Translate this page into:
Carriage of blaNDM-1 in Pseudomonas aeruginosa through multiple Inc type plasmids in a tertiary referral hospital of northeast India
* For correspondence: ab0404@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Pseudomonas aeruginosa is known to be a predominant opportunistic pathogen and also a frequent cause of nosocomial infection in patients with compromised immune system. Treatment option becomes complicated when this type of organism harbour resistance determinants such as New Delhi metallo-β-lactamase-1 (NDM-1). The genetic vehicles carrying this gene are often responsible for their horizontal spread, dissemination and maintenance within a broad host range1. Knowledge about transmission dynamics of blaNDM-1 is a key to succeed in the effort of infection control and slowing down the spread of multidrug resistance. This study was undertaken to characterize blaNDM-1 in clinical isolates of P. aeruginosa, their transmission dynamics and plasmid Inc types responsible for their horizontal transfer in a tertiary referral hospital of northeast India.
The samples for the present study were collected from the patients who were admitted or attended outpatient department of Silchar Medical College and Hospital, Silchar, Assam, India from October 2012 to September 2013. The protocol was approved by Institutional Research and Ethical Committee. During this period, a total of 290 consecutive non-duplicate clinical isolates of P. aeruginosa were collected, of which 88 isolates were found to be non-susceptible to carbapenem by minimum inhibitory concentration (MIC). Isolates with MIC above 4, 1, 8 μg/ml for imipenem, meropenem, and ertapenem, respectively were selected as per CLSI (Clinical Laboratory Standards Institute) guidelines2 and were subjected to modified Hodge test for detection of carbapenemase production3 and further confirmed for the presence of metallo-β-lactamase by imipenem-EDTA disc diffusion test4. PCR assay was performed to characterize the blaNDM gene as well as other metallo-β-lactamase genes blaVIM, blaIMP, blaSIM and blaSMB4567, and amplified products were sequenced to confirm the presence of resistant genes. The linkage of blaNDM-1 with insertion sequence ISAba125 was determined by using forward primer of ISAba125 (5/-GAAACTGTCGCACCTCAT GTTTG-3/) and reverse primer of blaNDM-1 (5/ -GTAGTGCTCAGTGTCGGCAT-3/)8. The class of integron carried out by blaNDM-1 was determined by integrase gene PCR9. blaNDM-1 positive bacterial isolates were cultured in Luria-Bertani (LB) broth (Hi-Media, Mumbai, India) containing 0.25 μg/ml of imipenem. After overnight incubation, plasmids were extracted by QIAprep Spin Miniprep Kit (Qiagen, Germany). Plasmids of blaNDM-1 were subjected to transformation by heat shock method10 using Escherichia coli JM107 as recipient. Transformants were selected on LB agar with 0.25 μg/ml of imipenem, which were then confirmed both by phenotypic as well as by PCR analysis. The plasmids were classified by PCR based replicon typing, carried out for determining the incompatibility group type of the plasmid in all blaNDM-1 harbouring strains. A total of 18 different replicon types such as FIA, FIB, FIC, HI1, HI2, I1/Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F and FIIA were targeted as described previously11. The antibiotic susceptibility was done by Kirby-Bauer disc-diffusion method10 against antibiotics viz. piperacillin-tazobactam (100/10 μg), co-trimoxazole (25 μg), amikacin (30 μg), gentamicin (10 μg), ciprofloxacin (5 μg), polymixin B (300 units), netilmicin (30 μg), carbenicillin (100 μg) and faropenem (5 μg) (Hi-Media, Mumbai, India). MIC was performed by agar dilution method against imipenem (MSD, India), ertapenem (MSD, India), meropenem (Lupin, India) and the results were interpreted as per CLSI guidelines2. The clonal relatedness among the blaNDM-1 producing P. aeruginosa isolates was determined by repetitive extragenic palindromic (REP) PCR12.
Of the 88 consecutive non-repetitive carbapenems non-susceptible (showing MIC of imipenem >4 μg/ml, meropenem >1 μg/ml and ertapenem >8μg/ml) Pseudomonas isolates, 16 were found carrying blaNDM-1 as confirmed by sequencing. The different clinical features of these 16 blaNDM-1 harbouring P. aeruginosa are given in Table I. In contrast, no other metallo-β-lactamase genes (blaVIM, blaIMP, blaSIM and blaGIM) were detected in any of the isolates. These blaNDM-1 positive isolates showed resistance towards most of the antibiotics including piperacillin/tazobactam, co-trimoxazole, faropenem, aminoglycoside and quinolone group of drugs. Seven isolates were resistant to polymixin B as well. Minimum inhibitory concentration results showed high MIC breakpoint (Table II) against all the tested antibiotics cephalosporins, monobactam and carbapenems. Integrase PCR showed that all the blaNDM-1 positive isolates were harbouring class 1 integron while in four isolates both class 1 and class 2 integrons were observed (Table I). In 11 isolates, ISAba125 was found in the upstream region of blaNDM-1. Plasmid analysis showed that blaNDM-1 gene was located within the plasmid of approximately 25-40 Kb in size and in transformation assay, NDM-1 gene was found to be horizontally transferable and the resistance determinant was carried within diverse Inc group viz. FIA, FIC and K types. However, in six transformants, the plasmid was untypable (Table I). REP PCR results revealed that these MBL producing isolates were heterogenous (Figure) and no particular clonal strain was responsible for any epidemic spread. No association could be established between REP PCR patterns and plasmid incompatibility types.
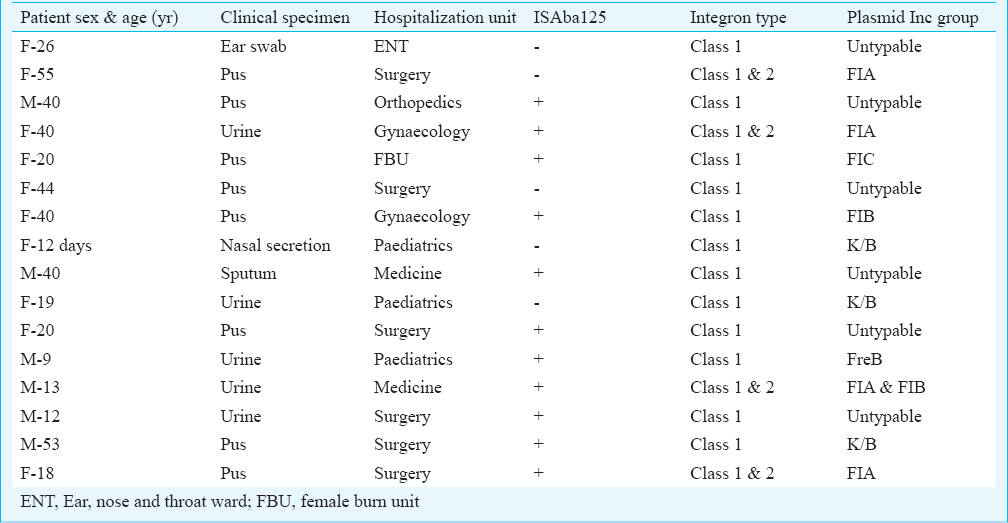

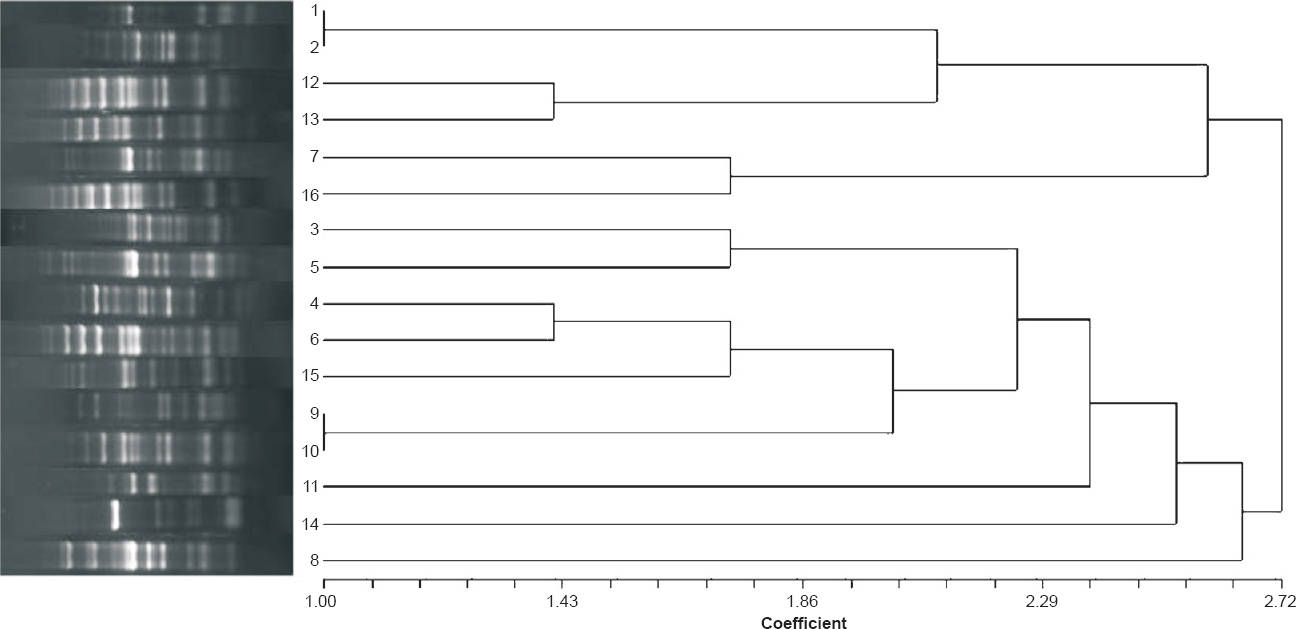
- Dendogram showing the clonal relatedness of 16 New Delhi metallo-β-lactamase producing Pseudomonas aeruginosa isolates based on REP-PCR band patterns.
After the first detection of NDM in Klebsiella pneumoniae it was also reported in Escherichia coli, Citrobacter fruendii, Enterobacter13 and in Acinetobacter spp14. Due to the rapid horizontal transmission this gene was also reported in P. aeruginosa from different parts of the world1516. In this study, we described the occurrence of blaNDM-1 gene among a number of MDR P. aeruginosa isolates indicating the spread of this resistant gene in the northeastern part of India. Toleman et al8 have described the association of insertion sequence ISAba125 in the upstream region of blaNDM-1 harbouring A. baumarnii and it is also established that whole ISAba125 or a truncated portion of it is excised along with the resistant gene when it is horizontally transmitted among the members of Enterobacteriaceae family. In our study also, similar kind of association of ISAba125 with blaNDM-1 in the upstream region was observed, which may be due to the horizontal transmission of this gene along with ISAba125 at interspecies level. Thus, this mobile genetic element may act as a unit of interspecies transmission in our setting. This horizontal transmission may also be facilitated due to the association with gene capture mechanism as it is known to be an important mean of spreading resistance in clinical isolates of Gram-negative bacilli17. An earlier study revealed that class 1 type of integron was mostly associated with clinical pathogens9, as also supported by our study where all the isolates were found carrying class 1 integron. It is observed that different incompatibility types of plasmid act as a genetic vehicle for transmission of this resistance gene, which reflects acquisition of blaNDM-1 harbouring plasmid from different sources. But in our study, in case of some transformants, we were unable to determine the incompatibility group of that plasmids. This may be due to the presence of any new incompatible type of plasmid that could not be detected by our target primers. Presence of new Inc type plasmids encoding blaNDM-1 corresponds their transplasmid expansion and diverse source of carriage. In an earlier study, it was reported that NDM-1 producing isolates were resistant to nearly all classes of antimicrobial agents except polymyxins and tigecycline13, but in our study the blaNDM-1 harbouring isolates showed resistance to all the antibiotics tested including polymixin B and tigecycline.
In conclusion, carriage of blaNDM-1 in different Inc type plasmids within a single hospital setting and their expansion may be a serious matter of concern in combating the carbapenem resistance.
Acknowledgment
The authors acknowledge the financial support provided by the Department of Biotechnology (DBT-NER twinning Scheme, BCIL/NER-BPCM/2011-1431) and Council of Scientific and Industrial Research, CSIR (Scheme no.37/1632/14/EMR-II), New Delhi, India.
Conflicts of Interest: None.
References
- Genetic features of blaNDM-1 positive Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:5403-7.
- [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI) In: Performance standards for antimicrobial susceptibility testing; 21st Informational Supplement. M100-S21. Wayne, USA: CLSI; 2011.
- [Google Scholar]
- Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother. 2010;65:249-51.
- [Google Scholar]
- Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046-54.
- [Google Scholar]
- Molecular characterization of metallo-β-lacatamase-producing Acinetobacter baumannii and Acinetbacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J Antimicrob Chemother. 2002;49:837-40.
- [Google Scholar]
- Novel acquired metallo-β-lactmase gene, blaSIM-1 in a class 1 integron from Acinetobacter baumanii clinical isolates from Korea. Antimicrob Agents Chemother. 2005;49:4485-91.
- [Google Scholar]
- SMB-1, a novel subclass B3 metallo- β-lactamase, associated with ISCR1 and a class 1 integron, from a carbapenem resistant Serratia marcescens clinical isolate. Antimicrob Agents Chemother. 2011;55:5143-9.
- [Google Scholar]
- blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56:2773-6.
- [Google Scholar]
- Vandenbroucke-grauls CMJE, Savelkoul PHM. Identification of epidemic strains of Acinetobacter baumannii by integrase gene PCR. J Clin Microbiol. 2001;39:8-13.
- [Google Scholar]
- Co-carriage of blaKPC-2 and blaNDM-1 in clinical isolation of Pseudomonas aeruginosa associated with hospital infections from India. PLoS One. 2015;10:e0145823.
- [Google Scholar]
- First description of KPC-2-producing Pseudomonas putida in Brazil. Antimicrob Agents Chemother. 2012;56:2205-6.
- [Google Scholar]
- Distribution of repetitve DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823-31.
- [Google Scholar]
- New Delhi metallo beta-Lactamase -1; Incidence and threats. Int J Biol Med Res. 2012;3:1870-4.
- [Google Scholar]
- NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J Antimicrob Chemother. 2011;66:1260-2.
- [Google Scholar]
- Detection of New Delhi metallo-beta lactamase-1 (NDM-1) carbapenemase in Pseudomonas aeruginosa in a single centre in southern India. Indian J Med Res. 2014;140:546-50.
- [Google Scholar]
- Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 2013;62:499-513.
- [Google Scholar]
- Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev. 2009;33:757-84.
- [Google Scholar]





