Translate this page into:
Characterization of the anopheline vector breeding habitats: Implications for elimination of malaria in tribal inhabited Car Nicobar Island
For correspondence: Dr I.P. Sunish, ICMR-Regional Medical Research Centre, Port Blair 744 103, Andaman and Nicobar Islands, India e-mail: sunish67@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Malaria is an important public health problem in Andaman & Nicobar archipelago. Among the three districts, Nicobar is the most endemic district where API is >2. In this district, the malaria incidence in Car Nicobar Tehsil has declined steadily over the past 10 years. A renewed initiative to consolidate this gain is being made with the ultimate objective of achieving zero indigenous transmission of malaria in Car Nicobar. So, the present study undertook a close environmental monitoring of water bodies for assessing changes in the risk potential of mosquito vector breeding habitats which can augment the elimination programme.
Methods:
The breeding habitats of anopheline mosquitoes were sampled in 16 areas of Car Nicobar Island for eight time periods during 2017-2020. Along with anophelines, various associated water parameters (n=60) were estimated, viz. physicochemical (n=13), and biological, which included culicine mosquito immatures, insect predators (n=5), phytoplanktons (n=31) and zooplanktons (n=10).
Results:
In the 16 study sites, overall 1126 surface water stagnating bodies constituting 21 different habitat types were surveyed. Of these, 17 were positive for anopheline breeding. Water bodies from three villages were consistently found to be positive for anopheline breeding. However, early instars of anopheline larvae were more abundant compared to the late instars. Four anopheline species were recorded, including Anopheles sundaicus, A. barbirostris, A. insulaeflorum and A. subpictus, in which 48 per cent were A. sundaicus. Multivariable analysis indicated that anopheline density was significantly higher in permanent water bodies than in temporary habitats (P<0.05) (high risk of anophelines). The highest pH (≥8.2), dissolved solids (≥0.39) levels showed significantly (P<0.05) decreased larval densities (lower risk of breeding), adjusted with breeding sites and season. Nitrite levels increased (P=0.022) larval densities.
Interpretation & conclusions:
The present study facilitated estimating the productive period of a larval habitat enabling target larval sources to reduce adult populations. Implementing larviciding strategy before monsoon season is presumably the most cost-effective strategy. The output can be utilized for environmental monitoring of mosquito breeding risk in other malaria endemic areas, particularly where medium/large water bodies are the predominant breeding sites for malaria vectors.
Keywords
Anophelines
Car Nicobar
malaria
risk factors
A system for elimination has been recommended by the Global Technical Strategy for Malaria Elimination, which includes strategy for malaria prevention diagnosis and treatment under universal coverage, strengthening surveillance, and thus accelerating towards its potential elimination1. The National Framework for Malaria Elimination2 was launched by the Government of India in 2016, to eliminate malaria in the country by 2030.
Malaria is one of the most important vector-borne diseases of concern in Andaman & Nicobar Islands. Due to severe devastation caused by the 2004 tsunami, malaria cases in this area increased, which has been attributed to the proliferation of vector breeding habitats3. In Nicobar district, labourers from mainland India who arrived for rehabilitation work were affected by malaria4, and their movement increased the risk of transmission. Car Nicobar, the headquarter of Nicobar district was particularly affected by the tsunami and witnessed widespread malaria. However, a declining trend was observed during the years 2011-2021 (API reduced from 4.4 to 0.33). In the other two tehsils, however, the endemicity is still high with an API >5 (personal communication). Nonetheless, Car Nicobar is maintaining vigil for the past few years against malaria imported from nearby endemic islands (Personal communication, Department of Health Services, Port Blair). Being on the verge of malaria elimination, a quantifiable determination of the potential factors responsible for vector breeding is important to devise intervention strategies as also is the sustenance of the gains achieved by continuous monitoring to prevent resurgence5,6. Of the 23 anopheline species reported in these islands7, Anopheles sundaicus cytotype ‘D’, which is known to breed in fresh as well as brackish water, is the causative malaria vector in this area8. This vector is primarily zoophagic (prefer animal blood), exophilic (rest outdoors) and exophagic (feed outdoors)9.
Vector control is recognized as the most effective way to interrupt parasite transmission. Vector control implemented in Car Nicobar Island was found to be effective, which was mainly attributed to the release of larvivorous fish; Gambusia affinis5. Even though abundance, dynamics and fitness of adult mosquitoes are determined by its immature stages, comparatively less studies are directed towards these aquatic stages. Immature stages of malaria vectors prefer various habitat types, viz., natural and cultivated swamps, puddles, river fringes, and burrow pits, open drains10, etc. Therefore, understanding the habitat characteristics can provide useful information for larval source management operations. Global climate change with higher temperature and sea levels are likely to create ecological impacts, which could, however, be pronounced in small island regions. Mapping the island for vector breeding risk can hence be used for monitoring the risk of malaria transmission, which can facilitate in the elimination of malaria from Car Nicobar. In this context the present study aimed to undertake an eco-geographical survey of the water bodies in and around the Car Nicobar island to characterize the anopheline breeding habitats.
Material & Methods
Study area and study design: This study was undertaken by ICMR-Regional Medical Research Centre, Port Blair, India. The study was carried out in 16 areas (15 villages and the Air Force base) of Car Nicobar island of Nicobar district, Andaman & Nicobar archipelago. Car Nicobar (District Head Quarter) spans 127 km2 in area, and is a flat terrain with the exception of small hilly areas in its interior. The climate is tropical with an average annual rainfall amounting to 2380 mm, and >100 rainy days per annum. The mean relative humidity is 79 per cent, and the mean minimum and maximum temperatures are reportedly 23.0°C and 30.2°C, respectively. The island is inhabited by the native aboriginal Nicobarese tribes, with a population of 17,125 as per census 201111. For the past decade, Car Nicobar has reported the least number of malaria cases [Annual Parasite Incidence (API) <1.0 from 2013], among the three tehsils of the Nicobar district (personal communication). A peak in malaria cases was observed subsequent to Tsunami (2004;API=90.59), which later declined steeply (API in 2007=8.51) (Fig. 1). After 2008, a gradual decline in API was observed and was <1 during the past six years. During the years, 2020 and 2021, the API was 0.33 and 0.11, respectively (personal communication, Directorate of Health Services, Port Blair).
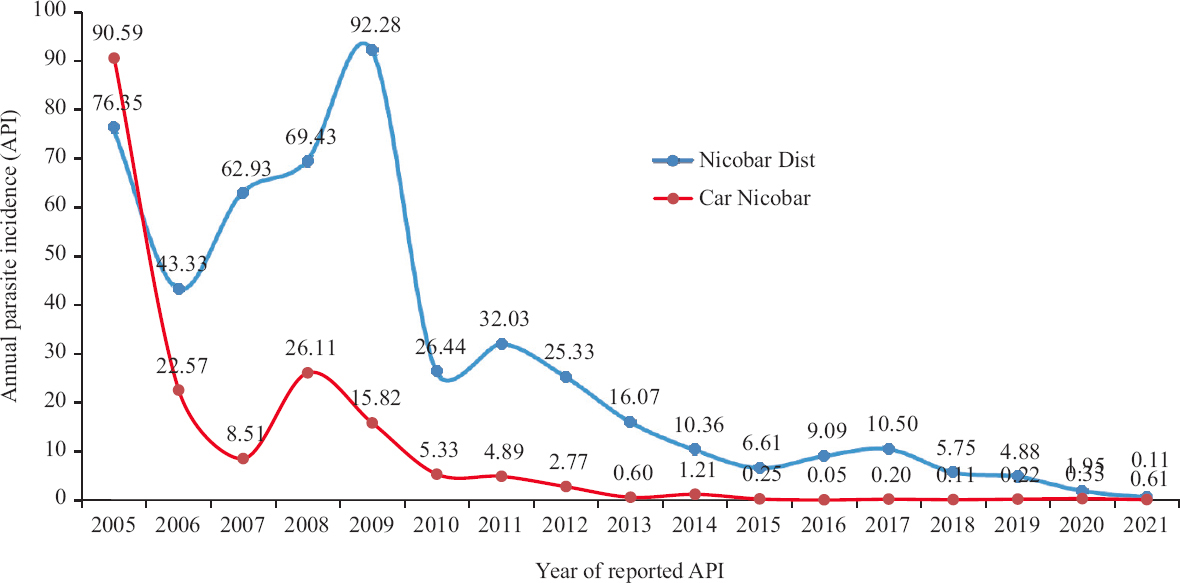
- API in Car Nicobar as compared to Nicobar District (2005-2021). Source: Directorate of Health Services, Port Blair. API, annual parasite incidence
Year-round larviciding in addition to indoor residual spray with DDT is biannually implemented in all these villages to control malaria-causing mosquito vectors. Gambusia affinis fishes are released in breeding habitats, from the nursery stock maintained in the district hospital. These fishes were introduced mainly during the malaria outbreak in the post-tsunami period by the State malaria department.
In the present study, all water bodies in the island were surveyed during the period 2017-2020, to estimate entomological, physicochemical and associated biological parameters, along with anopheline abundance. After obtaining permission from the tribal council, the respective village headman allocated one task force person to accompany the survey team, who was aware of the village surroundings and water bodies. A total of eight time periods covering three seasons were surveyed, in which 105-218 water bodies were sampled.
Entomological monitoring: The anopheline immatures were sampled by standard larval dipper and were expressed as per dip density. One dip/m2 area was considered for sampling. Late instars were brought to the laboratory, and the emerged adults were identified morphologically using standard taxonomic keys12.
Estimation of various parameters: Along with anopheline immatures, water samples from the same spots were collected to estimate various physicochemical parameters. These included pH, water temperature, dissolved oxygen, salinity, hardness, acidity, alkalinity, silicates, nitrates, nitrites, ammonia and phosphates, which were estimated by standard method13. Biological factors estimated included culicine immatures, insect predators (notonectids nymphs and adults, dytiscids, anisopterans and zygopterans), and planktonic forms. Insect predator density was estimated by the dipping method14. For algal composition in the water bodies, one litre of water sample was collected from each habitat, which was concentrated by sedimentation. Planktonic forms were observed for their composition and were enumerated.
Data analysis: Anopheles and Culex immature densities (per dip) were highly skewed and hence were square root-transformed. The linear Generalized Estimating Equation (GEE) was used to determine the association between the transformed immature densities and the breeding sites, season, physiochemical parameters and planktons. Both univariate and multivariable models were fitted, and estimates were reported with robust 95 per cent confidence intervals (95% CI). In linear GEE, independent variables (e.g., pH) were categorized into lower percentile (≤ 33 = lower), median and upper percentile (≥67 = Upper) for both univariate and multivariable analysis. The lower percentile was fixed as reference category (1) to compare with the upper percentile for all variables. All associated factors (breeding sites, season, etc.) which were significant at P<0.05 in the univariate GEE were further examined in multivariable GEE. The exchangeable correlation structure was adjusted in the GEE model. The quasi-information criterion (QIC) method was used to identify the best correlation structure. The data analyses were carried out using the software, Stata version 16 (StataCorp LLC, College Station, TX, USA).
Results
A total of eight time periods were surveyed during the period 2017-2020. There were 105 to 218 water bodies during each time period. From the whole island, 1126 water bodies were sampled, which were grouped into 21 habitat types. Of these, 17 habitat types were with anophelines. The per dip density of anopheline was high in temporary water habitats, viz., animal footprints, roadside ditches and vehicle track.
During the three seasons surveyed (viz., pre-monsoon, monsoon and post-monsoon), precipitation was recorded highest in post-monsoon (October-December) during 2018, while in 2019, it occurred during monsoon (July-September). In the former (2018), per dip density of both culicine and anopheline was highest, before the peak rainfall (Fig. 2). In the subsequent year (2019), the anopheline density in this period was low (0.39/dip), but peaked (0.56/dip) in October-December survey (post-monsoon), which later declined in the subsequent season.
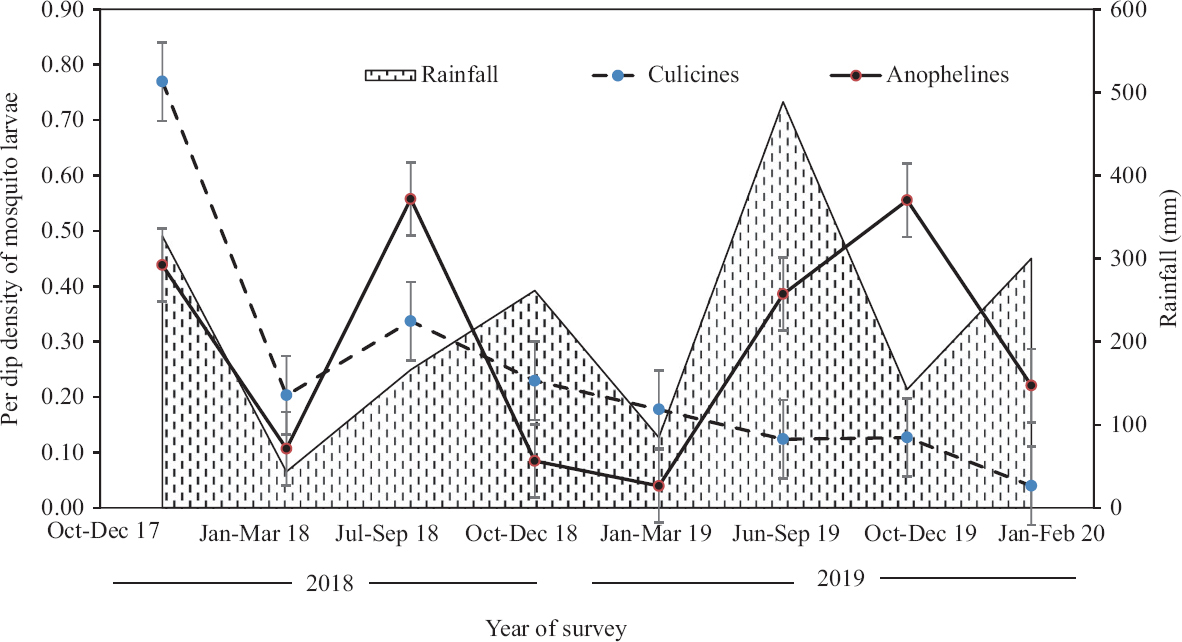
- Per dip density of mosquitoes during different time periods/seasons. Source: www.andaman.gov.in
All villages were found positive for anophelines at any one point of the survey. Among the 16 areas, three villages, viz., Kimious, Sawai and Teetop were positive for anophelines during all the surveys. Combining all the surveys, the highest density (per dip) was observed from Teetop, followed by Sawai village (Fig. 3). During 2018, five villages, viz. Kakana, Kimious, Kinmai, Sawai and Teetop, recorded anophelines in all the three seasons (pre-monsoon, monsoon and post-monsoon). Highest anopheline density was found in Teetop village during pre-monsoon season. In the subsequent year (2019), nine villages recorded anophelines in all the three seasons, with the per dip density ranging from 0.02/dip to 2.17/dip (Table I).
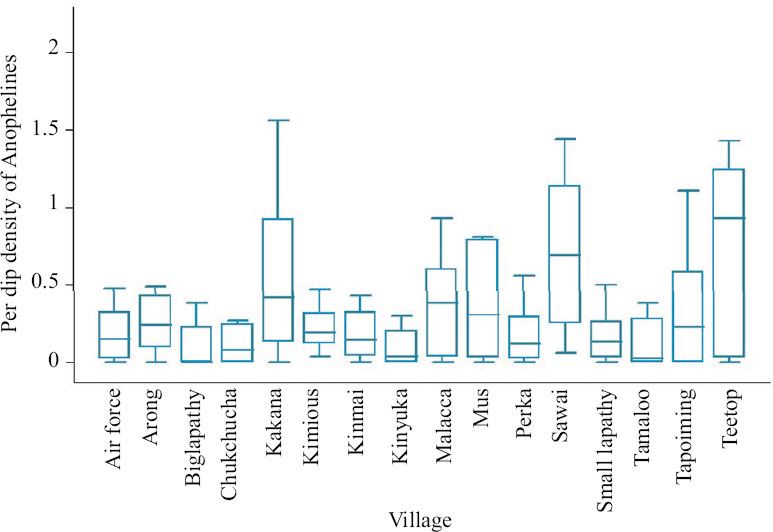
- Larval density of anophelines in the villages of Car Nicobar Island.
| Village | 2017 Post-M |
2018 | 2019 | 2020 Pre-M |
||||
|---|---|---|---|---|---|---|---|---|
| Pre-M | Monsoon | Post-M | Pre-M | Monsoon | Post-M | |||
| Arong | 1.28 | 0.49 | 0 | 0.18 | 0.02 | 0.30 | 0.37 | 0.18 |
| Sawai | 1.23 | 0.06 | 1.44 | 0.63 | 0.11 | 0.75 | 1.05 | 0.39 |
| Malacca | 0.93 | 0 | 0.31 | 0.03 | 0.04 | 0.69 | 0.45 | 0.52 |
| Teetop | 0.90 | 1.43 | 1.27 | 0.06 | 0 | 1.22 | 0.96 | 0 |
| Kakana | 0.85 | 0.08 | 1.00 | 0.18 | 0 | 1.56 | 0.18 | 0.66 |
| Kimious | 0.35 | 0.08 | 0.19 | 0.16 | 0.04 | 0.28 | 0.47 | 0.19 |
| Small Lapathy | 0.25 | 0.06 | 0.50 | 0 | 0.16 | 0 | 0.28 | 0.10 |
| Tapoiming | 0.24 | 1.11 | 0 | 0 | 0 | 0.91 | 0.26 | 0.22 |
| Kinmai | 0.21 | 0.97 | 0.04 | 0.05 | 0.08 | 0 | 0.43 | 0.22 |
| Mus | 0.17 | 0.81 | 0.03 | 0 | 0.03 | 0.44 | 2.17 | 0.78 |
| Perka | 0.17 | 0.07 | 0 | 0 | 0.05 | 0.35 | 0.56 | 0.24 |
| Air Force | 0.06 | 0 | 0 | 0.24 | 0.05 | 0.48 | 0.24 | 0.41 |
| Big Lapathy | 0 | 0.38 | 0 | 0 | 0 | 0 | 0.72 | 0.08 |
| Kinyuka | 0 | 0 | 0.05 | 0 | 0.03 | 0.61 | 0.30 | 0.11 |
| Chukchucha | 0 | 0 | 0 | 0.13 | 0.03 | 0.22 | 1.67 | 0.27 |
| Tamaloo | 0 | 0 | 0 | 0.05 | 0 | 0.38 | 1.17 | 0.18 |
Post-M, post-monsoon; Pre-M, pre-monsoon
Vehicle track habitat recorded high anopheline density, followed by culvert, Pucca drain, Kutcha drain (Fig. 4). The habitats: recreation pond, seashell and wells were found breeding once, during the eight time periods surveyed. Out of 17 habitat types found with anophelines, five habitats were positive during all the eight time periods, viz., culvert, pond, pucca drain, rainwater pool and roadside ditches (Table II). Anopheline pupae were found in ten habitat types, in which roadside ditches recorded the highest density (0.63/dip), followed by rainwater pools (0.3/dip).
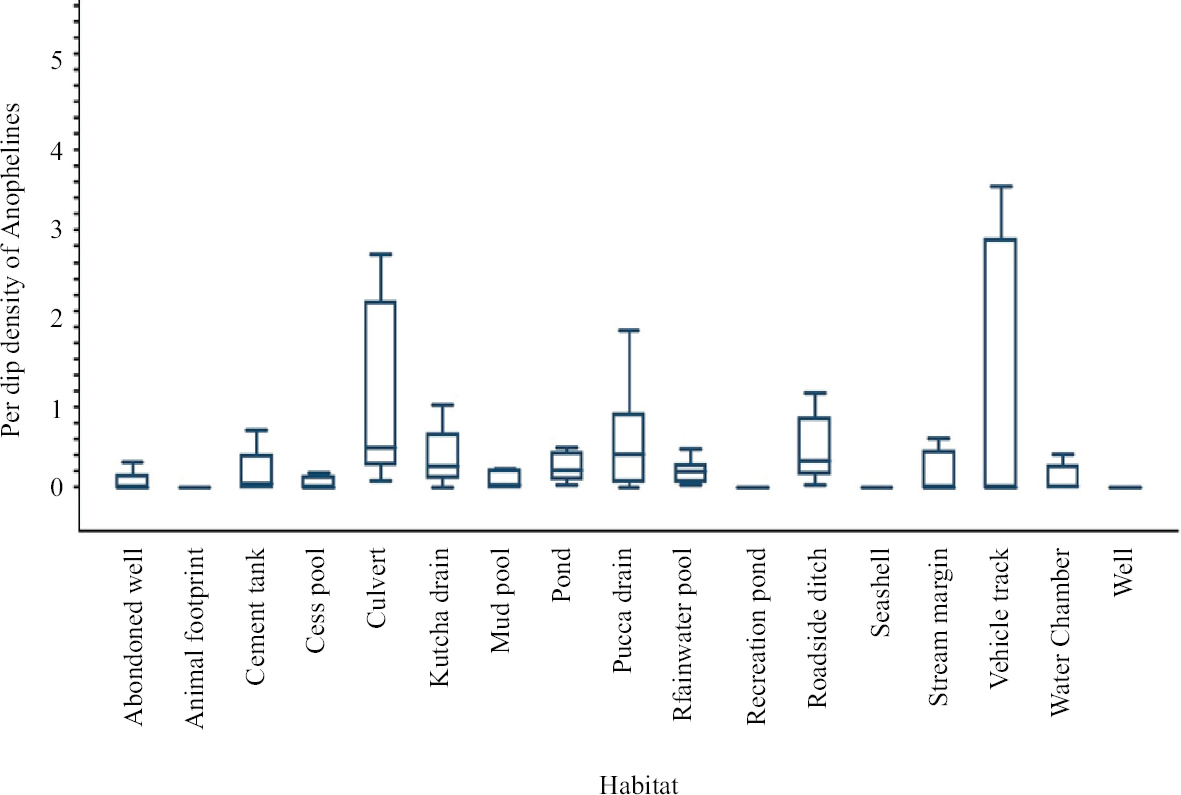
- Habitat wise larval density of anophelines in Car Nicobar Island.
| Habitat category | Habitats (number of +ve/number of surveyed) | 2017 Post-M |
2018 | 2019 | 2020 Pre-M |
||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-M | Monsoon | Post-M | Pre-M | Monsoon | Post-M | ||||
| Permanent | Kutcha drain (12/40) | 0.78 | 0.55 | 0.18 | 0.06 | 0 | 0.19 | 1.04 | 0.36 |
| Pond (69/181) | 0.42 | 0.14 | 0.15 | 0.06 | 0.03 | 0.44 | 0.50 | 0.28 | |
| Pucca drain (18/41) | 0.01 | 0.06 | 0.40 | 0.43 | 0.08 | 0.75 | 1.96 | 1.08 | |
| Cement tank (5/19) | 0 | 0.12 | 0 | 0 | 0.24 | 0.58 | 0 | 0.72 | |
| Well (1/10) | 0 | 0 | 0.06 | 0 | 0 | 0 | 0 | 0 | |
| Abandoned well (2/9) | 0 | 0 | 0 | 0 | 0.32 | 0.80 | 0 | 0 | |
| Recreation pond (1/4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.22 | |
| Temporary | Vehicle track (3/8) | 3.75 | 0 | 0 | 0 | 0 | 3.50 | 2.67 | 0 |
| Sea shell (1/5) | 2.50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Culvert (22/47) | 2.38 | 0.55 | 2.24 | 0.20 | 0.08 | 0.45 | 2.90 | 0.37 | |
| Road side ditch (47/224) | 0.56 | 0.14 | 0.38 | 0.21 | 0.04 | 0.28 | 1.18 | 4.05 | |
| Rain water pool (75/324) | 0.48 | 0.04 | 0.28 | 0.09 | 0.03 | 0.31 | 0.26 | 0.16 | |
| Water chamber (6/46) | 0.42 | 0.79 | 0.11 | 0.03 | 0 | 0 | 0 | 0 | |
| Mud pool (12/13) | 0.23 | 0.89 | 0.02 | 0 | 0.06 | 0 | 0 | 0.21 | |
| Stream margin (5/12) | 0 | 0.62 | 1.26 | 0 | 0 | 0 | 0 | 0.27 | |
| Cesspool (4/19) | 0 | 0 | 0 | 0.47 | 0.10 | 0 | 0 | 0.19 | |
| Animal foot print (1/2) | 0 | 0 | 0 | 0 | 0 | 7.0 | 0 | 0 | |
Altogether, 1386 mosquitoes were identified from 16 areas/villages (including the Air Force base). Eight mosquito species were identified, viz., A. sundaicus, A. barbirostris, A. insulaeflorum, A. subpictus, Culex quinquefasciatus, C. fragilis, Aedes aegypti and Aedes albopictus. Anophelines constituted 75 per cent of the total mosquitoes identified, of which 48 per cent belonged to A. sundaicus, followed by A. barbirostris (16.5%) (Fig. 5). Among the 16 areas, Sawai village recorded the highest number of mosquitoes. In all villages, A. sundaicus was recorded in high numbers. A. barbirostris was found in 13 areas, while A. subpictus in 10 areas. Habitat-wise analysis showed that ponds had the highest percentage of mosquitoes, including anophelines. Except two habitats, all other habitats were found with A. sundaicus. This species was found more in ponds, followed by roadside ditches . A. barbirostris was observed in six habitats, while A. insulaeflorum and A. subpictus from five and six habitats, respectively.
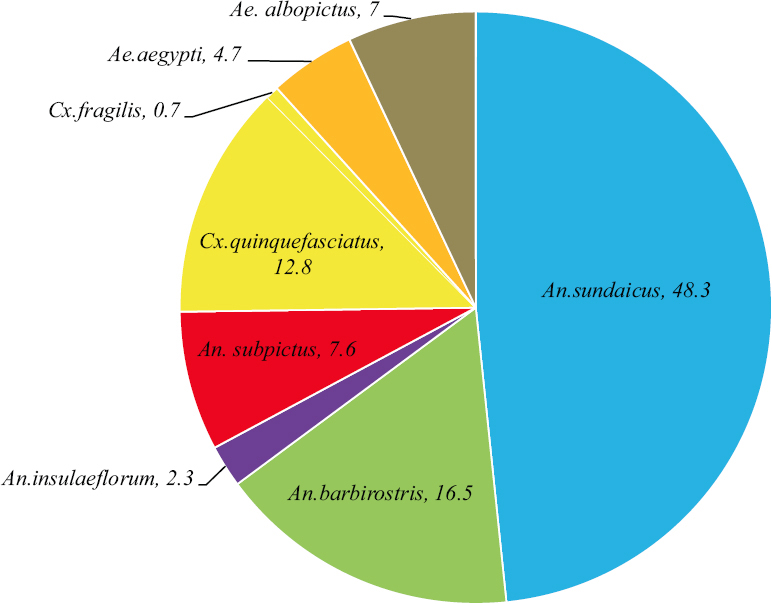
- Percentages of mosquito species identified from various habitats of Car Nicobar Island.
Physicochemical parameters in water bodies: There were 13 physicochemical parameters estimated from the water bodies. Nine parameters showed significant differences between the habitat types (P<0.05). The pH values ranged from 8.01 to 8.15, while the water temperature ranged from 19.1 to 39.1 (Table III). The pH was highest in monsoon season, while acidity and alkalinity were recorded to be highest during the post-monsoon period. Salinity was high from samples of lagoon margins (reached up to 9900 ppm). Total hardness ranged from 7.9 to 332.0 ppm, while silicate range was 1.1 to 13.9 ppm. Seasonal analysis showed that all parameters, except dissolved oxygen, were significantly different (P<0.05) between seasons. The maximum mean values for nitrates, nitrites and ammonia were 1.137, 0.078 and 0.452 ppm, respectively.
| Variables | Habitat type | Season | |||
|---|---|---|---|---|---|
| Temporary collections | Permanent water habitats | Pre-M | Monsoon | Post-M | |
| Culex# | 0.29±0.76 | 0.28±0.48 | 0.22±0.46 | 0.17±0.41 | 0.37±0.73*** |
| Anopheles# | 0.27±0.59 | 0.31±0.49* | 0.18±0.43 | 0.35±0.60 | 0.37±0.69*** |
| pH | 8.15±0.41 | 8.05±0.41 | 8.03±0.43 | 8.15±0.51 | 8.02±0.41** |
| Temperature (°C) | 30.75±1.9 | 29.91±1.38*** | 29.76±1.5 | 29.74±1.95 | 30.07±1.44** |
| Dissolved solids (ppm) | 0.38±0.09 | 0.36±0.08** | 0.36±0.08 | 0.36±0.09 | 0.38±0.08** |
| Dissolved oxygen (ppm) | 12.44±5.55 | 13.18±4.92*** | 12.11±4.74 | 12.91±5.49 | 12.08±4.29 |
| Acidity (ppm) | 42.0±31.0 | 25.0±19.0*** | 23.0±17.0 | 24.0±14.0 | 35.0±25.0*** |
| Alkalinity (ppm) | 30.0±19.0 | 21.0±12.0*** | 21.0±9.0 | 21.0±9.0 | 30.0±23.0*** |
| Salinity (ppm) | 1554.5±2897.5 | 56.0±432.6*** | 260.3±1248.8 | 99.5±526.1 | 23.3±13.1*** |
| Hardness (ppm) | 44.70±65.57 | 13.42±10.29*** | 18.09±27.66 | 13.52±12.07 | 11.47±1.79*** |
| Silicate (ppm) | 8.5±4.54 | 6.95±3.39*** | 8.05±4.05 | 6.04±3.49 | 7.94±2.74*** |
| Phosphate (ppm) | 0.06±0.09 | 0.05±0.06 | 0.07±0.07 | 0.07±0.08 | 0.03±0.02*** |
| Nitrate (ppm) | 0.93±1.52 | 0.68±0.66 | 0.71±0.63 | 0.68±0.64 | 0.89±0.93*** |
| Nitrite (ppm) | 0.05±0.04 | 0.06±0.05 | 0.06±0.05 | 0.05±0.05 | 0.03±0.03*** |
| Ammonia (ppm) | 0.26±0.10 | 0.31±0.14* | 0.32±0.14 | 0.34±0.18 | 0.25±0.12*** |
P*<0.05; **<0.01; ***<0.001; #Square root transformed density. Post-M, post-monsoon; Pre-M, pre-monsoon
Biological factors in water bodies: Among anophelines, early instars were more in the first two years, while in the subsequent year the density of late instars was higher. The per dip density of five groups of predators, viz., notonectids (nymphs), notonectids (adults), dytiscids, anisopterans and zygopterans, were highest during the first two years. There were 41 planktons identified from the water samples, of which 31 were phytoplanktons and 10 were zooplanktons. The phytoplanktons were grouped into six phyla, viz. Bacillariophyta, Charophyta, Chlorophyta, Cyanobacteria, Myzozoa and Ochrophyta. The phylum Ochrophyta constituted a greater number of organisms. All the six phyla of phytoplanktons were observed in 11 habitat types. Zooplanktons were grouped into five, viz. Arthropoda, Rotifer, Ciliophora, Protozoa and Amoebazoa.
Influence of various parameters on anopheline abundance: A total of 1,126 data sets were collected during eight time periods. The univariate GEE revealed that, breeding site types such as permanent water habitat had significantly higher (P<0.05) densities of anopheline immatures (Table IV). Compared to pre-monsoon, anophelines were significantly more during monsoon (β=0.16, P<0.001) and post-monsoon periods (β=0.20, P<0.001). Univariate analysis showed upper percentiles of pH (≥8.2), dissolved solids (≥0.39) and salinity (≥28) to be significantly (P<0.05) associated with decreased anopheline immatures (less risk for anopheline breeding), but higher nitrite level increased (P=0.035) larval densities. Few of the planktons, viz., navicula (>10), guinardia (>2), cymbella (>10), coscinodiscae (>10), fragilaria (>10), cyanobacteria (>33) and daphnia (>2) were independently associated with increased (P<0.05) anophelines.
| Anopheles immature densities | β | SE | P | 95% CI |
|---|---|---|---|---|
| Breeding sites | ||||
| Temporary collections | 1 | |||
| Permanent water habitats | 0.13 | 0.02 | 0.000*** | 0.09-0.18 |
| Season | ||||
| Pre-M | 1.00 | |||
| Monsoon | 0.17 | 0.04 | 0.000*** | 0.08-0.26 |
| Post-M | 0.24 | 0.04 | 0.000*** | 0.16-0.31 |
| pH | ||||
| ≤7.90 | 1.00 | |||
| >7.90-<8.20 | −0.07 | 0.05 | 0.157 | −0.16-0.03 |
| ≥8.20 | −0.20 | 0.04 | 0.000*** | −0.26-−0.12 |
| Dissolved solids | ||||
| ≤0.34 | 1.00 | |||
| >0.34-0.38 | −0.02 | 0.04 | 0.717 | −0.10-0.06 |
| ≥0.39 | −0.10 | 0.04 | 0.017* | −0.17-−0.02 |
| Salinity | ||||
| ≤18 | 1.00 | |||
| 19-27 | −0.13 | 0.04 | 0.003** | −0.21-−0.04 |
| ≥28 | −0.05 | 0.04 | 0.234 | −0.12-0.03 |
| Nitrite | ||||
| ≤0.026 | 1.00 | |||
| >0.026-0.33 | 0.05 | 0.04 | 0.261 | −0.04-0.13 |
| ≥0.034 | 0.10 | 0.04 | 0.015* | 0.02-0.17 |
P*<0.05; **<0.01; ***<0.001. CI, confidence interval; SE, standard error
In the multivariable analysis, permanent breeding sites significantly had higher larval density than temporary water bodies (P<0.05) (high risk of anophelines) adjusted with season and physicochemical parameters. Anopheles immature densities significantly increased in monsoon and post-monsoon season when compared with pre-monsoon after adjusting with breeding sites and physicochemical parameters. The pH (≥8.2) and dissolved solid (≥0.39 ppm) alone showed significantly (P<0.05) decreased larval densities (lower risk of breeding), adjusted with breeding sites and season. As observed in univariate, Nitrite level increased (P=0.022) larval densities and thus was risk for breeding. Phytoplanktons did not show any significance with anopheline density in multivariable analysis.
Discussion
Comprehending the ecology of anopheline larvae is fundamental to envisage and implement appropriate vector control strategies, which can target larval habitats in particular through physical or biocontrol approaches. These can eventually lead to the development of novel biocontrol methods that could complement the current malaria vector control programme. Mosquitoes move between their blood-meal source and breeding site for oviposition. Heterogeneity in man-biting activity will also influence the spatial distribution of larval habitats. One of the possible strategies for malaria control is to identify local vector species and selectively target water bodies supporting their breeding15.
In the present study, anopheline density was recorded as the highest during monsoon in the first year (2018), and post-monsoon in the subsequent year (2019). The amount of rainfall in 2019 was twice as compared to what was recorded in the previous year, which could have created more temporary habitats, and resulted in increased larval density. Multivariable analysis indicated an increase in anopheles immature density in monsoon and post-monsoon season when compared with pre-monsoon, after matching breeding sites and physicochemical parameters. Studies have reported a positive influence of rainfall on larval abundance16. Koenraadt et al17 observed rainfall to be significantly correlated with the number of A. gambiae s.l. larval habitat.
In the current study, anophelines were found in 50 per cent of the habitats across all seasons. Almost 1126 water bodies were surveyed, grouped into 21 habitat types, of which 17 types were positive for anophelines. Anopheline breeding was found in five habitat types, viz. culvert, pond, pucca drain, rainwater pool and roadside ditch, during all the survey periods. Early instars were more numerous than late instars. In many studies, small habitats were more productive for anopheline mosquitoes during the rainy season18. During the monsoon season, the anopheline larvae from transient habitats get washed off in the overflowing water from the habitats, as these are mostly present on the water surface. The permanent habitats, viz. ponds, cement tanks and drains were found with anopheline breeding.
The overall suitability of temporary habitats was principally influenced by the temperature of water, vegetation cover and the presence of predators/competitors. Aquatic insects demonstrate a significant reduction in the mosquito population and could facilitate the integrated vector control programmes19. Five groups of insect predators were sampled in the present study, but their predatory effect could not be demonstrated. This could be because most of the habitats surveyed were temporary, and since predators require a longer time to establish themselves in habitats, the predatory effect could not be demonstrated in a short period. Debrah et al20 in Kenya also did not find any significant influence of predators on A. funestus larval density and ascribed to the presence of other prey in larval habitats, due to which the larval consumption-ability of predators was significantly reduced. However, in the present study, Gambusia fish was widely observed in permanent water bodies and was considered the probable reason for low malaria incidence21.
The survival and development of vector larvae depend on the different properties of the habitats, including the stability of habitats for longer periods22 and increased dispersal of early instars23. Chemical properties of larval habitats, related to vegetation, pH, optimum temperature, ammonia, nitrate and sulphate have significant effects on larval development and survival24. Univariate analysis of the data collected from the present study showed upper percentiles of pH (≥8.2), dissolved solids (≥0.39 ppm) and salinity (≥28 ppm) were significantly (P<0.05) associated with decreased anopheline density (less risk for breeding), when compared with lower percentiles, but higher nitrite level was observed to increase (P=0.035) larval densities. However, salinity did not show any influence on anophelines in the multivariable analysis. Musonda and Sichilima25 reported a positive weak linear correlation (r=0.114) between pH and larval abundance in the breeding sites surveyed in Kapiri Mposhi. Dejenie et al26 in Ethiopia showed pH to be associated with increased anopheline larval densities in temporal water ponds, while a significant linear relationship existed for temperature. Kengluecha et al27 reported the influence of varied parameters on anopheline species in multiple regression analysis. The abundance of anopheline mosquito larvae significantly correlated with abiotic parameters such as temperature, dissolved oxygen (DO) and turbidity but not with TDS, salinity, hardness conductivity, and pH28. This finding is in contrary to that by Olayemi et al29 who reported a strong correlation between anopheline larval abundance with temperature, TDS, DO, transparency, conductivity, nutrient level, salinity and pH. Gunathilaka et al30 from Sri Lanka, reported A. culicifacies and other potential malaria vectors breeding in drains containing waste water.
In the present study, few planktons were independently associated (P<0.05) with increased anopheline densities but did not show any significance in multivariable analysis. Most of the phytoplanktons are ideal food for mosquito larvae. Certain zooplanktons (copepods31, daphnia) are predators for mosquito larvae, but in the present study, this relationship was not investigated. The environmental parameters, viz., precipitation, air temperature and humidity, were not estimated and analyzed with other parameters. This is a limitation of this study, as including these factors could have provided a holistic representation on the factors influencing the abundance of anopheline immatures. Habitats producing high adult vectors may eventually contribute to malaria transmission, and the ability to identify these habitats is crucial in implementing targeted control32.
The findings of this present study showed that nitrite levels significantly enhanced larval densities posing a risk for anopheline breeding in this area. Thus by estimating nitrite levels in similar eco-geographical locations can help predict the vulnerability of an area for anopheline abundance. Second, anopheline immature density significantly increased in monsoon and post-monsoon season, and hence, implementing larviciding before monsoon season will be more effective in reducing anopheline density and thus, future transmission.
Financial support & sponsorship: The study was funded by the extramural grant from Department of Science & Technology, MoHFW, GoI [DST/CCP/NHH/116/2017(G)].
Conflicts of Interest: None.
References
- Global technical strategy for malaria 2016-2030. Available from: https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf
- National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. National framework for malaria elimination in India (2016–2030). Delhi: NVBDC, MoHFW, GoI; 2016.
- Altered environment and risk of malaria outbreak in South Andaman, Andaman &Nicobar Islands, India affected by tsunami disaster. Malar J. 2005;4:32.
- [Google Scholar]
- Tsunami, post-tsunami malaria situation in Nancowry group of islands, Nicobar district, Andaman and Nicobar Islands. Indian J Med Res. 2011;133:76-82.
- [Google Scholar]
- Malaria in the Andaman and Nicobar Islands:Challenges and opportunities for elimination. Asian Pac J Trop Dis. 2015;5:837-40.
- [Google Scholar]
- Cytogenetic characterization of Anopheles sundaicus (Diptera:Culicidae) population from Car Nicobar Island, India. Ann Entomol Soc Am. 2004;97:171-6.
- [Google Scholar]
- Resting and biting habits of Anopheles sundaicus in Car Nicobar Island. Indian J Malariol. 1994;31:103-14.
- [Google Scholar]
- Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS One. 2011;6:e19473.
- [Google Scholar]
- Car Nicobar Tehsil population, caste, religion data - Nicobars district, Andaman and Nicobar. Available from: https://www.censusindia.co.in/subdistrict/car-nicobar-tehsil-nicobars-andaman-and-nicobar-5916
- Indian Council of Medical Research. Pictorial identification key for Indian anophelines 2005:40.
- [Google Scholar]
- Standard methods for the examination of water and wastewater. (18th ed). Washington, DC: APHA; 1998. p. :45-60.
- [Google Scholar]
- Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara river, Kenya and Tanzania. SpringerPlus. 2015;4:136.
- [Google Scholar]
- The unexpected importance of mosquito oviposition behaviour for malaria:Non-productive larval habitats can be sources for malaria transmission. Malar J. 2005;4:23.
- [Google Scholar]
- Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the MatapíRiver, Amapá, [corrected] Brazil. Med Vet Entomol. 2009;23:335-49.
- [Google Scholar]
- The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s and Anopheles arabiensis in a Kenyan village. Acta Trop. 2004;90:141-53.
- [Google Scholar]
- Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. J Vector Borne Dis. 2007;44:44-51.
- [Google Scholar]
- Larval ecology and bionomics of Anopheles funestus in highland and lowland sites in western Kenya. PLoS One. 2021;16:e0255321.
- [Google Scholar]
- Declining trend of malaria in Car Nicobar Island, inhabited by the Nicobarese tribe:Plausible factors. J Vector Borne Dis. 2015;52:178-81.
- [Google Scholar]
- Factors influencing differential larval habitat productivity of Anopheles gambiae complex mosquitoes in a western Kenyan village. J Vector Borne Dis. 2011;48:52-7.
- [Google Scholar]
- Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar J. 2009;8:62.
- [Google Scholar]
- Ammonium sulphate fertiliser increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Trop. 2004;89:187-92.
- [Google Scholar]
- The effects of pH and temperature parameters of water on abundance of Anopheles mosquito larvae in different breeding sites of Kapiri Mposhi District of Zambia. Acad J Entomol. 2019;12:14-21.
- [Google Scholar]
- Characterization of mosquito breeding sites in and in the vicinity of Tigray microdams. Ethiop J Health Sci. 2011;21:57-66.
- [Google Scholar]
- Water quality and breeding habitats of anopheline mosquito in northwestern Thailand. Southeast Asian J Trop Med Public Health. 2005;36:46-53.
- [Google Scholar]
- Occurrence of major and potential malaria vector immature stages in different breeding habitats and associated biotic and abiotic characters in the district of Trincomalee Sri Lanka. J Vector Borne Dis. 2020;57:85-95.
- [Google Scholar]
- Distribution of mosquito larvae in relation to physico-chemical characteristics of breeding habitats in Minna, north central Nigeria. Rev Infect. 2010;1:49-53.
- [Google Scholar]
- Anopheles culicifacies breeding in polluted water bodies in Trincomalee district of Sri Lanka. Malar J. 2013;12:285.
- [Google Scholar]
- Use of cyclopoid copepods for control of Anopheles (Diptera:Culicidae) mosquito larvae to prevent re-emergence of malaria in Sri Lanka. J Vector Borne Dis. 2019;56:200-6.
- [Google Scholar]
- Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546-52.
- [Google Scholar]






