Translate this page into:
Commercial serological tests for the diagnosis of active tuberculosis in India: Time for introspection
*Address for correspondence: sarman_singh@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
India accounts for one fifth of the global TB burden – a total of 9.2 million new cases and 1.7 million deaths every year12. There are two main components of effective TB control programme: the rapid and sensitive diagnosis of the disease and containment of its spread to the uninfected population. The sputum smear microscopy, still a backbone of TB diagnosis, is less sensitive and can miss half of the pulmonary TB cases. The conventional culture method which uses egg based medium (Lowenstein-Jensen, L-J) is time consuming and lacks desirable detection level. Liquid automated culture methods, such as Bactec-MGIT (BD, USA) and MB-Bact (Biomerieux, France) have highly improved detection rates, comparatively much faster (median detection time 10-24 days) but are costly requiring air handling and infection control measures1. Molecular methods such as polymerase chain reaction (PCR) and its modified versions have come as boon in the diagnosis of tuberculosis. Despite the limitation of detecting dead bacilli, PCR is rapid (report can be made available on the same day) and precise, depending on the gene sequences targeted and protocols used. These methods can be used on various clinical samples such as sputum, tissue biopsies, cerebrospinal and other body fluids, lymph nodes and other tissue aspirates, urine, faeces, etc1.
In resource limited settings like India, tuberculosis detection rates are suboptimal mainly because the diagnosis is usually made by less sensitive tools such as sputum microscopy and chest X-rays. The poor detection rates lead to mismanagement of infectious cases and possibility of drug resistance development. However, non-availability of affordable, rapid and precise diagnostic tools at peripheral level have led to mushrooming of commercial serological tests2–4. Extensive reviews and meta-analyses have concluded that the presently available antibody detection based serological tests are no good for the diagnosis of tuberculosis while helpful for other diseases. In view of this, the WHO has recently issued an advisory2. This editorial provides an academic overview of the issue.
Commercially available serological tests
A search showed more than 73 manufactures of TB serological test kits45. Most of these (60) are prepared in rapid test (lateral flow or flow through) format as compared to microwell ELISA (only 13). The Table shows widely publicized manufactures and trade names of these test kits.
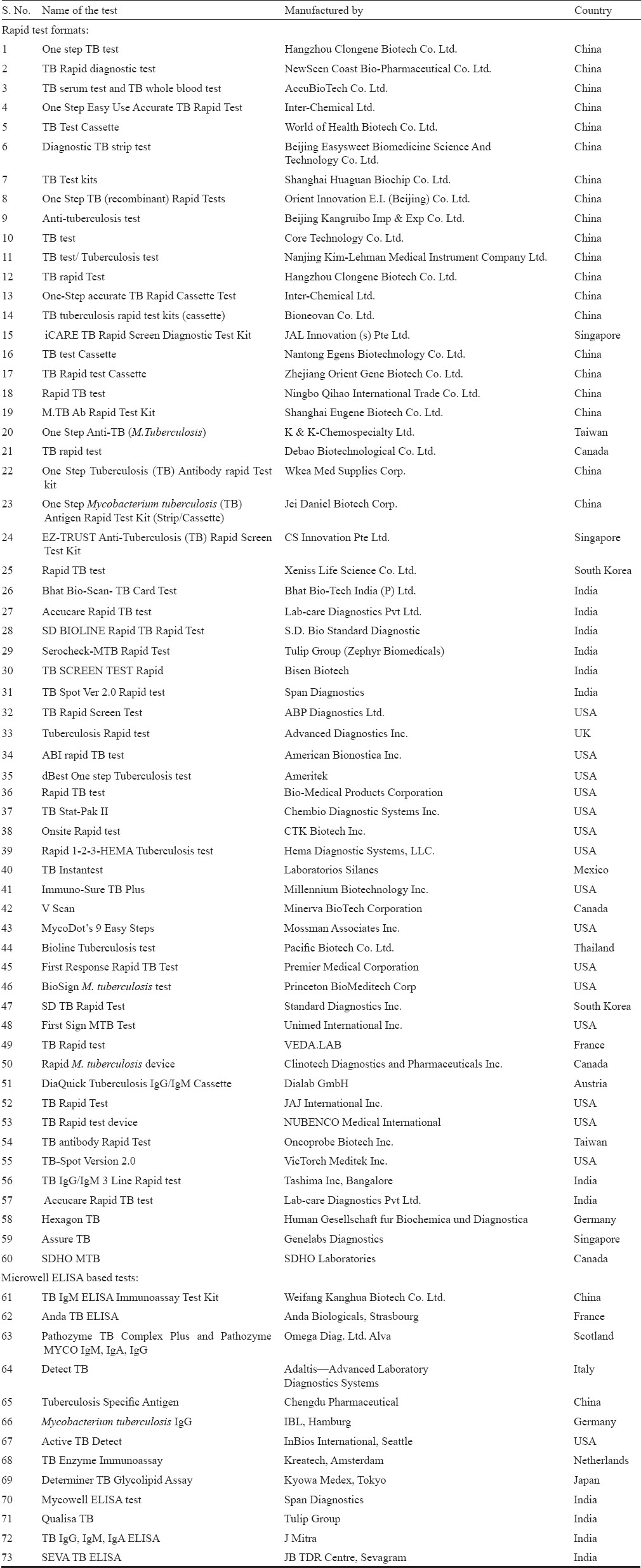
This non-exhaustive list clearly indicates that China has over taken all other countries for rapid test marketing. There are at least 24 TB rapid test kit manufactures from China alone. However, only two manufacturers market microwell ELISA. China is followed by USA, with 15 rapid test kits and one ELISA kit, probably understanding that rapid test market is more lucrative. India has eight rapid test manufacturers and four ELISA manufacturers (Table).
In 2008, the WHO started a kit evaluation programme for various infectious diseases including HIV, hepatitis and malaria and TB rapid test kits5. Only 19 firms responded to WHO evaluation programme and submitted their kits (Serial no. 31-49) and six firms refused to provide their kits for WHO evaluation (serial no. 50-55), presumably because these firms do not market directly. No microwell ELISA kit was evaluated by the WHO under this programme. However, recently several scientists have analysed both rapid and ELISA formats67. The results of these analyses are scary. Beside these listed commercial tests, there are a number of in-house assays claiming high sensitivity and specificity8. Most of these tests are developed in unaccredited basic biology laboratories with little cross-validation by third parties.
Sensitivity of commercially available serological tests
The claims of every manufacturer that their product is better are extremely tall and misguiding. For example, manufacture no. 8 [Orient Innovation E.I. (Beijing) Co., Ltd.] (Table) claims 99 per cent sensitivity and 99 per cent specificity; manufacture no. 28 (Standard Diagnostics) claims 98 per cent sensitivity and 99 per cent specificity; Tulip Group (manufacturer no. 29) goes further ahead in claiming 100 per cent sensitivity and 99 per cent specificity; manufacturer no. 30 claims 94 per cent sensitivity and 98 per cent specificity4. Of the 13 commercial kits based on ELISA, two are from China, one each from France, Scotland, Germany, Netherlands, Italy, USA and Japan while four are from India. All Indian manufacturers have claimed high accuracy. Tulip g0 roup for its Qualisa TB kit claims 100 per cent sensitivity and 100 per cent specificity; J Mitra claims only 80 per cent sensitivity and 97 per cent specificity. A new entrant in the Indian market claims 97 per cent sensitivity and 99 per cent specificity for its in-house SEVA-ELISA test8. Indeed all these claims are based on in-house or small studies with no proper validation.
Sensitivity or ability to diagnose true TB cases is very critical and any test which has lesser detection rate than sputum microscopy does not warrant serious attention. The WHO advisory2 is based on exhaustive literature search and evaluation of most commercially available kits by third party mandated by WHO. Pottumarthy et al3, evaluated seven commercially available serological tests, and found that the diagnostic sensitivities of these tests with patients with active tuberculosis ranged from as low as 16 per cent and maximum up to to 57 per cent. The Pathozyme Tuberculosis IgA EIA had the highest sensitivity (57%) and the immunochromatographic rapid tests had a sensitivity of 41 per cent. We also carried out an extensive study (442 microbiologically proven cases) and found that a commercially available ELISA kit [Pathozyme MYCO IgM, IgA, IgG (Omega Diag., Ltd, Scotland)] had dismally poor sensitivity. Among the culture proven pulmonary TB cases, the sensitivity of Pathozyme Myco IgM was only 50.23 per cent, IgA 26.36 per cent and IgG 24.5 per cent (Singh et al, unpublished data). Steingart et al6, while olymeriz the published data found that compared with ELISA which had pooled sensitivity of 60 per cent, rapid assays yielded lower pooled sensitivity (53%). The situation was worst in HIV-TB co-infected patients in whom the performance of one rapid test had poorer detection rate than smear microscopy. It could detect (sensitivity) only 68 per cent smear positive and culture proven cases and the overall sensitivity was only 16 per cent, that too in Africa6. The WHO expert group while deciding to issue a policy to ban all serological tests for TB diagnosis after olymeriz the data of 67 publications, observed that even for pulmonary tuberculosis, the sensitivity was highly variable ranging from as low as 0 to 100 per cent2. The 76 per cent pooled sensitivity of Anda-TB IgG, which is the most commonly evaluated test in smear-positive patients and 59 per cent in smear-negative patients. Based on another set of data analysis Dowdy et al7 estimated that even the best kit (Anda60) has poorer impact on TB diagnosis than the most cost-effective and rapid smear microscopy. They also concluded that due to poor sensitivity even in open pulmonary TB cases smear microscopy will be capable of averting more number of secondary cases (containing spread from infected patients to uninfected population) than serology. The sensitivity was no better for extra-pulmonary cases, an argument most often put forward in support of serology. Indeed the liquid culture and molecular techniques take priority for pulmonary as well as extra-pulmonary cases, both in terms of detection rate and cost-effectiveness.
Specificity of serological tests
Specificity of all diagnostic tests is very important, but it becomes critical in diseases that warrant treatment or with a social stigma. TB comes under both categories. Any test which can label an uninfected person as infected is most undesirable. Hence, WHO has justifiably taken specificity into consideration while banning all serological tests2. In our unpublished study we included 789 healthy family contacts of pulmonary TB patients to evaluate the usefulness of a commercially available ELISA kit [Pathozyme MYCO IgM, IgA, IgG (Omega Diag., Ltd, Scotland)]. As many as 28 per cent healthy contacts were found to be reactive to MYCO IgM, IgA, or IgG. We found a devastating cross-reactivity (up to 72%) in kala-azar patients (details not shown here). Dowdy et al7, estimated that due to high false positivity of commercially available serological tests, as many as 1,57,000 false (non-diseased) cases may be wrongly treated in India alone. This costs billions of rupees to Government of India and thousands of such wrongly treated cases may develop side effects of the anti-tuberculosis treatment. The WHO in its report also slams the serology based studies by mentioning that “a vast majority of studies were either sponsored by industry, involved commercial test manufacturers, or failed to provide information on industry sponsorship”. Therefore, the WHO made a policy statement that commercial serological tests provide inconsistent and imprecise findings resulting in highly variable values for sensitivity and specificity adversely impacting patient safety. Overall data quality was graded as very low and it is strongly recommended that these tests should not be used for the diagnosis of both pulmonary and extra-pulmonary TB.
The basic premise of serological tests was ease, rapidity and ever increasing demand in TB endemic countries. Hence, these tests have always been the first choice for small time laboratories wanting to mint the easy money from poor patients. Unfortunately, unethical medical practices provided major boost to these kits in recent years, without bothering much on quality of tests and implications of false-positive and false- negative results. Few credible academic institutions have promoted use of these tests. Our own data (unpublished) and WHO evaluation unambiguously show that TB serology results confuse more than providing any diagnostic clue. The I that serology is a cheaper has no basis, as serology profile (IgG, IgA, IgM) costs more than even liquid culture and PCR test, combined.
However, despite WHO guidelines endorsed by TB Division of Government of India banning these serological tests, not much is expected. It is mainly because, the Central TB Division has no control over the import or manufacturing of these kits in Indian market which are licensed for marketing by Drug Controller General of India. Hence, until the import and manufacturing of these kits is banned, these kits will continue to confuse the Indian markets and interested parties making huge profits. Nevertheless, it is hoped that none of the presently marketed serological tests will be prescribed or used in India and other TB endemic countries without proper re-validation on well characterized samples, despite tall claims by the companies. This editorial is not the obituary for serology. Immunological detection with appropriate sensitivity and specificity will remain an attractive research option for developing immunodiagnosis of tuberculosis. Certainly the antigen targets have to be properly chosen and, this has not been explored so far. Techniques for antigen detection will continue to have edge over antibody detection methods due to obvious reasons. Same yardsticks also will apply to techniques based on delayed type of hypersensitivity (tuberculin, quantiferon type of assays). Finally, these aspects need to be addressed for all the diseases alike.
References
- Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other Mycobacterial species directly from clinical specimens. J Appl Microbiol. 2009;107:425-35.
- [Google Scholar]
- World Health Organization. 2011. Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. Geneva: WHO; Available from: http://www.who.int/tb/features_archive/20july11_end_to_inaccurate_tb_blood_tests/en/index.html
- [Google Scholar]
- A comparison of seven tests for serological diagnosis of tuberculosis. J Clin Microbiol. 2000;38:2227-31.
- [Google Scholar]
- Rapid Diagnostic Devices Manufacturers. Available from: http://www.alibaba.com/products/tb_test.html?qrwKey=tb_test&oriKey=TB_serology_test&qrw=1
- [Google Scholar]
- WHO. Diagnostic Evaluation Series, 2: Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. 2008. Available from: http://apps.who.int/tdr/publications/tdr-researchpublications/diagnostics-evaluation-2/pdf/diagnosticevaluation-2.pdf
- [Google Scholar]
- Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: An updated systematic review and meta-analysis. PloS Med. 2011;8(8):e1001062.
- [Google Scholar]
- Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PloS Med. 2011;8(8):e1001074.
- [Google Scholar]
- Improved laboratory diagnosis of tuberculosis - The Indian experience. Tuberculosis. 2011;91:414-26.
- [Google Scholar]





