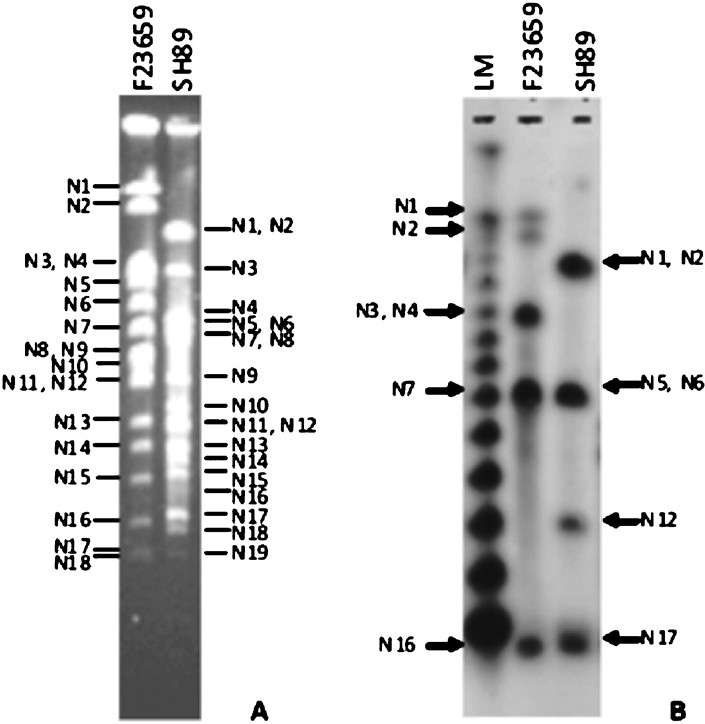
- Identification of NotI fragments carrying rrn operon(s). A. Ethidium bromide-stained gel containing the NotI-digested genomic DNAs of S. dysenteriae type 2 (F23659) and type 7 (SH89) isolates. NotI fragments of the isolates are as indicated. B. The gel shown in ‘A’ was transferred to a nylon membrane, hybridized with the rrn gene and autoradiographed. NotI fragments carrying rrn operon were marked, for F23659 (type 2) and SH89 (type 7) in the left and right margins, respectively. LM is the lambda concatameric DNA used as molecular size markers.
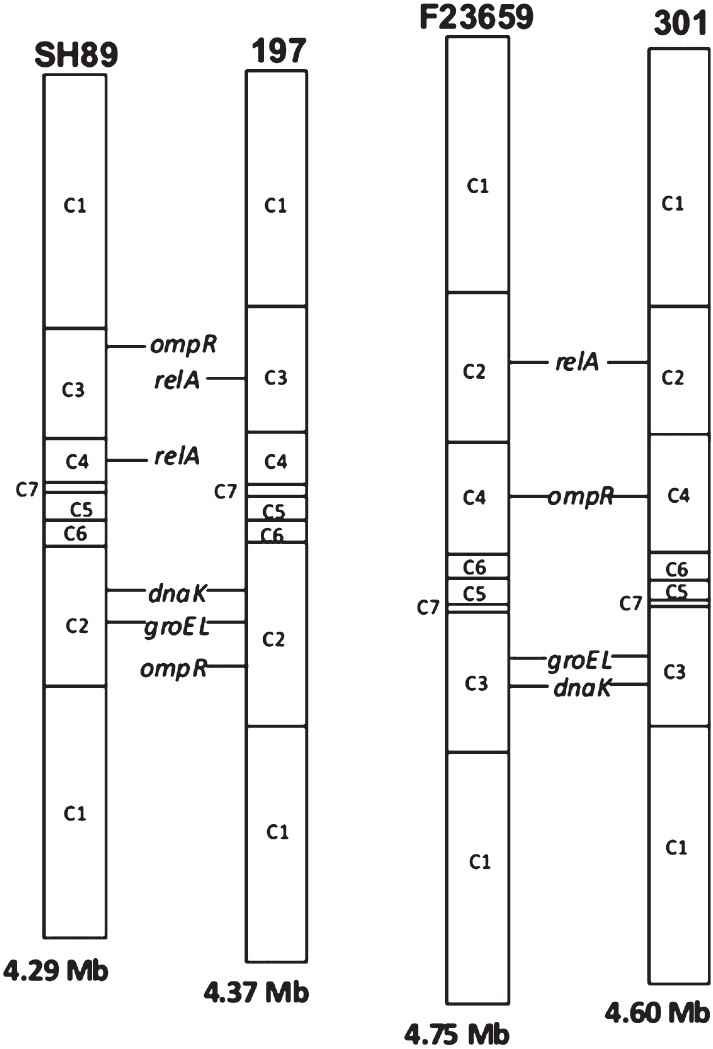
- Comparisons of the I-CeuI maps of S. dysenteriae type 2 isolate F23659, type 7 isolate SH89, S. dysenteriae type 1 isolate 197 and S. flexneri type 2a isolate 301. The I-CeuI map information for the whole genome sequenced isolates 197 and 301 were obtained from public database (www.tigr.org). For simplicity, the circular maps of Shigella genomes were shown as linear maps at an arbitrarily chosen point. The order of the I-CeuI fragments as well as positions of some selected genes, namely, dnaK, groEL, relA and ompR were also shown. Genome size of each Shigella isolate is indicated at the bottom of each linear map.
Comparative genome analysis of S. dysenteriae: For this, I-CeuI-digested genomic DNAs of S. dysenteriae isolates belonging to different serotypes were subjected to Southern blot hybridization experiments. The extent of RFLP could be detected in greater details by positioning various genes on the I-CeuI profiles of the isolates F23659 and SH89. The dnaK13 and the groEL14 genes, which code for the heat-shock protein Hsp70 and Hsp60, respectively, hybridized with the C3 fragment of the isolate F23659, but in the case of SH89 the same probes hybridized with the C2 fragment (Fig. 3). The relA gene16, which codes for the (p)ppGpp synthetase enzyme, hybridized with the C2 and C4 fragments of the isolates F23659 and SH89, respectively. The ompR gene which codes for a response regulator, positioned in the C4 and C3 fragments of the isolates F23659 and SH89, respectively (data not shown). The positions of all these genes in the genomes of isolates 301 and 197 were determined mainly from their whole genome sequence information (www.tigr.org) and shown in Fig. 4. All these position specific genes indicate that during evolution some rearrangement had occurred among C2, C3 and C4 fragments of S. dysenteriae type 1, type 2 and type 7 isolates. Moreover, the order of the I-CeuI fragments of F23659 matched with that of 301, while SH89 showed similarity (except relA and ompR) with that of 197 (Fig. 4). Considering the similarities between the genomes of S. dysenteriae type 2 isolate F23659 and S. flexneri type 2a isolate 301, it may be hypothesized that although SH89 (type 7) and F23659 (type 2) are taxonomically identical up to the species level, their evolutionary origins might be different. The gene probing data also reflect that S. dysenteriae type 2 may have originated from an ancestor closely linked with S. flexneri type 2a (Fig. 4).
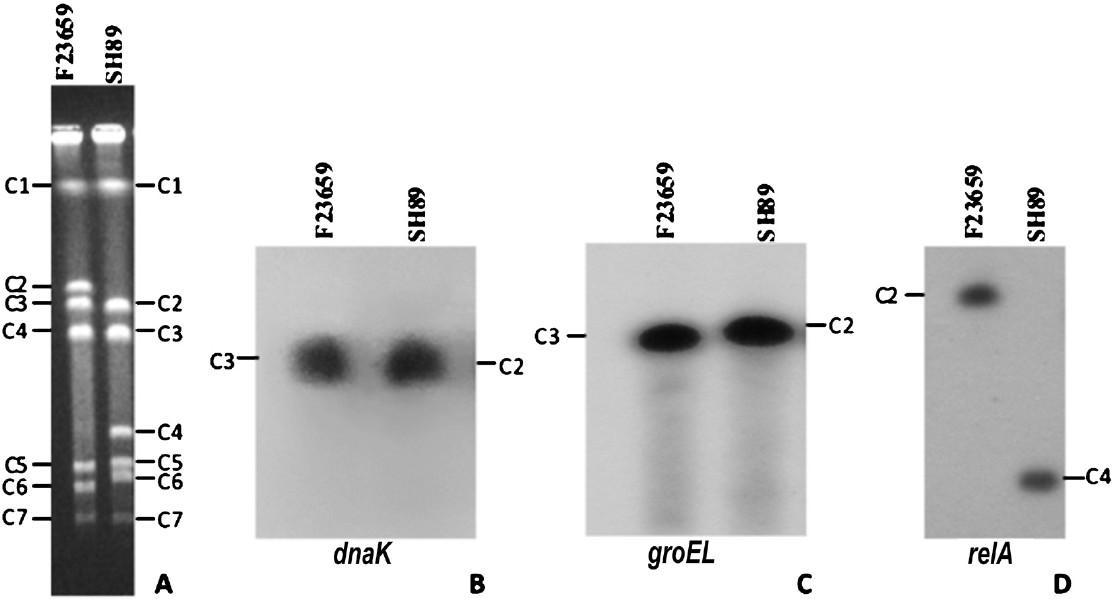
- RFLP in the genomes of S. dysenteriae isolates. A. Ethidium bromide stained PFGE gel containing I-CeuI-digested chromosomal DNAs of S. dysenteriae type 2 isolate F23659 and the type 7 isolate SH89. Electrophoresis conditions were same as described in the legend of Fig. 1C. Seven I-CeuI fragments (C1 to C7) of each isolate were marked. B-D. I-CeuI-digested chromosomal DNAs of the isolates F23659 and SH89 shown in ‘A’ were transferred to a membrane and hybridized with dnaK (B), groEL (C) or relA (D) gene as a probe. The same membrane was successively used after deprobing each time. Both dnaK and groEL genes hybridized with the I-CeuI fragment C3 of the F23659, but in the case of the SH89 the genes were hybridized with the I-CeuI fragment C2. The relA gene hybridized with the C2 and C4 fragments of the F23659 and SH89, respectively.
Discussion
Recent clinical studies in Bangladesh5 indicated that the prevalence of S. dysenteriae serotypes has been changed. Previously S. dysenteriae type 1 was the most frequently isolated serotype from clinical samples, which was gradually replaced by serotypes 2 and 46. Recently, Shigella like strains were identified in Dhaka, Bangladesh, which are biochemically typical Shigella but could not be serotyped by using the commercially available serotyping kit5. The S. dysenteriae type 2 strains in Bangladesh may be emerging from a common ancestral clone25. Analysis of the serotyping results for the S. dysenteriae strains isolated between 1999 and 2000 at Dhaka had also shown an abrupt decrease of serotype 1 from 76.4 to 6.5 per cent, while the isolation rate of other S. dysenteriae types (types 2 to 12) was increased from 19 to 87 per cent6. This observation reflects that there is a temporal shift in the dominance of S. dysenteriae serotypes in recent years6.
PFGE has been a valuable typing method for epidemiological investigation of several bacterial pathogens and clonality studies26. The current genomic RFLP data among the S. dysenteriae isolates belonging to serotypes 1, 2 and 7 indicate usefulness of the genomic RFLP method in identification of Shigella strains at the molecular level. We constructed the combined I-CeuI physical and genetic maps of two S. dysenteriae isolates, F23659 and SH89, belonging to serotypes 2 and 7, respectively. In this study, rDNA was detected on the basis of the number of fragments obtained in the I-CeuI digested chromosomal DNA of the strains. The number of the rDNA loci is seven in all the Shigella isolates, which suggest that the numbers of rrn loci are well conserved among the genus. It is noted that the genome size of S. dysenteriae type 2 isolate was larger than both type 1 and type 7 and similar to that of the genome size of S. flexneri type 2a strain. Moreover, comparative genome analysis revealed that the type 2 isolate F23659 was closely related with that of S. flexneri type 2a and the genome of type 7 isolate SH89 matched with that of S. dysenteriae type 1 isolate 197. Considering the facts that the S. dysenteriae type 2 isolate F23659 was similar to the whole genome sequenced strain27 of S. flexneri type 2a (isolate 301) with respect to genome size, number of rrn operons and positions of genetic loci specific for house-keeping genes studied in this study, it could be possible that the S. dysenteriae type 2 and the S. flexneri type 2a isolate 301 are genetically similar. The ancestry of the S. dysenteriae type 2 may largely account for the differences in its genome with that of serotypes 1 and 7 studied here. The S. dysenteriae type 2 strains may have evolved from a primitive strain that was genetically linked with the ancestor of S. flexneri type 2a. Recent study involving O-antigen modification of S. flexneri serotype 1c identified a gtrlC gene cluster. Analysis of the sequences surrounding this cluster revealed that it was carried by a novel bacteriophage28. This finding provided a possible clue about the evolution of serotypes within S. flexneri. Similar mechanism(s) could be associated in the evolution of S. dysenteriae type 2. However, further genetic analysis is required to get an insight into the genome structure of isolates belonging to S. dysenteriae type 2. The emergence of S. dysenteriae type 2 with epidemic potential is of high concern and should be carefully monitored in countries of Indian subcontinent, which is believed to be an epicenter for the spread of Shigella infection.
Acknowledgment
Authors thank Prof. Siddhartha Roy for his constant encouragement. The work was supported by research grant from Council of Scientific and Industrial Research (CSIR), New Delhi, Government of India. The first two authors (PP and AP) acknowledge CSIR and DBT (Government of India), respectively, for the research fellowships.
References
- Clonal multidrug-resistant Shigella dysenteriae type 1 strains associated with epidemic and sporadic dysenteries in eastern India. Antimicrob Agents Chemother. 2004;48:681-4.
- [Google Scholar]
- Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 2005;33:6445-58.
- [Google Scholar]
- Escherichia, Shigella, and Salmonella. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, eds. Manual of clinical microbiology (8th ed). Washington D.C: ASM Press; 2003. p. :654-71.
- [Google Scholar]
- A novel serovar of Shigella dysenteriae from patients with diarrhoea in Bangladesh. J Med Microbiol. 2007;56:654-8.
- [Google Scholar]
- Temporal shifts in the dominance of serotypes of Shigella dysenteriae from 1999 to 2002 in Dhaka, Bangladesh. J Clin Microbiol. 2003;41:5053-8.
- [Google Scholar]
- Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651-66.
- [Google Scholar]
- Mechanism of Shigella entry into epithelial cells. Curr Opin Microbiol. 1999;2:51-5.
- [Google Scholar]
- Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infect Immun. 2003;71:2775-86.
- [Google Scholar]
- Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of Shigellae strains isolated from Kolkata, India during 1995-2000. Epidemiol Infect. 2002;129:235-43.
- [Google Scholar]
- Highly plastic chromosomal organization in Salmonella typhi. Proc Natl Acad Sci USA. 1996;93:10303-8.
- [Google Scholar]
- Construction of physical map and mapping of chromosomal virulence genes of the biovar 3 Agrobacterium (Rhizobium vitis) strain K-Ag-1. Genes Genet Syst. 2006;81:373-80.
- [Google Scholar]
- Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987;169:283-90.
- [Google Scholar]
- The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379-85.
- [Google Scholar]
- Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981;6:112-8.
- [Google Scholar]
- Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protective mechanism for starvation stress in Escherichia coli. J Biol Chem. 1993;268:2307-11.
- [Google Scholar]
- Rearrangements in the genomes of Vibrio cholerae strains belonging to different serovars and biovars. Int J Syst Bacteriol. 1997;47:858-62.
- [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T, eds. Molecular cloning - a laboratory manual (2nd ed). CSH NJ: Cold Spring Harbor Laboratory Press; 1989.
- Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Struhl K, eds. Current protocols in molecular biology. New York, USA: John Wiley and Sons, Inc; 1994.
- Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insersions. Mol Microbiol. 1991;5:2171-80.
- [Google Scholar]
- Cholera toxin (CTX) genetic element in Vibrio cholerae O139. Microbiology. 1995;141:1977-83.
- [Google Scholar]
- Genesis of variants of Vibrio cholerae O1 biotype El Tor: role of CTXΦ array and its position in the genome. Microbiology. 2003;149:89-97.
- [Google Scholar]
- Molecular typing of Shigella strains using pulsed field gel electrophoresis and genome hybridization with insertion sequences. Res Microbiol. 1991;142:489-98.
- [Google Scholar]
- Evaluation of pulsed-field gel electrophoresis for typing of Shigella dysenteriae type 1. J Med Microbiol. 1999;48:781-4.
- [Google Scholar]
- The emerging strains of Shigella dysenteriae type 2 in Bangladesh are clonal. Epidemiol Infect. 2006;134:1249-56.
- [Google Scholar]
- Clonal lines of Salmonella enterica serotype enteridis documented by IS200-ribo-, pulsed-field gel electrophoresis and RFLP typing. J Med Microbiol. 1994;40:15-22.
- [Google Scholar]
- Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 2002;30:4432-41.
- [Google Scholar]
- A novel glucosyl transferase involved in O-antigen modification of Shigella flexneri serotype 1c. J Bacteriol. 2009;191:6612-7.
- [Google Scholar]






