Translate this page into:
Clinicopathologic features of undifferentiated round cell sarcomas of bone & soft tissues: An attempt to unravel the BCOR-CCNB3- & CIC-DUX4-positive sarcomas
For correspondence: Dr Bharat Rekhi, Department of Surgical Pathology, R. No. AB-818, 8th Floor, Annex Building, Tata Memorial Hospital, Dr E.B. Road, Parel, Mumbai 400 012, Maharashtra, India e-mail: rekhi.bharat@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Certain genetically defined undifferentiated round cell sarcomas, namely BCOR-CCNB3 and CIC-DUX4 positive, have been described. Here we present detailed clinicopathologic features and molecular results in such cases.
Methods:
Fifty one cases of undifferentiated round cell sarcomas, including 32 cases, tested for BCOR-CCNB3 and CIC-DUX4 fusions, by reverse transcription polymerase chain reaction technique and 44 tumours, for CCNB3 immunostaining, were analyzed.
Results:
Twenty seven (52.9%) tumours occurred in males and 24 (47%) in females; in soft tissues (38; 74.5%), commonly, trunk and extremities and bones (13; 25.4%), frequently, femur and tibia. Five of 32 (15.6%) tested cases were positive for BCOR-CCNB3 fusion and seven (21.8%) for CIC-DUX4 fusions. Histopathologically, CIC-DUX4-positive sarcomas comprised nodular aggregates of round to polygonal cells, containing hyperchromatic nuclei, prominent nucleoli and moderate cytoplasm, with focal myxoid stroma and variable necrosis, in certain cases. BCOR-CCNB3-positive sarcomas mostly comprised diffusely arranged, round to oval to short spindly cells with angulated nuclei, vesicular chromatin, inconspicuous nucleoli and interspersed vessels. Immunohistochemically, tumour cells were positive for MIC2 in 24 of 49 (48.9%) and CCNB3 in 12 of 44 (27.2%) cases. Four of five BCOR-CCNB3-positive sarcomas showed CCNB3 immunostaining and 6 of 7 CIC-DUX4-positive sarcomas displayed WT1 immunostaining. Most patients (27/37) (72.9%) underwent surgical resection and chemotherapy. Median overall survival was 12 months, and disease-free survival was seven months.
Interpretation & conclusions:
Undifferentiated round cell sarcomas are rare; mostly occur in soft tissues of extremities, with CIC-DUX4 positive, as these are relatively more frequent than BCOR-CCNB3 positive sarcomas. CCNB3 and WT1 are useful immunostains for triaging such cases for BCOR-CCNB3 and CIC-DUX4 fusion testing, respectively. Overall, these are relatively aggressive tumours, especially CIC-DUX4-positive sarcomas.
Keywords
BCOR-CCNB3
CIC-DUX4
disease free survival
Ewing sarcoma
‘Ewing-like’ sarcoma
synovial sarcoma
undifferentiated round cell sarcoma
According to the World Health Organization (WHO) classification of tumours of soft tissue and bone, Ewing sarcoma family of tumours (ESFTs) are a group of small round cell sarcomas showing varying degree of neuroectodermal differentiation, detected by light microscopy, immunohistochemistry (IHC) and/or by electron microscopy1. Microscopically, these tumours are composed of uniform, small cells with round nuclei, scant cytoplasm and fine nuclear chromatin. By IHC, most of these tumours display diffuse cytoplasmic membranous immunostaining for CD99/MIC2 and intranuclear staining for Friend leukemia integration 1 (Fli1)12. Both these markers are sensitive, however, not diagnostically specific2.
Ewing sarcoma is characterized by recurrent translocations between EWSR1 (Ewing sarcoma RNA binding protein 1) and ETS (E26 transformation-specific) family of transcription factors. Most of the tumours harbour t(11; 22)-EWSR1-FLI1 or t(21; 22)-EWSR1-ERG transcripts123. Detection of these chimeric transcripts constitutes the diagnostic gold standard for this tumour. In the WHO classification, a new category has emerged, namely undifferentiated/unclassified round cell sarcomas, lacking any distinct line of differentiation, as well as genetic abnormalities, seen in Ewing sarcomas. Clinically, these tumours usually occur in younger patients, at various sites, more frequently in somatic soft tissues, mimic other round cell sarcomas and are characterized by a relatively more frequent rapid growth and a variable response to presently available conventional chemotherapy (CT) regimens1.
Gene expression profiling has unravelled specific transcripts within these undifferentiated, round cell sarcomas, namely CIC-DUX4, CIC-FOXO4, BCOR-CCNB3, BCOR-MAML3 and ZC3H7B-BCOR456789101112. Some studies have shown certain small round cell sarcomas, displaying EWSR1 rearrangement and fusion with non-ETS fusion genes, such as PATZ1, POU5F, NFATc2 or SP31314.
CIC (capicua homolog)-DUX4 (double homeobox 4) is a relatively newly identified gene fusion, resulting from either t(4; 19)(q35; q13)or t(10; 19)(q26; q13) translocations1011121516. Tumours, characterized by these fusions, mainly affect young adults in the third or fourth decades of life; frequently involve soft tissues and are clinically agressive111517.
Pierron et al7 identified recurrent gene fusions of BCOR (encoding Bcl-6 interacting corepressor) and CCNB3 (cyclin B3), leading to BCOR-CCNB3 fusions in certain unclassifiable small round cell sarcomas, occurring in adolescents and young adults89. These tumours are seen in young or adolescent males, involve long bones and have a relatively less aggressive clinical course791819. On the other hand, sarcomas characterized by CIC- rearrangements have been found to be associated with aggressive outcomes2021.
Currently, patients afflicted with undifferentiated round cell sarcomas are treated on the lines of ESFTs. Therefore, it is imperative to identify their underlying fusion, to differentiate these from other round cell sarcomas, treated differently89161718.
Subclassifying undifferentiated round cell sarcomas into these genetic subgroups can also help in appropriate risk stratification and further development of therapeutic approaches targeted towards the specific fusion transcripts and/or downstream signalling pathways, regulated by those fusion genes, thus affecting their overall survival (OS) rate9171819. The present study was conducted to study clinicopathologic features of undifferentiated round cell sarcomas of bone and soft tissue and to identify BCOR-CCNB3 and CIC-DUX4 fusion-positive sarcomas from within those cases.
Material & Methods
This retrospective study approved by the Institutional Ethics Committee was conducted in the department of Surgical Pathology and in the division of Molecular Pathology and Translational Medicine, Tata Memorial Hospital, Mumbai, India. The institutional electronic search engine was used to retrieve cases using key words ‘undifferentiated sarcoma’ and ‘undifferentiated round cell sarcoma’ for cases registered from January 2008 to April 2019 (11 yr and 3 months). Clinical details were obtained from Electronic Medical Records and case files from the medical records department. Cases included were of undifferentiated round cell sarcoma, round to spindle cell sarcomas, not otherwise specified, with no age, site or gender preference, as per WHO definition and morphologic features, including immunophenotyping1. Cases of well-defined round cell sarcoma, such as Ewing sarcoma, rhabdomyosarcoma (RMS) and poorly differentiated synovial sarcoma were excluded.
Assuming the incidence of undifferentiated ‘Ewing-like’ small round cell tumours from a study conducted by Machado et al10 as 2.5 per cent for BCOR-CCNB3 and three per cent for CIC-DUX4; of 200 cases, sample size of minimum 23 was calculated using two-sided 95 per cent confidence interval for one proportion, a precision of five per cent and a sample proportion of three per cent. Fifty nine cases diagnosed as undifferentiated round cell sarcoma were retrieved. Haematoxylin and Eosin (H and E) stained slides, along with corresponding immunostains in every case, were reviewed. Diagnosis of Ewing sarcoma was ruled out, based on immunohistochemical features and molecular results in 28 cases12.
After review, eight cases were excluded. Among these, four cases were positive for EWSR1 gene rearrangement; two cases were uninterpretable for EWSR1 rearrangement test (where Ewing sarcoma was highly suspected, on morphology with immunohistochemical results); a single case was diagnosed as Ewing sarcoma, based on immunohistochemical results and another case was finalized as a small cell osteosarcoma.
Fifty one cases included 22 patients triaged at our institution with 15 patients, who underwent only biopsy and seven patients, who underwent a biopsy, followed by post-CT tumour resections. Twenty eight cases constituted as referrals, submitted in the form of paraffin blocks of biopsy specimens (n=13) and excision specimens (n=15). The remaining single case was submitted in the form of an excision specimen.
Types of surgical resections were defined as follows: Resection (R)0 : wide resection, with both gross and microscopically clear margins; R1 : marginal resection, with grossly free and microscopically positive margin and R2 : intracapsular resection, with grossly and microscopically positive margins. Resection specimens wherein marginal status could not be identified were labelled as RX.
Various immunohistochemical antibody markers performed in the cases are enlisted in Table I. Apart from the available immunostained sections in each case, 44 (86.2%) cases were additionally subjected to immunohistochemical staining for CCNB3. Besides, 10 cases of Ewing sarcoma and nine of synovial sarcoma (specific translocation positive) were also tested for these three fusion transcripts and CCNB3 immunostaining, as controls.
| Antibody marker | Clone | Dilution (vol/vol) | Antigen retrieval | Manufacturer |
|---|---|---|---|---|
| MIC2 (CD99) | 12E7 | 1:100 | Tris-EDTA, Pascal | Dako, Produktionsvej, Glostrup, Denmark |
| Fli-1 | BV4 | 1:75 | Sodium citrate, Pascal | Cell Marque, Rocklin, CA, USA |
| WT1 antigen | 6FH2 | 1:100 | Sodium citrate, microwave | Dako |
| Calretinin | 5A5 | 1:50 | Sodium citrate, microwave | Leica, Newcastle, UK |
| S100P | Polyclonal | 1:1500 | Pepsin | Dako |
| AE1/AE3 (PanCK) | AE1/AE3 | 1:100 | Sodium citrate, microwave | Biocare, Concord, CA, USA |
| EMA | E29 | 1:200 | Pepsin | Dako |
| Desmin | Monoclonal, D33 | 1:200 | Heat. Pascal (Tris-EDTA) | Dako |
| MyoD1 | Monoclonal, 5.8A | 1:40 | Heat. Pascal (Tris-EDTA) | Dako |
| Myogenin | Monoclonal, L026 | 1:50 | Heat. Pascal (Sodium citrate) | Leica, Novacastra |
| INI1/SMARCB1 | Monoclonal, 3E10 | 1:600 | Tris-EDTA, Pascal | Acris, San Diego, CA, USA |
| CCNB3 | Polyclonal | 1:200 | Trisodium citrate, pressure cooker | Sigma, Gillingham, UK |
EMA, epithelial membrane antigen; EDTA, ethylenediaminetetraacetic acid; WT1, Wilm’s tumour-1; Fli1, friend leukaemia integration-1; S100P: S100 protein
Interpretation of CCNB3 immunostaining: Strong and diffuse nuclear positivity of CCNB3 was considered as positive immunostaining. Mere cytoplasmic staining or focal nuclear immunostaining was not considered as positive. Testicular tissue, with seminiferous tubules, including spermatogonia (in meiosis), showing nuclear positivity was included as a positive control. Besides, a case which was positive for BCOR-CCNB3 fusion with positive intranuclear CCNB3 immunostaining was included as a control822.
Paraffin blocks of all 51 cases were available for reverse transcription polymerase chain reaction (RT-PCR), for testing CIC-DUX4 and BCOR-CCNB3 gene fusions.
Molecular analysis
RNA extraction and cDNA synthesis: For RNA, macrodissected tissue sections (4 μ×10 μ) were collected in sterile 1.5 ml microfuge tubes. Deparaffinization and digestion of the tissue with proteinase K extraction was performed; RNA was extracted using Recover All Total nucleic acid extraction kit (Ambion, Thermo Fisher Scientific, USA) and quantified using Nanodrop (Thermo Fisher Scientific, USA). RNA (100 ng) was reverse transcribed using Revertaid H-minus first strand cDNA synthesis kit (Fermentas, Thermo Fisher Scientific, USA). No reverse transcriptase control (no RT) was included with each sample as a control for genomic DNA contamination. The integrity and the quality of the extracted RNA were assessed by performing PCR for β-actin (ACTNB) housekeeping gene 208 and 432 bp using cDNA as template.
PCR for CIC-DUX4 and BCOR-CCNB3: Qualitative gel-based PCR assay was performed, with primers for both the fusions, enlisted in Table II817. Briefly, reaction was performed in 20 μl total volume containing 2 μl of cDNA, 1.6 μl of 10 mM deoxynucleoside triphosphate (dNTP) and 0.4 μl of GXL-Taq polymerase (Takara Bio USA, Inc., USA), 4 μl of 5x PCR buffer, and 1 μl each of 10 pmol of each primer (Sigma Genosys, USA) was added. Each PCR run included appropriate controls and reagent control along with the samples. Samples were analyzed by running on 10 per cent polyacrylamide gel (PAGE) and interpreted using gel documentation system (Alpha Innotech, Cell Biosciences, USA).
| Gene | Sequence | Primer sequences817 | PCR product size (bp) |
|---|---|---|---|
| CIC (1) | Forward | 5’- CTCACCCAGCTCGGACTCT-3’ | 165 |
| DUX4 (1) | Reverse | 5’- CAGGGGAGTGCAGACCAG-3’ | |
| CIC (2) | Forward | 5’- GAGGACGTGCTTGGGGAGCTACAGT-3’ | 230 |
| DUX4 (2) | Reverse | 5’- CGCTGTGTGGAGTCTCTCACCCG-3’ | |
| BCOR | Forward | 5’ -GGCTCCACCCCAGTGATCT-3’ | 140 |
| CCNB3 | Reverse | 5’- GGGTGTTTTGGAGGTGGTGGAT-3’ |
PCR, polymerase chain reaction
Sanger sequencing: BCOR-CCNB3 and CIC-DUX4 fusion-positive cases (n=4) were sequenced, using capillary electrophoresis. Fusion products were purified and cycle sequenced using BigDye® Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher Scientific, USA). Products were purified using the Optima DTR™ system (Edge Biosystems, Gaithersburg, USA) and subjected to capillary electrophoresis on ABI 3500 Genetic Analyzer (Thermo Fisher Scientific, USA). The sequencing reads were uploaded in the BLAST search engine of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and showed 100 per cent alignment with BCOR-CCNB3 and CIC-DUX4 fusion transcripts from the NCBI database.
Statistical analysis: This was a descriptive study that included analysis of retrospectively diagnosed cases. Events were recorded in the form of recurrences, metastasis and death. Finally, clinical outcomes of patients were stated as alive with no evidence of disease (AWNED), alive-with-disease (AWD) and died-of-disease (DOD). SPSS software IBM SPSS statistics v25 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Chi-square or Fisher's exact test was used for categorical variables. Kaplan-Meier analysis was used to estimate survival, and the log-rank test was used to assess differences by groups. Disease-free survival (DFS) was defined from the date of diagnosis, till the date of first occurrence of relapse/recurrence. OS was defined from the date of diagnosis, till the last follow up. Patients not experiencing an event (recurrence and/or metastasis) were censored at the date of their last follow up. Cases with follow up duration of less than three months were excluded from the survival analysis. Various clinicopathologic parameters were associated with DFS and OS using Chi-square/Fisher's exact test and Kaplan-Meier analysis.
Results
Among 51 patients, there were 27 (53%) males (M) and 24 (47%) females (F) patients, with an overall M:F ratio of 1.1:1. Age of the patients ranged from one to 63 yr (average=23.9; median=20). Forty three per cent patients (22/51) were below 18 yr of age and 56.8 per cent (29/51) were more than 18 yr of age. The tumour locations included various soft tissue sites in 38 (74.5%) cases and bones in 13 (25%) cases. Among soft tissue sites, eight (21%) tumours were located in the trunk, including chest wall and back; nine (23.6%) were located in the upper extremities; 10 (26.3%) in the lower extremities; four in the head-neck region; six in the abdominopelvic region and remaining one tumour was located in the retroperitoneum. In the upper extremity, arm (n=6) was the most common site, followed by axilla (n=1). In the lower extremity, thigh was involved in four cases and leg in five cases. Of the 13 tumours occurring within the bones, eight (61.5%) were located in the lower extremity, with femur (n=4) being the most common site, followed by tibia (n=3) and foot (n=1). In three cases, tumours occurred in the upper extremity, including humerus (n=1), clavicle (n=1) and phalynx (n=1). The remaining one case each occurred in the costovertebral junction and ilium, respectively.
Clinical details regarding tumour (T) size were known in 33 of 51 (64.7%) cases. T-size varied from 2.6 to 20 cm (average=8.6; median=8.6 cm). T-size more than five cm in 26 of 33 (78.7%) cases and less than 5 cm was seen in 7 of 33 (21.1%) cases.
RT-PCR for BCOR-CCNB3 and CIC-DUX4 (1 and 2) fusion transcripts was performed in all 51 cases. Of these, 11 cases had limited tissue, leading to a low concentration of extracted RNA, therefore, could not yield an amplifiable cDNA. Overall, cDNA synthesis was performed in the remaining 40 cases, after which 34 cases showed a band for β-actin (ACTNB) housekeeping gene.
Finally, 32 cases were interpretable. Five of 32 (15.6%) cases were positive for BCOR-CCNB3 fusion. Seven (21.8%) cases displayed CIC-DUX4 (1) (n=3)and CIC-DUX4 (2) (n=4) fusions. Three cases positive for BCOR-CCNB3 fusion and a single case positive for the CIC-DUX4 fusion on further testing for sequencing displayed positive results. None of the seven cases of Ewing sarcoma and eight cases of synovial sarcoma displayed BCOR-CCNB3 or CIC-DUX4 (1 and 2) fusions. Forty two of 51 cases (47 different molecular and/ or molecular cytogenetic tests), tested for Ewing sarcoma (n=40), desmoplastic small round cell tumour (DSRCT) (n=2) and synovial sarcoma (n=3) were negative for the specific genetic results, namely EWSR1, EWS-FLI, EWS-ERG, EWS-WT1 and SS18, respectively.
Among the five cases harbouring BCOR-CCNB3 fusions, there were four males and a single female patient (M:F=4:1), with age ranging from 6 to 29 yr (average=26; median=25 yr). Site-wise, a single case, each, was identified in the vertebra, tibia, leg, back and arm, respectively. There were two cases involving bone and three involving soft tissues. Among the seven cases harbouring CIC-DUX4 fusions, there were five female and two male patients, with age ranging from 8 to 53 yr (average=31.5, median=25 yr). Site-wise, all seven cases occurred in the soft tissues, mostly lower extremities (4), including thigh (2) and leg (2), followed by a single case, each, occurring in the hand, pelvic soft tissues and gluteal region, respectively.
Histopathologic findings: Gross findings were available in the form of resected specimens in 13 of 22 (59%) cases, including five cases of ‘chemo naïve’ excised specimens (referral material) and eight cases of post-CT-treated resection specimens. Gross examination of five ‘chemo naïve’ specimens revealed well-circumscribed, nodular, homogenous and grey-white cut surfaces, while five post-CT-treated specimens revealed ill-defined variegated appearance, as a result of haemorrhage and necrosis. In the remaining three cases, there was no residual tumour.
Microscopically, the tumour cells were arranged in diffuse/sheet-like pattern in 29 of 51 (56.8%) cases, multinodular pattern with fibrous septae in 17 (33.3%) cases and a mixed pattern comprising nodular, pseudoglandular, cords, trabeculae and perivascular arrangement of cells in six (11.7%) cases. Variable amount of myxoid stroma was seen in 22 (43.1%) tumours. Variable necrosis, including focal, duct-like and geographic patterns, was seen in 24 (47.05%) tumours. Prominent single cell apoptosis was seen in 12 (23.5%) tumours. Large areas of haemorrhage were seen in three tumours.
On microscopic examination of post-CT specimens of eight cases, five showed no residual viable tumour; a single case showed 25 per cent residual viable tumour and the remaining two cases showed 42 and 91 per cent residual tumour, respectively (poor chemo response).
By IHC, tumour cells showed positive staining (incomplete to complete membranous) pattern for CD99/MIC2 in 24 of 49 (48.9%) tumours; ‘dot-like’ immunoreactivity in 16 (32.6%) tumours and negativity in nine tumours. In addition, tumour cells showed positivity for Fli1 immunostaining in 25 of 32 (78.12%) tumours; Wilm's tumour protein 1 (WT1) (paranuclear to intranuclear) in 17 of 24 (70.8%) tumours; calretinin (focal) in 7 of 16 (43.7%) tumours; desmin (focal) in 1 of 33 tumours, including MyoD1 and myogenin negativity; S100 protein in 6 of 25 (24%) tumours, pan cytokeratin (AE1/AE3) (focal) in 12 of 39 (30.7%), epithelial membrane antigen (EMA) 1 of 19 (5.2%) tumours and synaptophysin in none of 21 tumours.
In 19 of 22 (86.3%) cases, tumour cells displayed retained expression of INI1/SMARCB1 immunostaining, while in three cases, tumour cells showed complete loss and in a single case, tumour cells showed weak to absent expression (reduced).
CCNB3 immunostaining was tested in 44 cases and 19 controls [10 Ewing sarcomas and 9 synovial sarcomas (translocation confirmed)]. In 12 of 44 (27.2%) test cases, tumour cells showed diffuse CCNB3 immunostaining and in 2 of 13 control cases, tumour cells showed focal immunostaining, including a single Ewing sarcoma and synovial sarcoma.
BCOR-CCNB3 fusion-positive sarcomas: On gross examination of a single, completely resected specimen of tibia, a grey-white, fleshy tumour (T size=7 cm) was seen involving the meta-diaphysis, extending into the soft tissues. Another post-CT-treated case did not reveal any residual tumour.
Microscopically, in all cases, tumour cells were arranged in a diffuse pattern with interspersed thin-walled blood vessels. Three cases showed myxoid stroma. The cytomorphology varied from round to oval, to spindle shaped and angulate nuclei with inconspicuous nucleoli and vesicular chromatin in most cases. A single case (case 34) revealed cells with hyperchromatic nuclei and prominent nucleoli.
Immunohistochemically, tumour cells showed weak cytoplasmic membranous to ‘dot-like'-positive expression for CD99/MIC2 diffuse immunostaining for CCNB3 in four of five cases (80%) and focal intranuclear staining for WT1 (1/1).
Diffuse immunostaining for CCNB3 was also observed in another four cases, negative for BCOR-CCNB3 fusions, a single case of CIC-DUX4-positive sarcoma and three cases, wherein gene fusions could not be tested. Sensitivity and specificity of CCNB3 immunostaining for BCOR-CCNB3 fusion-positive sarcomas was 80 and 84.6 per cent, respectively.
CIC-DUX4 fusion-positive sarcomas
Microscopically, these tumours were characterized by a predominant nodular, followed by diffuse and nesting growth pattern; round to polygonal cells, containing moderate amount of cytoplasm, focal ‘rhabdoid-like’ appearance (two cases), hyperchromatic nuclei, prominent nucleoli and focal necrosis (2 cases). Three cases displayed focal myxoid stroma. Immunohistochemically, 3 of 7 tumours displayed focal ‘dot-like’ immunostaining for AE1/AE3; 6 of 7 tumours displayed WT1 immunostaining (mostly multifocal) and 1 of 4 tumours displayed focal calretinin immunostaining (Figs 1-6).
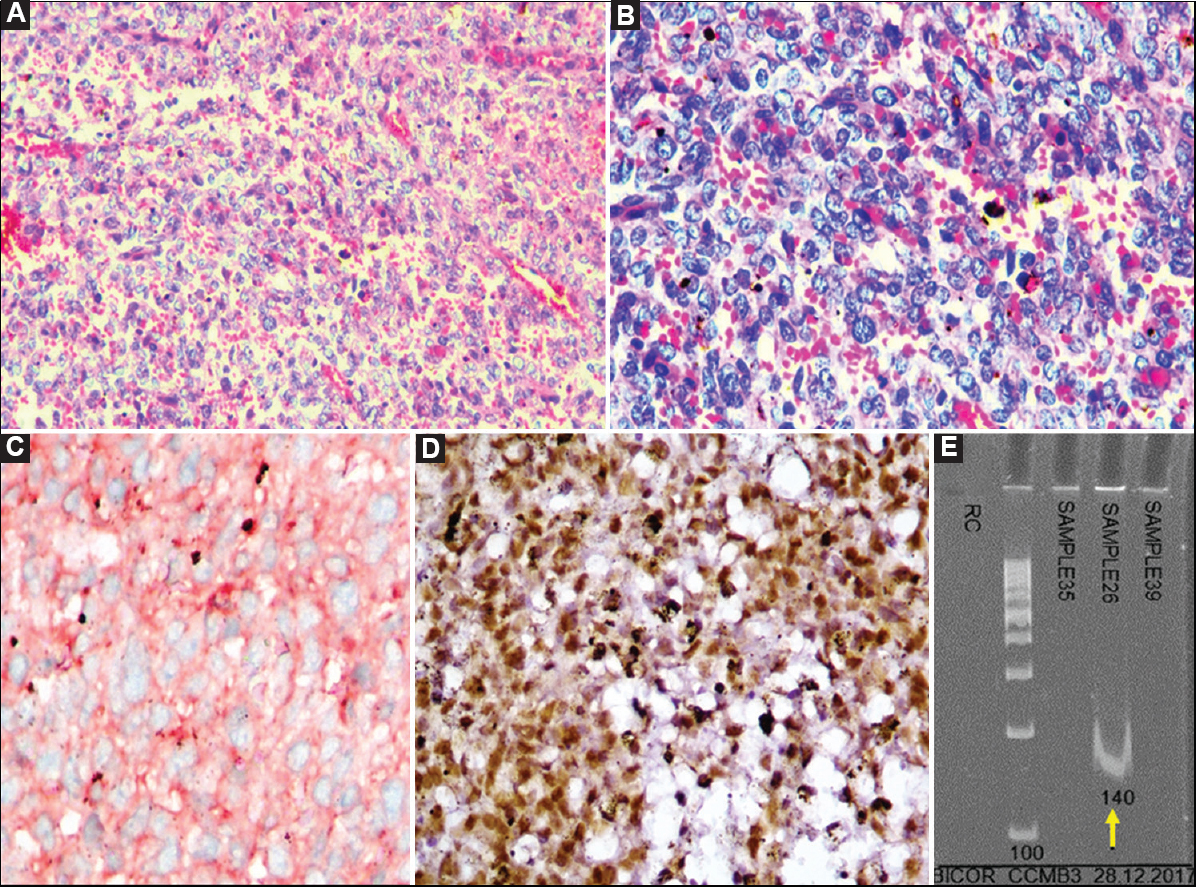
- Case 20: Undifferentiated sarcoma showing positive BCOR-CCNB3 fusion. (A) Malignant tumour composed of round to spindly cells in a richly vascularized stroma (H and E, ×200), (B) Higher magnification showing round to oval cells with vesicular to hyperchromatic nuclei, indistinct nucleoli and scant cytoplasm in a vascularized stroma (H and E, ×400), (C) Tumour cells showing diffuse cytoplasmic to ‘dot-like', focally membranous staining for MIC2/CD99 (DAB, ×400), (D) CCNB3 positivity (DAB, ×400), and (E) Polymerase chain reaction result showing positive band for BCOR-CCNB3 fusion transcript (140 bp) (arrow).
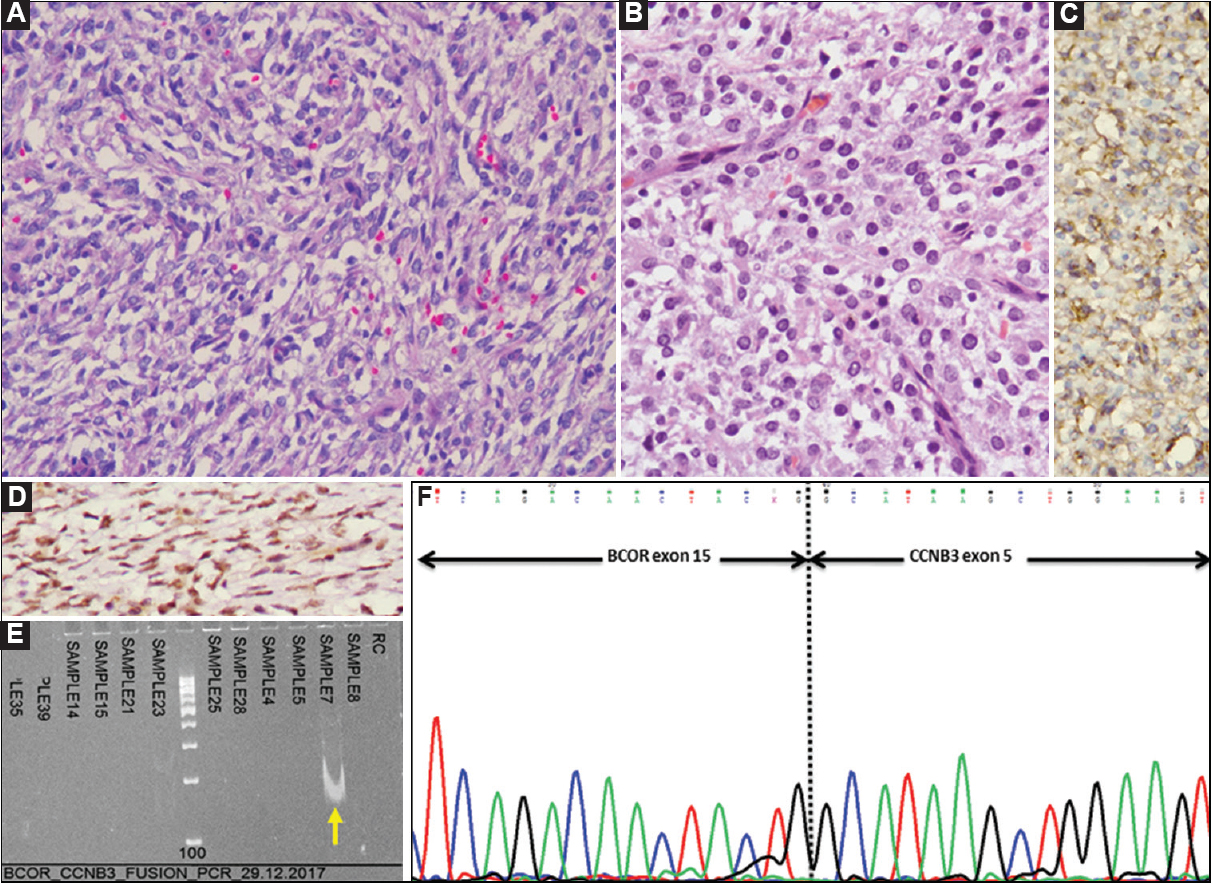
- Case 24: BCOR-CCNB3-positive undifferentiated round cell sarcoma. (A) Microscopically, a tumour comprising round to spindle shaped cells, including a few angulate forms in a vascularized stroma (H and E, ×200), (B) Tumour cells with round nuclei and scant to moderate, eosinophilic cytoplasm (H and E, ×400), (C) By immunohistochemistry (IHC), tumour cells showing cytoplasmic and ‘dot-like’ MIC2/CD99 positivity (DAB, ×400). (D) CCNB3 positivity (DAB, ×400), (E) Polymerase chain reaction result showing positive band for BCOR-CCNB3 fusion transcript (140 bp) (arrow), and (F) Sanger sequencing result showing BCOR-CCNB3 fusion.
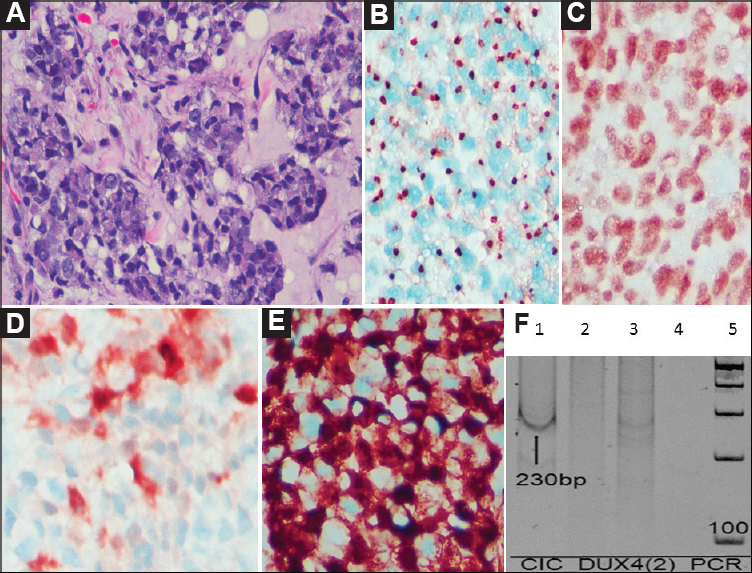
- Case 37: Undifferentiated round cell sarcoma, (A) Round to polygonal cells arranged in a nesting pattern (H and E, ×400), (B) By IHC, tumour cells display ‘dot-like’ positivity for MIC2/CD99 (DAB, ×400), (C) INI1/SMARCB1 is diffusely retained in the tumour cells (DAB, ×400), (D) Focal distinct patchy positivity for calretinin (DAB, ×400), (E) Strong intranuclear and paranuclear positivity for WT1 (DAB ×400), and (F) Polymerase chain reaction result showing positive band for CIC-DUX4 fusion transcript (230 bp) (arrow).
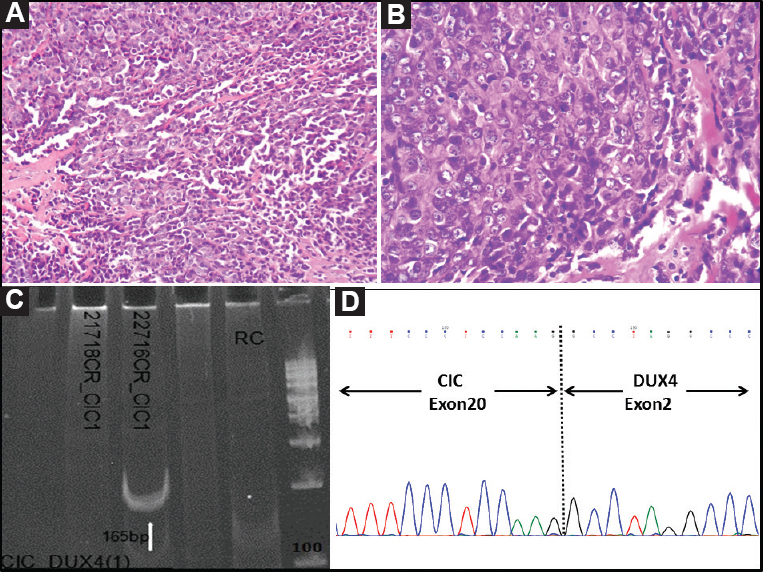
- Case 44: Undifferentiated round cell sarcoma. (A) Nodular tumour composed of round to polygonal cells arranged in a diffuse pattern (H and E, ×200), (B) Higher magnification showing presence of polygonal cells with scant to moderate cytoplasm and discernable nucleoli (H and E, ×400), (C) Polymerase chain reaction result showing positive band for CIC-DUX4 type I fusion transcript (165 bp) (arrow), and (D) Sanger sequencing result showing CIC-DUX4 fusion, on electropherogram.
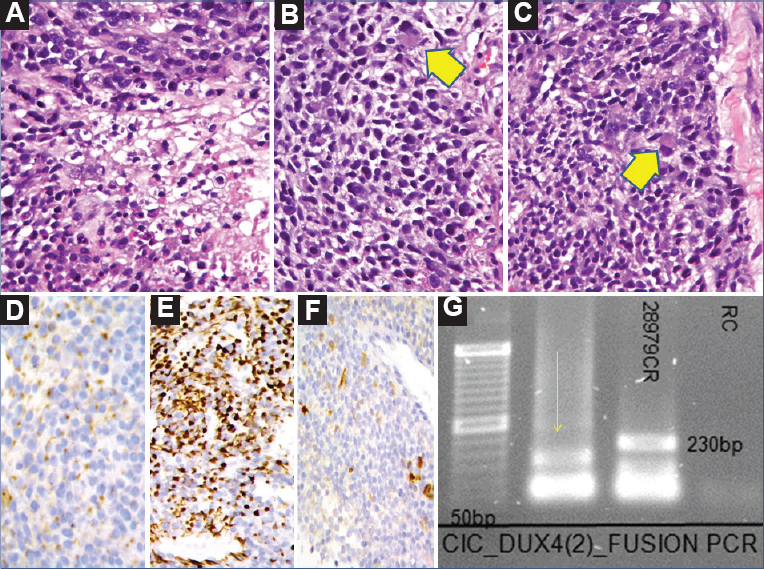
- Case 45: Undifferentiated round cell sarcoma. (A) Malignant tumour comprising round to polygonal cells with central necrosis (H and E, ×200), (B) Higher magnification showing predominant round to oval cells with interspersed large cell containing abundant eosinophilic cytoplasm, reminiscent of ‘rhabdoid-like’ cell (arrow head) (H and E, ×400), (C) Epithelioid to ‘rhabdoid-like’ cells (H and E, ×400), (arrow head), (D) ‘Dot-like’ positivity for AE1/AE3 (AB, ×400), (E) WT1 positivity (DAB, ×400), (F) Focal calretinin positivity (DAB, ×400), and (G) Polymerase chain reaction result showing positive band for CIC-DUX4 type II fusion transcript (165 bp) (arrow).
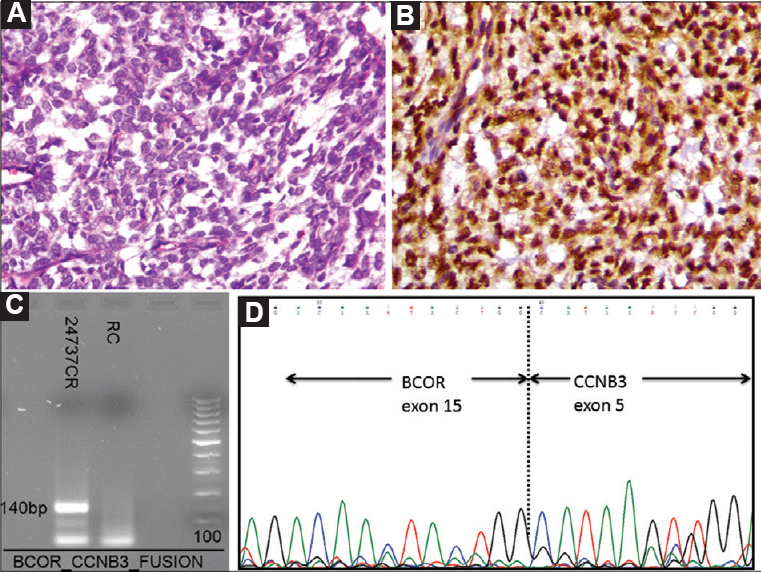
- Case 46: (A) Sarcoma comprising round to oval and spindly cells arranged in a diffuse manner with intervening thin-walled blood vessels (H and E, ×200) (B). Intranuclear and paranuclear positivity for CCNB3 (DAB, ×400). (C) Polymerase chain reaction result showing positive band for BCOR-CCNB3 fusion transcript (140 bp), and (D) Sanger sequencing result showing BCOR-CCNB3 fusion, on electropherogram.
Treatment details were available in 37 (72.5%) cases. Most patients (21/37) (56.7%) were treated with surgical resection and CT, including 16 in pre-operative/neoadjuvant CT (NACT) and five in post-operative/adjuvant settings. Seven patients received adjuvant RT. Two patients were offered NACT and radiotherapy (RT), as a result of inoperable sites. Twenty five patients were treated by Ewing family tumours (EFT)-2001 protocol CT regimen23 and two were treated with CT regimen of conventional high-grade osteosarcoma24. EFT 2001 included treatment in the form of four cycles, over eight weeks, starting with vincristine (V), ifosfamide (I) and etoposide (E), as first cycle, followed by vincristine after one week; vincristine on second week; VIE on third week (second cycle); vincristine on fourth week; vincristine on fifth week; vincristine, doxorubicin (A) and cyclophosphamide (C) on sixth week (third cycle); vincristine on seventh week and VAC on eighth week (fourth cycle), followed by vincristine. Subsequently, local therapy including RT (continue CT)/surgery and RT was offered. The CT regimen for high-grade osteosarcoma included cisplatin and adriamycin for the first two weeks, preceding surgery (neoadjuvant setting), followed by two cycles of adriamycin and ifosfamide, also in neoadjuvant setting. This was followed by surgical resection and subsequently cisplatin and ifosfamide in the fifth, sixth, seventh and eighth week, in the form of adjuvant settings.
Three patients were offered only surgical resection and two, induced on definitive CT, including two patients (cases 50 and 51; one patient on ifosfamide and adriamycin, in view of her age and another on EFT 2001). Two patients with multiple pulmonary metastasis at the time of initial presentation received best supportive, in the form of metronomic CT and palliative CT, respectively.
Of the 31 patients who underwent surgery, 11 patients (35.4%) underwent R0 resection. In the remaining 20 patients (64.5%), type of tumour resection was not known. In four patients, no treatment could be initiated as unfortunately, they died within 1-3 months of the diagnosis.
Follow up and outcomes: Follow up was available in 39 of 51 (76.4%) cases (1-45 months. median=7, average=12.9 months). Six of 39 (11.4%) patients developed recurrences and 12 (30.7%) patients developed metastases (including cases with multiple metastases), most commonly in lungs (9), lymph node (2), bone marrow (3), brain (3), bones (2) and abdomen (1).
Of the 39 patients, 14 (35.8%) were AWD over 4-36 months; 10 (25.6%) were AWNED over 5-45 months. Fourteen patients DOD were over 1-21 months. A single patient died of septic shock and acute renal failure. Seven of 15 deceased patients harboured metastatic lesions and one had tumour recurrence.
Among three cases of BCOR-CCNB3-positive sarcomas, where follow up details were available, a single patient each was AWNED (6 months); AWD (4 months) and DOD (8 months). Among five cases of CIC-DUX4-positive sarcomas, where follow up details were available, two patients were AWD (6 and 9 months), two DOD (1 and 8 months) and a single patient was AWNED (5 months) (Table III).
| Age yr/sex | Site | Bone/soft tissues (ST) | Immunohistochemical results | Molecular tests | Treatment | Clinical outcome |
|---|---|---|---|---|---|---|
| 26/male | Neck mass | ST | AE1/AE3-N, MIC2-weak P, WT1-focal P, Calretinin-P, INI1-lost, S100-focal P, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
Sx+AdjCT | AWD (5 months) |
| 61/male | Back | ST | MIC2-NP, WT1-P, S100P-weak P, INI1-R, Desmin-NP |
EWSR1-N, HK-NW | Sx+AdjCT+RT (palliative) | Metastasis (lung, abdomen) DOD (16 months) |
| 1/male | Chest wall | ST | MIC2-P, Fli1-P, CCNB3-focal P, Desmin-N |
EWSR1-N, BCOR- CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | AWNED (16 months) |
| 41/male | Leg | ST | MIC2-‘Dot-like’ P, WT1- P, Calretinin-N, AE1/AE3-focal P, S100P-N, Desmin-N, CCNB3-P |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | Metastasis (lung, brain) AWD (12 months) |
| 27/female | Thigh | ST | MIC2-‘Dot-like’ P, WT1- P, Calretinin-N, INI1-R, S100P-N, AE1/AE3-N, Desmin-N, CCNB3-P |
BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NA | LTFU |
| 4/female | Retroperitoneum | ST | MIC2-‘Dot-like’ P, Fli1-P, WT1-P, EMA-focal P, INI1-R, AE1/AE3-N, Desmin-N, CCNB3-N |
EWSR1-N, BCOR-N, CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NA | LTFU |
| 48/female | Arm | ST | MIC2-N, Fli1-N, AE1/AE3-N, S100P-N, Desmin-N, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
Sx+AdjCT (ifosfamide+ adriamycin)+RT | AWNED (12 months) |
| 37/female | Arm | ST | MIC2 Membrane-P ‘Dot-like’ P, Fli1-P, WT1-P, Calretinin-P, S100P-N, AE1/AE3-focal P, INI1-R, Desmin-N, CCNB3-P |
EWSR1-N, HK-NW |
NACT+Sx | Metastasis (lung, LN) AWD (4 months) |
| 11/female | Pelvis | ST | MIC2-Membrane-P ‘Dot-like’- P, Desmin-N, CCNB3-N |
HK-NW | Sx+AdjCT | Recurrence DOD (6 months) |
| 22/male | Chest wall | ST | MIC2-weak P, Fli1-P, Calretinin-N, AE1/ AE3-N, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | DOD (13 months) |
| 12/male | Axilla | ST | MIC2 ‘Dot-like’- P; AE1/AE3-N, S100P-focal P, INI1-R, Desmin-N CCNB3-N |
HK-NW | NACT+Sx | Metastasis (lung, brain) DOD (19 months) |
| 21/male | Back | ST | MIC2 ‘Dot-like’, Fli1-P, WT1-P, Calretinin-focal P, S100P-N, AE1/AE3-N, INI1-R, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx+RT | AWD (1 month) |
| 8/male | Arm | ST | MIC2-focal P, AE1/AE3-focal P, EMA-N, S100P-N, INI1-R, Desmin-N |
HK-NW | NACT+Sx | Metastasis (lung, brain) DOD (21 months) |
| 16/female | Pelvis | ST | MIC2-P, S100P-N, AE1/AE3- N, Desmin-N, CCNB3-N |
EWS-FLI1-N, EWS-WT1-N, BCOR-CCNB3-U, CIC-DUX4 (1)-UI, CIC-DUX4 (2)-UI |
Sx+AdjCT | DOD (7 months) |
| 45/female | Foot | Bone | MIC2 ‘Dot-like’- P, Fli1-focal weak P, INI1-R, S100P-N, AE1/AE3-focal P, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
Sx | AWNED (27 months) |
| 43/male | Chest wall | ST | MIC2-weak P, Fli1-weak P, AE1/AE3-N, S100P-N, Desmin-N, CCNB3-focalP |
HK-NW | Palliative CT | Metastasis (skeletal, LN) DOD (2 months) |
| 15/male | Femur | Bone | MIC2-‘Dot-like’ P, EMA-N, S100P-N, Desmin-N, CCNB3-P |
EWS-FLI1-N, EWS-WT1-N HK-NW |
NACT (OGS)+Sx | Metastasis (lung) DOD (9 months) |
| 6/female | Tibia | Bone | MIC2‘Dot-like’- P, S100P-N, AE1/AE3-N, INI1-Reduced, Desmin-N, CCNB3-focal P |
EWS-FLI1-N, EWSR1-N, HK-NW |
Sx | DOD (17 months) |
| 4/female | Temporal region | ST | MIC2-Membrane-P ’Dot-like’ P, AE1/AE3-focal P, INI1-lost, Desmin-N, CCNB3-N |
HK-NW | Sx+AdjCT+RT | AWNED (45 months) |
| 19/male | Back | ST | MIC2-weak P, Fli1-weak P, EMA-N, WT1-N, INI1-R, Desmin-NP, CCNB3-P |
EWSR1-N, BCOR-CCNB3-P, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
LTFU | LTFU |
| 62/male | Tibia | Bone | MIC2-N, Fli1-N, AE1/AE3-N, Desmin-N, CCNB3-N |
HK-NW | LTFU | LTFU |
| 40/female | Abdomino-pelvic region | ST | MIC2-P, Fli1-N, Desmin-N, CCNB3-N |
HK-NW | NACT+Sx | Metastasis (lung, skeletal). AWD (32 months) |
| 14/male | Clavicle | Bone | MIC2- focal P, Fli1-N, AE1/AE3-N, INI1-R, Desmin-N, CCNB3-N |
HK-NW | No Tx | Metastasis (bone marrow) DOD (3 months) |
| 6/male | Tibia | Bone | MIC2-Membrane P ‘Dot-like’- P, Fli1-P, S100P-N, AE1/AE3-N, WT1-N, Calretinin-N, INI1-R, Desmin-NP, CCNB3-P |
EWSR1-N, BCOR-CCNB3-P, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | AWD (4 months) |
| 5/female | Pelvic mass | ST | MIC2-N, Fli1-N, AE1/AE3-N, Desmin-N CCNB3-N |
HK-NW | NA | Metastasis (bone marrow) AWD (3 months) |
| 16/male | Femur | Bone | MIC2‘Dot-like’P, Fli1-weak P, AE1/AE3-N, Desmin-NP CCNB3-P |
EWS-FLI1-N, EWS-ERG-N, EWSR1-N, HK-NW |
NACT+Sx+RT | AWNED (41 months) |
| 63/male | Chest wall | ST | MIC2‘Dot-like’- P, Fli1-N, WT1-N, S100P-P, AE1/AE3-focal P, EMA-N, INI1-R, Desmin-focal P, Myogenin-N, MyoD1-N, CCNB3-focalP |
EWSR1-N, HK-NW |
Meteronomic CT | Metastasis (lung) AWD (36 months) |
| 25/female | Arm | ST | MIC2 ‘Dot-like’- P, S100-N, Desmin-N, CCNB3-focal P |
EWSR1-N, BCOR-CCNB3-P, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NA | LTFU |
| 23/female | Phalynx | Bone | MIC2 ‘Dot-like’- P, Fli1-P, Desmin-N, CCNB3-UI |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx+RT | AWNED (31 months) |
| 14/male | Femur | Bone | MIC2-N, Desmin-NP, CCNB3-UI |
EWSR1-N, HK-NW | NA | LTFU |
| 8/female | Back | ST | MIC2-P, Fli1-weak P, INI1-R, AE1/AE3-N, Desmin-N, CCNB3-P |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
No Tx | DOD (3 months) |
| 14/male | Humerus | Bone | MIC2 ‘Dot-like’- P, WT1-N, S100P-N, AE1/AE3-N, Desmin-NP |
HK-NW | NACT+Sx | AWD (6 months) |
| 57/male | Arm | ST | MIC2-weak P, Fli1-weak P, WT1-P, S100P-weak P, AE1/AE3-N, Desmin-N CCNB3-focal P |
EWSR1-N, BCOR-CCNB3-UI, CIC-DUX4 (1)-UI, CIC-DUX4 (2)-UI |
NACT+Sx | DDOC (7 months) |
| 25/male | Costovertebral junction | Bone | MIC2-focal P, S100P-N, AE1/AE3-focal P, Desmin-N, CCNB3-P |
EWSR1-N BCOR-CCNB3-P, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | DOD (8 months) |
| 20/female | Thigh | ST | MIC2-Membrane P ‘Dot-like’- P, Fli1-P, WT1-P, INI1-R, AE1/AE3-N, Desmin-N, CCNB3-focal P |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-P, CIC-DUX4 (2)-N |
No Tx | Metastasis (lung, bone marrow) DOD (1 month) |
| 46/female | Arm | ST | MIC2-focal P, Fli1-P, Calretinin-N, S100P-N, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
Sx+AdjCT | AWD (7 months) |
| 8/female | Thigh | ST | MIC2 ‘Dot-Like’- P, WT1-P Calretinin-P, S100P-N, AE1/AE3-N, INI1-R, Desmin-N, CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-P |
No Tx | DOD (3 months) |
| 53/female | Leg | ST | MIC2-P, Fli1-P, WT1-P, S100P-N, AE1/AE3-focal P, Desmin-NP, CCNB3-focal P |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-P, CIC-DUX4 (2)-N |
Sx | AWNED (5 months) |
| 21/male | Leg | ST | MIC2-NP, Fli1-P, WT1-N, Calretinin-N, S100P-focal P, AE1/AE3-N, Desmin-NP, CCNB3-P |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT+Sx | AWD (4 months) |
| 12/male | Femur | Bone | MIC2 ‘Dot-like’- P, Fli1-N, WT1-focal P, AE1/AE3-N, Desmin-N CCNB3-N |
EWSR1-N, BCOR-CCNB3-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N |
NACT (OGS)+Sx | AWNED (5 months) |
| 3/female | Neck mass | ST | MIC2-focal P, Fli1-P, WT1-P, Calretinin-focal P, S100P-focal P, AE1/AE3-focal P, Desmin-N |
EWSR1-N, HK-NW | NA | LTFU |
| 5/female | Abdominal wall | ST | MIC2-N, INI1-R, AE1/AE3-N, Desmin-NP, CCNB3-N |
EWSR1-N, BCOR-CCNB3-UI, CIC-DUX4 (1)-UI, CIC-DUX4 (2)-UI |
NACT+Sx | Recurrence AWNED (17 months) |
| 20/female | Thigh | ST | MIC2-P, Fli1-P, Calretinin-P, WT1-N, AE1/AE3-N. INI1-R, Desmin-NP, CCNB3-N |
EWSR1-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N BCOR-CCNB3-N |
NA | LTFU |
| 25/female | Hand (palm) | ST | MIC2-focal P, Fli1-P, AE1/AE3-N, EMA-N, GFAP-N, CD34-N, CD31-N, S100P-N, Calretinin-N, WT1-N, Synaptophysin-N, Desmin-NP, CCNB3-N |
EWSR1-N, CIC-DUX4 (1)-P, CIC-DUX4 (2)-N BCOR-CCNB3-N |
Sx+AdjCT | Recurrence (7 months) AWD (9 months) |
| 20/male | Leg | ST | MIC2 ‘Dot-like’- P, AE1/AE3 ‘Dot-like’- P, Fli1-P WT1-P, S100P-N, CD34-N PAX8-N, INI 1-R, Desmin-N |
EWSR1-N, SS18-N CIC-DUX4 (1)-N, CIC-DUX4 (2)-P BCOR-CCNB3-N |
NACT+RT | AWD (6 months) |
| 29/male | Leg | ST | MIC2-N, Fli1-Focal, weak P, EMA-N, CD34-N, BCL2-N, S100P-N, INI1-R, Desmin-N, CCNB3-P |
EWSR1-N, SS18-N CIC-DUX4 (1)-N, CIC-DUX4 (2)-N BCOR-CCNB3-P |
NACT+Sx+RT | AWNED (6 months) |
| 23/male | Hand | ST | MIC2 Membrane-P ‘Dot-like’- P, Fli1-P, WT1-P, Calretinin-focal P INI1-R, AE1/AE3-N, Desmin-N, CCNB3-P |
EWSR1-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N BCOR-CCNB3-N |
NACT+Sx | AWNED (3 months) |
| 15/female | Orbit | ST | MIC2-P, Fli1-focal P, synaptophysin-focal P, Desmin-N, CCNB3-N |
EWSR1-N, N-myc-N |
NACT+RT | AWD (36 months) |
| 45/male | Pelvic | ST | MIC2-N, WT1-focal P, Calretinin-N, AE1/ SE3-N, INI1-R, Desmin-N, CCNB3-N |
EWSR1-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-P BCOR-CCNB3-N |
NA | NA |
| 50/female | Gluteal | ST | MIC2-N, WT1-P, Calretinin-N, AE1/ AE3 ‘Dot-like’- P, S100P-focal P, Desmin-NP, CCNB3-N |
EWSR1-N, SS18-N CIC-DUX4 (1)-N, CIC-DUX4 (2)-P BCOR-CCNB3-N |
CT (ifosfamide + adriamycin) | On Tx |
| 14/male | Ilium | Bone | MIC2-N, Fli1-P, AE1AE3 ‘Dot-like’- P, Desmin-NP, CCNB3-N |
EWSR1-N, CIC-DUX4 (1)-N, CIC-DUX4 (2)-N BCOR-CCNB3-N |
CT | On Tx |
P, positive; N, negative; R, retained; UI, uninterpretable; NA, not available; NP, not performed; HK-NW, housekeeping gene for CIX-DUX4 and BCOR-CCNB3 fusions, not worked; Sx, surgery; CT, chemotherapy; AdjCT, adjuvant CT; NACT, neo-adjuvant CT; LTFU, lost to follow up; LN, lymph node; AWD, alive with disease; AWNED, alive with no evidence of disease; DOD, died of disease; DDOC, died due to other cause; Tx, treatment; EWSR1, Ewing sarcoma RNA binding protein 1; EMA, epithelial membrane antigen; FOD, free of disease; OGS, osteosarcoma
Survival analysis: The median OS was 12 months, and median disease-free survival (DFS) was seven months, during an average follow up of 12 months. Furthermore, there was a significant association between recurrences and DFS, and OS, as well as between distant metastasis and DFS, indicating that patients without recurrences and metastasis had a significantly improved DFS (P=0.034, P=0.008, respectively). There was no significant association between patient age group (<18 and ≥18 yr); gender; T-size (≤5 cm vs. >5 cm; ≤8 cm vs. >8 cm); presence of necrosis and treatment with NACT; with regard to OS and DFS (Table IV).
| Variable | Number of cases, n (%) | P (OS) | P (DFS) |
|---|---|---|---|
| Age (yr) | |||
| <18 | 22 (43) | 0.564 | 0.242 |
| ≥18 | 29 (56.8) | ||
| Gender | |||
| Male | 27 (52.9) | 0.521 | 0.524 |
| Female | 24 (47) | ||
| Site | |||
| Bone | 13 (25.4) | 0.227 | 0.606 |
| Soft tissue | 38 (74.5) | ||
| T-size (cm) | |||
| ≥5 | 26/33 (78.7) | 0.471 | 0.848 |
| <5 | 7/33 (21.1) | ||
| Outcomes | |||
| Recurrences | 6/39 (11.4) | 0.011 | 0.034 |
| Metastases | 12/39 (30.7) | 0.697 | 0.008 |
| AWD | 14/39 (35.8) | ||
| AWNED | 10/39 (25.6) | ||
| DOD | 14/39 (35.8) | ||
| DDOC | 1/39 (2.5) |
DFS, disease-free survival; OS, overall survival; AWD, alive with disease; AWNED, alive with no evidence of disease
Discussion
It is important to correctly diagnose undifferentiated round cell sarcomas because these tumours mimic various other sarcomas, associated with differing treatment regimens and clinical outcomes. We have earlier observed that in cases where molecular testing for Ewing sarcomas was requested, only 71 per cent cases turned out to be Ewing sarcoma2. In a study from the French sarcoma group, the authors observed modification of diagnosis in 12 per cent cases of Ewing sarcomas, after molecular testing, reinforcing importance of molecular testing in sarcomas25. The spectrum of undifferentiated round cell sarcomas has been evolving, especially with the identification of newer gene fusions underlying these tumours, such as BCOR-CCNB3, CIC-DUX4 and CIC-FOXO435789111222262728.
The present study describes clinicopathologic features of 51 cases of undifferentiated round cell sarcomas, including 32 cases, tested for BCOR-CCNB3 and CIC-DUX4 fusions. Nearly 38 per cent cases were positive for these mutually exclusive, specific fusion transcripts, namely BCOR-CCNB in 16 per cent cases and CIC-DUX4, in 22 per cent cases. Pierron et al7 identified BCOR-CCNB3 fusion in four per cent cases. Subsequently, various investigators identified BCOR-CCNB3 fusions in 1.4-13 per cent, in different studies92228. Machado et al10 observed CIC-DUX4 fusions in six and BCOR-CCNB3 fusions in five cases, respectively, of 200 undifferentiated sarcomas, with an overall frequency of 5.5 per cent of these transcripts. Yamada et al20 observed either BCOR-CCNB3 or CIC-DUX4-positive fusions in 16 of 164 (9.7%) cases of unclassified tumours with a round cell component. We observed a relatively higher percentage of these fusion-positive tumours, as a result of including critically reviewed cases and also due to being the largest cancer referral centre of our country.
Clinically, a wide age range was observed in the present study (average=23.9 yr, median=20 yr), as noted by some authors82229. However, others observed these tumours in relatively older patients with median age, ranging from 30 to 37 yr1721223031. Gender-wise, male preponderance was noted in BCOR-CCNB3-positive sarcomas, as similarly reported81718212230, while CIC-DUX4-positive sarcomas occurred more frequently in females21.
Site-wise, most tumours occurred in the soft tissues (75%), especially lower and upper extremities, followed by bony sites, as previously documented6111721. Among the tumours involving the bone, lower extremities were involved in eight cases with femur and tibia as the most commonly involved bones, as similarly reported1820222830. In a study, 86 per cent cases of CIC-rearranged sarcomas were identified within soft tissues20. Similar to the present study, a significant number of BCOR-CCNB3-positive sarcomas have been reported in bones822.
Average tumour size observed in the present study was large, i.e. 8.6 cm as has been reported by others81926. Seventy nine per cent cases had a tumour size exceeding 5 cm, as reported by Antonescu et al21. During gross examination, CT-naïve tumour s pecimens were well circumscribed, nodular, homogenous and greyish white on cut surface, whereas post-NACT resection s pecimens showed a variegated appearance, due to haemorrhage and necrosis82229. Microscopically, in most cases, tumour cells were arranged in various growth patterns. While CIC-DUX4-positive sarcomas more frequently exhibited a nodular growth pattern, BCOR-CCNB3-positive sarcomas displayed consistently diffuse growth pattern of cells with interspersed thin-walled blood vessels. Variable myxoid stroma and areas of necrosis, seen more frequently in CIC-DUX4-positive sarcomas, as previously reported69111520212930.
We observed a varied cytomorphology, including predominantly round, to focally polygonal/epithelioid to spindle cells with angulated nuclei and vesicular chromatin in BCOR-CCNB3-positive sarcomas and polygonal to ‘rhabdoid-like’ (2 cases) and rarely, pleomorphic giant cells, with hyperchromatic nuclei, showing prominent nucleoli, in CIC-DUX4-positive sarcomas, as observed by different investigators815202229.
Immunohistochemically, in a significant number of cases, the tumour cells showed a ‘dot-like’ immunoreactivity for MIC2/CD99, followed by focal cytoplasmic membranous and a mixed-type of membranous and ‘dot-like’ positivity. In four different studies, respective authors17212930 observed similar patterns of MIC2/CD99 immunoexpression, ranging from focal to multifocal membranous to patchy positivity. Most cases of Ewing sarcoma display diffuse cytoplasmic membranous immunostaining for MIC22.
WT1 immunostaining was noted in 70 per cent cases in the present study, including 6/7 (86%) CIC-DUX4 positive cases, as previously reported6152129. Yoshida et al29 recommended WT1 and calretinin as surrogate markers for genetically defined undifferentiated round cell sarcomas. We observed variable calretinin positivity in 44 per cent tumours.
We also noted focal epithelial differentiation (AE1/AE3) positivity in 32 per cent cases, as observed by others2128. However, EMA was rarely positive, focally in five per cent cases. Other immunohistochemical markers, previously reported in some of these cases include p63, CD56, TLE1, NKX2.2, ERG and BCOR20. NKX2.2 is reported to be useful in differentiating Ewing sarcomas from undifferentiated sarcomas, including BCOR-CCNB3 and CIC-DUX4-positive, in view of its positive expression in the former and negative expression in the latter tumours31.
As a result of a histopathologic spectrum and overlapping immunohistochemical markers, there is a need to differentiate the undifferentiated round cell sarcomas from other more well-defined sarcomas, which constitute as their differential diagnoses. Among these, Ewing sarcoma is their closest mimic. EWSR1 rearrangement and/or specific fusion transcripts for ESFTs were negative in 40 cases, of the present study.
Other round cell tumours, such as a DSRCT and a RMS also are histologic mimics of these tumours, the former especially in abdominal tumours32. DSRCT displays polyphenotypic differentiation, including immunoexpression of epithelial markers, neuroendocrine markers, desmin and WT1, along with EWSR1 rearrangement32. While WT1 immunostaining was observed in 70 per cent cases of our study, lack of EWSR1 rearrangement in nine cases and polyphenotypic differentiation in four cases helped in ruling out a DSRCT.
The presence of variable myxoid stroma in certain cases led to consideration of myoepitheliomas and extraskeletal myxoid chondrosarcomas33. Lack of co-expression of epithelial markers, S100 protein and GFAP helped in ruling out a myoepithelial tumour, especially in two cases of CIC-DUX4-positive sarcomas, which can have overlapping features with the former tumour2134. An epithelioid sarcoma is characterized by epithelioid and spindle cells with central necrosis and a loss of immunohistochemical expression of INI1/SMARCB1 in more than 80 per cent cases35. In this study, INI1/SMARCB1 was retained in 86 per cent tumours; completely lost in two and showed weak to absent immunoexpression (reduced) in a single tumour. Differential diagnosis of an extrarenal malignant rhabdoid tumour (MRT) was also considered. However, in view of lack of rhabdoid cells, these tumours were diagnosed as ‘INI1-deficient’ undifferentiated sarcomas. Despite complete loss of INI1, these tumours show a relatively better clinical course, possibly in view of lack of rhabdoid cells. Therefore, it is important to differentiate these tumours from extrarenal MRTs36. Epithelioid sarcoma was a close differential diagnosis, especially in CIC-DUX4-positive tumours.
Poorly differentiated synovial sarcoma constituted another differential diagnosis, especially in BCOR-CCNB3- positive cases (e.g., case 46), in view of overlapping histopathological features8. Seven cases, where synovial sarcoma was a close differential diagnosis, showed negative t(X; 18) translocation results.
Identification of the specific transcripts underlying undifferentiated round cell sarcomas has led to the discovery of certain immunohistochemical markers, namely CCNB3 and BCOR, expressed in some of these sarcomas78910202237. We observed a strong and diffuse CCNB3 immunostaining in 27 per cent tumours, including four out of five cases of BCOR-CCNB3-positive sarcomas and in one of the four cases of CIC-DUX4-positive sarcomas. While CCNB3 has been recommended as a reasonably sensitive immunohistochemical marker for BCOR-CCNB3-positive sarcomas, with sensitivity ranging from 60 to 90 per cent; its focal expression is also seen in some cases of Ewing sarcoma and solitary fibrous tumours781022. Machado et al10 observed CCNB3 immunoexpression in three of five cases of BCOR-CCNB3-positive sarcomas, while Matsuyama et al22 observed the same in nine of 11 such cases. We observed positive CCNB3 immunostaining in seven cases, lacking BCOR-CCNB3 fusion. Therapeutically, most patients in the present study were treated with EFT 2001 CT regimen. During follow up, 11 per cent patients developed recurrences and 31 per cent patients developed metastasis. Fourteen patients died of disease, with seven such patients harbouring metastatic lesions, most commonly in lung, as reported by other authors1121.
The median OS was 12 months and DFS was seven months. Median OS for seven cases of CIC-DUX4-positive cases was five months. In one of the studies from our country, the estimated OS and DFS rates in cases of metastatic Ewing sarcoma were 65.6 and 37.5 per cent, while in non-metastatic Ewing sarcoma were 83 and 62 per cent, respectively23. These results clearly showed that undifferentiated round cell sarcomas, especially CIC-DUX4 positive, had a relatively poorer clinical outcome, as compared to Ewing sarcoma, irrespective of metastatic and non-metastatic tumours. Two previous studies2129 reported a significantly worse outcome in patients harbouring CIC-rearranged sarcomas.
Puls et al8 observed that the five year survival rates in patients with Ewing sarcoma and BCOR-CCNB3 were 54 and 75 per cent, respectively. However, they observed a longer OS in cases harbouring tumours in the lower extremities, as compared to the axial skeleton and soft tissues. Among five BCOR-CCNB3-positive cases in our study, a single patient died of disease; single patient was alive with disease and another patient was AWNED. Among five cases of CIC-DUX4-positive sarcomas, two patients died of the disease, two were alive with disease, while a single case was AWNED during five months of follow up. CIC-DUX4-positive sarcomas are invariably clincially aggressive1117.
Cohen-Gogo et al18 observed that that the OS and DFS in cases with BCOR-CCNB3-positive sarcomas, was significantly better for the patients who received induction CT according to Ewing sarcoma protocol. Antonescu et al21 observed that cases of CIC-DUX4-positive tumours, treated by NACT, had a poor survival as compared to the patients who were managed with only surgery first followed by adjuvant CT. Patients with recurrences, in the present study had significantly lower DFS and OS. Moreover, it is reported that patients with complete resection have improved outcomes17.
The limitation of the study was that all cases could not be subjected to a consistent panel of immunostains and molecular tests, due to resource and technical constraints (older paraffin blocks, suboptimal fixation in a few referral cases). Moreover, we could not test the cases, negative for EWSR1, CIC-DUX4 and BCOR-CCNB3 fusions (15 cases), for the other fusions and CIC and BCOR rearrangements, which have various other fusion partners. It would be worthwhile to study those cases for a wider gene panel, including newly described fusions with a high-throughput technique, such as next generation sequencing, as well as CIC and BCOR rearrangements, by fluorescence in-situ hybridization (FISH) technique4513141521.
To conclude, undifferentiated round cell sarcomas are rare and display a wide clinicopathologic spectrum; occur more frequently in the soft tissues of extremities, with CIC-DUX4 positive, more frequent than BCOR-CCNB3-positive sarcomas, including in our population. Even though, presently, CIC-DUX4-positive sarcomas are treated similar to Ewing sarcomas, these tumours should be distinguished from the latter, in view of their relatively aggressive outcomes. Recurrences and metastasis significantly affect clinical outcomes.
Acknowledgment
Authors thank Ms Manisha Chavan for immunohistochemical staining, especially for CCNB3 immunostaining.
Financial support & sponsorship: This study was financially supported by intramural grant, Tata Memorial Centre, Mumbai
Conflicts of Interest: None
References
- Undifferentiated/ Unclassified sarcomas. In: Tumours of soft tissue and bone: Pathology and genetics. World Health Organization classification of tumours (4th ed). Lyon: IARC Press; 2013. p. :236-8.
- [Google Scholar]
- Clinicopathological and molecular spectrum of Ewing sarcomas/PNETs, including validation of EWSR1 rearrangement by conventional and array FISH technique in certain cases. Pathol Oncol Res. 2014;20:503-16.
- [Google Scholar]
- Round cell sarcomas beyond Ewing: Emerging entities. Histopathology. 2014;64:26-37.
- [Google Scholar]
- Novel ZC3H7B-BCOR, MEAF6-PHF1, and EPC1-PHF1 fusions in ossifying fibromyxoid tumours-molecular characterization shows genetic overlap with endometrial stromal sarcoma. Genes Chromosomes Cancer. 2014;53:183-93.
- [Google Scholar]
- Novel BCOR-MAML3 and ZC3H7B-BCOR gene fusions in undifferentiated small blue round cell sarcomas. Am J Surg Pathol. 2016;40:433-42.
- [Google Scholar]
- Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumours compared to EWSR1-rearranged Ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer. 2014;53:622-33.
- [Google Scholar]
- A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461-6.
- [Google Scholar]
- BCOR-CCNB3 (Ewing-like) sarcoma: A clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J Surg Pathol. 2014;38:1307-18.
- [Google Scholar]
- BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of male children. Mod Pathol. 2015;28:575-86.
- [Google Scholar]
- Defining Ewing and Ewing-like small round cell tumours (SRCT): The need for molecular techniques in their categorization and differential diagnosis. A study of 200 cases. Ann Diagn Pathol. 2016;22:25-32.
- [Google Scholar]
- High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer. 2012;51:207-18.
- [Google Scholar]
- Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum Mol Genet. 2006;15:2125-37.
- [Google Scholar]
- Undifferentiated small round cell sarcomas with rare EWS gene fusions: Identification of a novel EWS-SP3 fusion and of additional cases with the EWS-ETV1 and EWS-FEV fusions. J Mol Diagn. 2007;9:498-509.
- [Google Scholar]
- The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res. 2009;15:2259-68.
- [Google Scholar]
- ETV4 is a useful marker for the diagnosis of CIC-rearranged undifferentiated round-cell sarcomas: A study of 127 cases including mimicking lesions. Mod Pathol. 2016;29:1523-31.
- [Google Scholar]
- Superficial EWSR1-negative undifferentiated small round cell sarcoma with CIC/DUX4 gene fusion: A new variant of Ewing-like tumours with locoregional lymph node metastasis. Virchows Arch. 2013;463:837-42.
- [Google Scholar]
- The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol. 2012;43:180-9.
- [Google Scholar]
- Ewing-like sarcomas with BCOR-CCNB3 fusion transcript: A clinical, radiological and pathological retrospective study from the Société Française des Cancers de L'Enfant. Pediatr Blood Cancer. 2014;61:2191-8.
- [Google Scholar]
- Screening of BCOR-CCNB3 sarcoma using immunohistochemistry for CCNB3: A clinicopathological report of three pediatric cases. Pathol Int. 2015;65:410-4.
- [Google Scholar]
- Histological and immunohistochemical characteristics of undifferentiated small round cell sarcomas associated with CIC-DUX4 and BCOR-CCNB3 fusion genes. Virchows Arch. 2017;470:373-80.
- [Google Scholar]
- Sarcomas with CIC-rearrangements are a distinct pathologic entity with aggressive outcome: A clinicopathologic and molecular study of 115 cases. Am J Surg Pathol. 2017;41:941-9.
- [Google Scholar]
- Clinicopathologic diversity of undifferentiated sarcoma with BCOR-CCNB3 fusion: Analysis of 11 cases with a reappraisal of the utility of immunohistochemistry for BCOR and CCNB3. Am J Surg Pathol. 2017;41:1713-21.
- [Google Scholar]
- Analysis of bone and soft-tissue sarcomas registered during the year 2012 at Tata Memorial Hospital, Mumbai, with clinical outcomes. Indian J Cancer. 2018;55:37-44.
- [Google Scholar]
- Chemotherapy compliance in patients with osteosarcoma. Pediatr Blood Cancer. 2013;60:41-4.
- [Google Scholar]
- Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): A prospective, multicentre, observational study. Lancet Oncol. 2016;17:532-8.
- [Google Scholar]
- A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: A genetically distinct variant of Ewing-like sarcoma. Am J Surg Pathol. 2014;38:1571-6.
- [Google Scholar]
- Clinicopathologic features of a second patient with Ewing-like sarcoma harboring CIC-FOXO4 gene fusion. Am J Surg Pathol. 2014;38:1724-5.
- [Google Scholar]
- BCOR-CCNB3 undifferentiated sarcoma-does immunohistochemistry help in the identification? Pediatr Dev Pathol. 2017;20:321-9.
- [Google Scholar]
- CIC-rearranged sarcomas: A study of 20 cases and comparisons with Ewing sarcomas. Am J Surg Pathol. 2016;40:313-23.
- [Google Scholar]
- Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Mod Pathol. 2016;29:1324-34.
- [Google Scholar]
- Evaluation of NKX2-2 expression in round cell sarcomas and other tumours with EWSR1 rearrangement: Imperfect specificity for Ewing sarcoma. Mod Pathol. 2016;29:370-80.
- [Google Scholar]
- Desmoplastic small round cell tumour-clinicopathological spectrum, including unusual features, and immunohistochemical analysis of 45 tumours diagnosed at a tertiary cancer referral centre, with molecular results (EWS-WT1) in select cases. Pathol Oncol Res. 2012;18:917-27.
- [Google Scholar]
- EWSR1-POU5F1 fusion in soft tissue myoepithelial tumours. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-24.
- [Google Scholar]
- Myoepithelial tumours of soft tissue: A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-96.
- [Google Scholar]
- Immunohistochemical validation of INI1/SMARCB1 in a spectrum of musculoskeletal tumours: An experience at a tertiary cancer referral centre. Pathol Res Pract. 2013;209:758-66.
- [Google Scholar]
- Loss of INI1 expression defines a unique subset of pediatric undifferentiated soft tissue sarcomas. Mod Pathol. 2009;22:142-50.
- [Google Scholar]
- BCOR overexpression is a highly sensitive marker in round cell sarcomas with BCOR genetic abnormalities. Am J Surg Pathol. 2016;40:1670-8.
- [Google Scholar]






