Translate this page into:
Carbon ions of different linear energy transfer (LET) values induce apoptosis & G2 cell cycle arrest in radio-resistant melanoma cells
* Reprint requests: Dr Aleksandra Ristić-Fira, Vinča Institute of Nuclear Sciences, University of Belgrade P.O. Box 522, 11001 Belgrade, Serbia e-mail: aristic@vin.bg.ac.rs
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The main goal when treating malignancies with radiation is to deprive tumour cells of their reproductive potential. One approach is to induce tumour cell apoptosis. This study was conducted to evaluate the ability of carbon ions (12C) to induce apoptosis and cell cycle arrest in human HTB140 melanoma cells.
Methods:
In this in vitro study, human melanoma HTB140 cells were irradiated with the 62 MeV/n carbon (12C) ion beam, having two different linear energy transfer (LET) values: 197 and 382 keV/μm. The dose range was 2 to 16 Gy. Cell viability was estimated by the sulforhodamine B assay seven days after irradiation. The cell cycle and apoptosis were evaluated 48 h after irradiation using flow cytometry. At the same time point, protein and gene expression of apoptotic regulators were estimated using the Western blot and q-PCR methods, respectively.
Results:
Cell viability experiments indicated strong anti-tumour effects of 12C ions. The analysis of cell cycle showed that 12C ions blocked HTB140 cells in G2 phase and induced the dose dependent increase of apoptosis. The maximum value of 21.8 per cent was attained after irradiation with LET of 197 keV/μm at the dose level of 16 Gy. Pro-apoptotic effects of 12C ions were confirmed by changes of key apoptotic molecules: the p53, Bax, Bcl-2, poly ADP ribose polymerase (PARP) as well as nuclear factor kappa B (NFκB). At the level of protein expression, the results indicated significant increases of p53, NFκB and Bax/Bcl-2 ratio and PARP cleavage. The Bax/Bcl-2 mRNA ratio was also increased, while no change was detected in the level of NFκB mRNA.
Interpretation & conclusions:
The present results indicated that anti-tumour effects of 12C ions in human melanoma HTB140 cells were accomplished through induction of the mitochondrial apoptotic pathway as well as G2 arrest.
Keywords
Apoptosis
carbon ions
cell cycle
LET
melanoma
Metastatic melanoma is one of the most aggressive cancers resistant to most modalities of cancer therapy. Therefore, the development of a therapeutic modality for the treatment of this disease is of great interest1. Carbon (12C) ions efficiently eliminate radio-resistant tumours2 and have a higher dose effect on malignant tumours compared to conventional radiotherapy3. Accelerated charged particles, including 12C ions, lose their energy through interactions with atoms along their path in irradiated tissue. These release a large amount of energy at the end of their range, therefore, forming the Bragg peak4. The energy loss per unit of length along the particle track, i.e. linear energy transfer (LET) is at the origin of induced biological effects. For 12C ions, the LET values of about 200 keV/μm produce the largest biological effectiveness4.
Several oncogenes and tumour suppressor genes play a pivotal role in modulating response of tumour cells to radiation. The product of the p53 tumour suppressor gene regulates genomic stability and cellular response to DNA damage, but can also affect the sensitivity of tumour cells to radiation-induced apoptosis5. Wild-type p53 protein appeared to be a positive regulator of Bax (Bcl-2 associated X protein) expression. In addition, p53 can transcriptionally downregulate the expression of Bcl-2. Thus, p53 expression may influence the ratio of Bax/Bcl-2 and subsequently determine the induction of apoptosis6. Constitutive activation of nuclear factor kappa B (NFκB) induce overexpression of its downstream targets such as Bcl-xL (B-cell lymphoma-extra large), Bcl-2, vascular endothelial growth factor and interleukin-8, which may in turn mediate resistance to apoptosis induced by chemotherapy and radiation7.
Understanding the specific biological effects of high LET radiation on cancer cells could provide valuable data for the design of novel therapeutic applications in the treatment of cancers which are resistant to conventional clinical approaches8. Available data suggests that heavy ions induce DNA double-strand breaks (DSBs) and have inhibitory effects on malignant cells9. However, their clinical application is limited by the side effects caused by secondary particles in the “tail part” of the Bragg curve, thus increasing dose. In contrast to other heavy ions, 12C ions are a promising tool for cancer radiotherapy and these are under investigation at various research and therapeutic centers. Radiation with 12C ions is efficient in elimination of hypoxic tumours, since oxygen enhancement ratio is reduced for this type of radiation91011.
This study was undertaken to estimate the effectiveness of 12C ions of different LET values, on human HTB140 melanoma cells which are highly radio-resistant to conventional radiation and protons1213. Since molecular mechanisms involved in 12C ions induced apoptosis and cell cycle arrest are important for the elimination of tumour cells, these were also investigated.
Material & Methods
Irradiations with 12C ions, viability assays and cell sample preparations for biological tests were carried out at National Institute for Nuclear Physics (INFN), Southern National Laboratory (LNS), Catania, Italy. Biological assays i.e., flow cytometry, Western blot analysis and real time-polymerase chain reaction (q-PCR) were performed in the Laboratory of Molecular Biology and Endocrinology, Vinča Institute of Nuclear Sciences, University of Belgrade, Belgrade, Serbia.
Cell culture: Human HTB140 melanoma cells were obtained from the American Tissue Culture Collection (ATCC, Rockville, MD, USA) and cultured in the RPMI 1640 cell culture medium supplemented with 10 per cent foetal bovine serum and penicillin/streptomycin (all from Sigma-Aldrich Chemie GmbH, Steinheim, Germany), in a humidified atmosphere of 5 per cent CO2 at 37 °C (Heraeus, Hanau, Germany).
Irradiation conditions: Exponentially growing HTB140 human melanoma cells were irradiated with the 62 MeV/n 12C ion beams produced by the superconducting cyclotron at INFN - LNS. Two positions for irradiation within the Bragg curve were obtained by interposing Perspex plates (Polymethyl methacrylate - PMMA) of different thickness between the final collimator and the cell monolayer. The Perspex plates were 7.7 and 7.9 mm thick, giving relative doses of 49 and 87.5 per cent, respectively. Cells were irradiated with single dose levels of 2, 4, 8, 12 and 16 gray (Gy), with the average dose rate of 11.45 ± 0.31 Gy/min. Reference dosimetry was performed by the plane-parallel Markus ionization chamber (Advanced Markus Chamber, 0.02 cm2, Type 34045, PTW Freiberg, Germany), calibrated according to the International Atomic Energy Agency Technical Report Series (IAEA-TRS) code of practice1415. For both positions the corresponding LET values were calculated by numerical simulations carried out with the GEANT4 (GeometryANd Tracking) code16 and were 197 and 382 keV/μm, respectively. Cell monolayers were fixed vertically in a special device for irradiation, facing the horizontal beam.
Since the range of the 62 MeV/n 12C ions is short, with a very narrow Bragg peak, the precision of positioning of the cell samples was checked by placing GafChromic HS films (ISP Technologies, Wayne, New Jersey, USA) before and after each irradiation. Irradiations were carried out in air at room temperature.
Cell viability assay: Sulforhodamine B (SRB) assay, based on the measurement of cellular protein content, is used for estimating cell viability17. Control and irradiated cells were seeded in 96-well plates at a density of 3000 cells per well. After an incubation period of seven days, cell monolayers were fixed with 10 per cent trichloroacetic acid and stained with 0.4 per cent SRB for 30 min. The excess dye is removed by washing with 1 per cent acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution for absorbance determination at 550 nm with a reference wavelength at 650 nm, using an ELISA plate reader (Wallac, Victor2 1420 Multilabel counter, PerkinElmer, Turku, Finland).
Flow cytometric analysis of cell cycle and apoptosis: For flow cytometric evaluation of the cell cycle status, control and irradiated cells were washed with phosphate-buffered saline (PBS), pH 7.4 and fixed overnight with cold 70 per cent ethanol, following incubation at 37 °C in PBS containing 500 μg/ml ribonuclease A (RNase A, Sigma-Aldrich Chemie GmbH) for 30 min and staining with 50 μg/ml propidium iodide (PI, Sigma-Aldrich Chemie GmbH). After incubation at room temperature for 30 min, cells were analyzed by flow cytometry (CyFlow, Partec, Münster, Germany) using FloMax software (Partec, Germany). For each sample, 10 000 cells were analyzed. Sub-G1 cells were scored as apoptotic population.
Western blot analysis: For the Western blot analysis cells were collected, washed with PBS, lysed in a cell lysis buffer and samples were prepared as previously described18. The membranes were incubated with primary antibodies against p53, Bcl-2, Bax, NFκB and PARP (poly ADP ribose polymerase) (Cell Signaling, Danvers, MA, USA) (1:1000 dilution) at 4°C overnight. The membranes were subjected to three 10 min washes with PBS with Tween 20 (PBST) and then incubated with horse radish peroxidase (HRP)-conjugated secondary antibody (HRP, Cell Signaling, 1:5000 dilution) at 4°C for 2 h. The proteins were visualized with an enhanced chemiluminescence (BM Chemiluminescence western blotting kit, Sigma-Aldrich Chemie GmbH) and exposed on to an X-ray film. The densitometry of the protein bands on the X-ray film was conducted using Image J Analysis PC software (National Institutes of Health, USA).
Real time polymerase chain reaction (q-PCR): Total RNA was isolated from the melanoma cells using Qiagen RNeasy Mini Kit (Qiagen Gmbh, Hilden, Germany) and quantified by measurement of the absorbance at 260 nm using NanoDrop 1000 (Thermo Scientific, Waltham, MA, USA). Purity was assessed from the 260/280 nm absorbance ratio. RNA was converted to cDNA using The RevertAid™ First strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania) according to manufacturer's instructions. Real-time PCR was done using the The SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 50 ng of cDNA and forward and reverse primer in final concentration of 50 nM in a 10 μl reaction volume. The PCR was performed using the following conditions - two min step at 50 °C, 10 min step at 95 °C, followed by 40 cycles of 95 °C for 15 sec, and finally one min at 60 °C. Expression of the housekeeping ribosomal protein L19 (RPL19) gene was used for normalization of bax, bcl-2 and NFκB to enable cross-comparisons among the samples. The sequences for primers were as follows192021: RPL19 - forward 5’TCGCCAATGCCAACTCTCGTC3’; reverse 5’AGCCCGGGAATGGACAGTCAC3’.
Bax - forward 5’GGGGACGAACTGGACAGTAA3’; reverse 5’CAGTTGAAGTTGCCGTCAGA3’.
Bcl-2 - forward 5’ATGTGTGTGGAGAGCGTCAA3’; reverse 5’ACAGTTCCACAAAGGCATCC3’.
NFκB - forward 5’AAACACTGTGAGGATGGGATCTG3’; reverse 5’CGAAGCCGACCACCATGT3’.
The relative changes in gene expression data were analyzed by the ΔΔCT method. The results were analyzed by Standard 7500 System (Applied Biosystem, USA).
Statistical analysis: The measurements were made in triplicate during each experiment, while each experiment was repeated three times, excluding the cell viability experiment which was performed in quadruplicate. The significance of the differences among original values of the experimental groups was assessed by the independent Student's t test. The mean value of control sample was arbitrarily calculated as 100 per cent, while mean values of irradiated samples were presented as percentage of control ± S.D. For cell cycle analysis number of cells in G1, S and G2 phases was presented as percentage of diploid cells. Number of diploid cells was arbitrarily calculated as 100 per cent.
Results
Since HTB140 cells are radio-resistant cells12, irradiation doses used in this study were adapted to the specificity of the cell line, thus being higher than those commonly used for the analysis of cell growth inhibition22 and ranged from 2 to 16 Gy. In our previous study23, the effects of 12C ions on HTB140 cells were analyzed for a broad spectrum of LET values (82-742 keV/μm), and the anti-tumour effect was highest after irradiation with about 200 keV/μm. Therefore, LET value of 197 keV/μm was chosen for the analysis of pro-apoptotic ability of carbon ions.
Effectiveness of carbon ions on inactivation of HTB140 melanoma cells: The efficiency of 12C ions on HTB140 melanoma cells was examined by measuring cell viability seven days after irradiation. This time point was reported to be optimal for the evaluation of radiation effects on HTB140 cells24. Percentage of viable cells varied from 31.6 to 39 per cent and from 37.1 to 56.6 per cent compared to control for LET values of 197 and 382 keV/μm, respectively. These results indicated that the viability of HTB140 melanoma cells was significantly suppressed by 12C ions (P<0.001 for all irradiated samples), with more prominent inhibitory effects observed after irradiation with 12C ions of 197 keV/μm (Fig. 1).
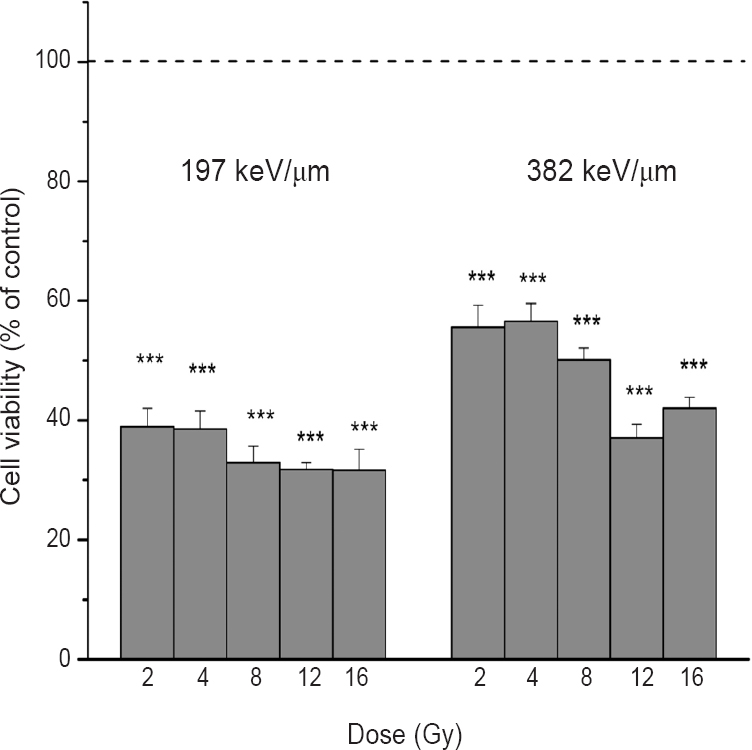
- Viability of HTB140 cells obtained by Sulforhodamine B (SRB) assay, seven days after irradiation with 12C ions of 197 and 382 keV/μm. Applied irradiation doses were from 2 to 16 Gy. Mean values obtained from four experiments are presented as percentage of control ± SD. Control value was set as 100 per cent. ***P<0.001 compared to control.
Cell cycle distribution: In the control samples, 17 per cent of cells were detected in G2 phase. The increase in the percentage of G2 phase cells was obtained in irradiated samples ranging from 23 to 29 per cent for 12C ions of 197 keV/μm and from 21.8 to 26.2 per cent for 12C ions of 382 keV/μm (Fig. 2). The results showed that the population of cells in G2 phase increased in all irradiated samples, with more pronounced changes detected after irradiation with the LET of 197 keV/μm.
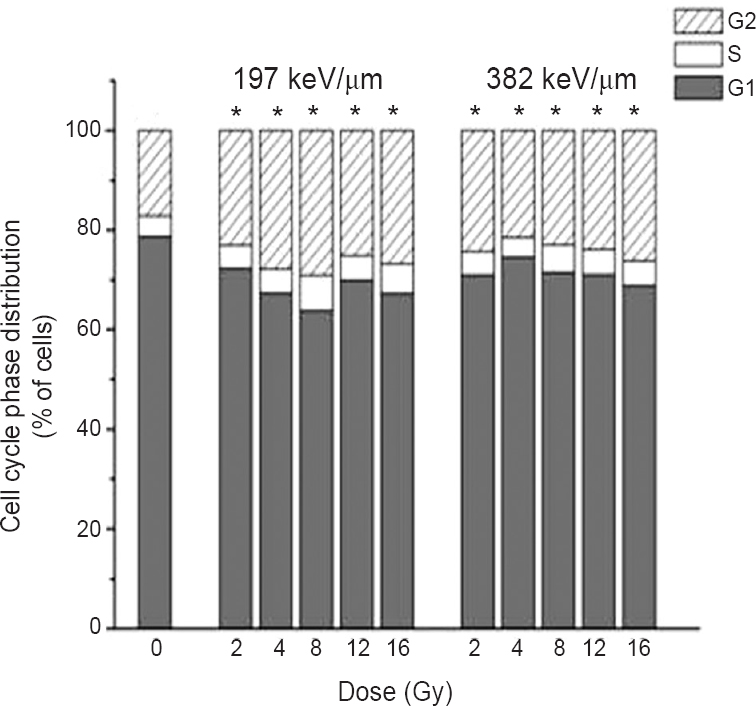
- Cell cycle analysis of HTB140 cells estimated by flow cytometry, 48 h after irradiation with 12C ions of 197 and 382 keV/μm. Applied irradiation doses were from 2 to 16 Gy. The percentage of cells in G1, S and G2 phases was obtained with the FloMax software. Mean values obtained from three experiments are presented. *P<0.05; G2 phase at different irradiation doses compared to control.
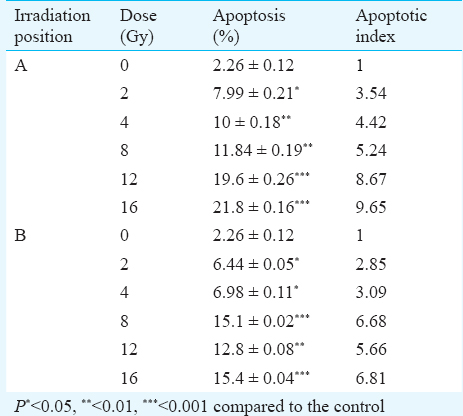
Analysis of apoptotic signaling pathway induced by carbon ions: Nuclear staining with PI was performed to detect apoptosis in irradiated cells. The percentage of apoptotic cells and corresponding apoptotic indexes of HTB140 cells were evaluated 48 h after irradiation. Cells exposed to 12C ions exhibited a significant number of cells in sub-G1 phase. Dose dependent increase in the level of apoptosis was observed in both irradiation positions. The highest values obtained after irradiation with 16 Gy were 21.8 ± 0.16 and 15.4 ± 0.04 for 12C ions of 197 and 382 keV/μm, respectively (Table).
A remarkable rise of p53 protein was detected after irradiation with 12C ions of 197 keV/μm, reaching an increase of 327 to 737 per cent when compared to the control (P<0.05 for 2 Gy; P<0.01 for 4 and 8 Gy; P<0.001 for 12 and 16 Gy) (Fig. 3A). Irradiations with 12C ions of 382 keV/μm induced the rise of p53 when doses higher than 4 Gy were applied. The obtained rise was from 137 to 295 per cent, when compared to the control level (P<0.05 for 4 Gy; P<0.01 for 8, 12 and 16 Gy) (Fig. 3B).
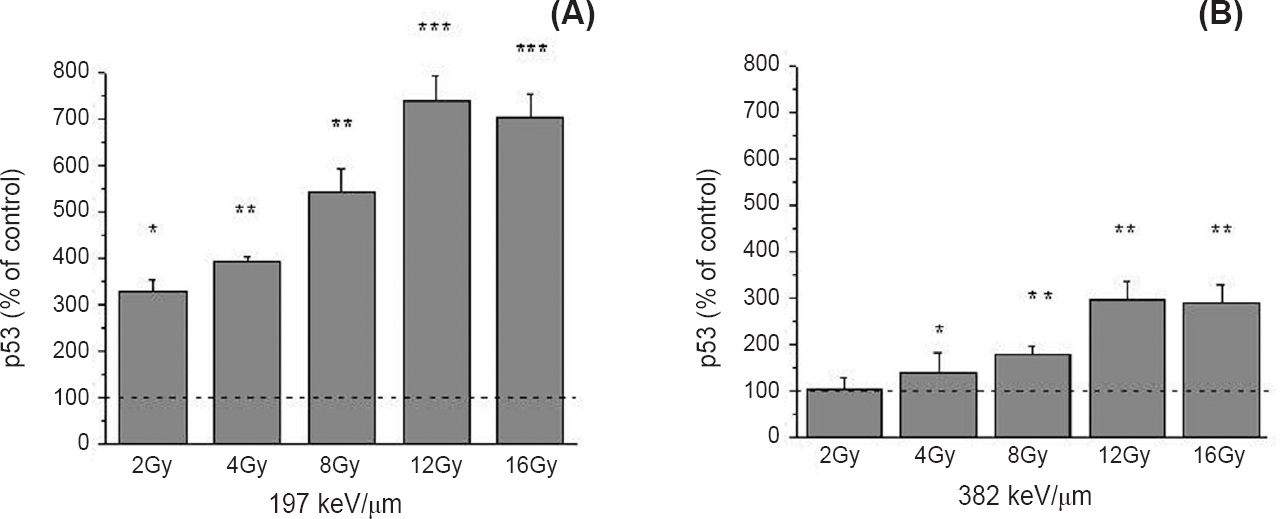
- Level of the p53 protein 48 h after irradiation with 12C ions of 197 (A) and 382 keV/μm (B), obtained by Western blot. β - Actin was used as internal loading control. Irradiation doses were from 2 to 16 Gy. Mean values obtained from three experiments are presented as percentage of control ± SD. Control value was set as 100 per cent. *P<0.05, **<0.01, ***<0.001 compared to the control.
Fig. 4A shows significant dose dependent increase of Bax/Bcl-2 ratio, after irradiation with 12C ions of 197 keV/μm. The increase was from 1.24 to 3.38 when compared to the control value which was set to 1 (P<0.05 for 2 Gy; P<0.01 for 4 Gy; P<0.001 for 8-16 Gy). The ratio of Bax/Bcl-2 was increased after irradiation with 12C ions of 382 keV/μm, for the doses of 8, 12 and 16 Gy and ranged from 1.65 to 2.52 (Fig. 4B).
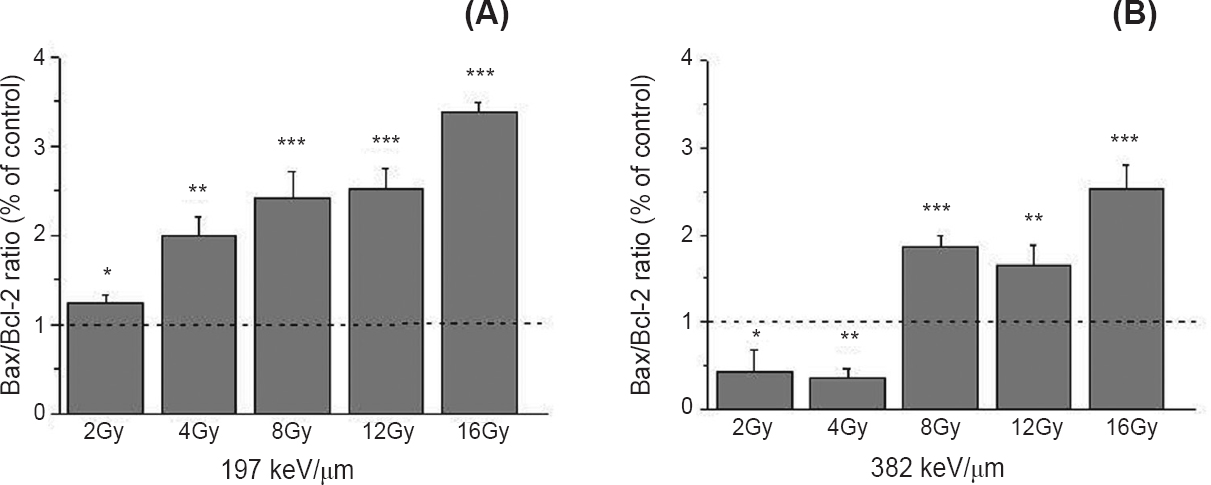
- Bax/Bcl-2 ratio 48 h after irradiation of HTB140 cells with 12C ions of 197 (A) and 382 keV/μm (B), obtained by Western blot. Irradiation doses were from 2 to 16 Gy. β-Actin was used as internal loading control. Mean values, obtained from three experiments, of Bax/Bcl-2 ratios for irradiated samples were normalized to Bax/Bcl-2 ratio for control sample which was set as 1. P*<0.05, **<0.01, ***<0.001 compared to the control.
Western blot experiments demonstrated an increase in the cleavage of PARP in all irradiated cells. Greater dose-dependent pattern of PARP cleavage was detected following the exposure to 382 keV/μm 12C ions. In our experiments, expression of the p65 subunit of NFκB was detected by Western blot. The obtained results indicated its increase in all irradiated samples. Irradiation with 12C ions of 197 keV/μm led to an increase of 246-410 per cent (P<0.05 for 2-4 Gy; P<0.01 for 8-16 Gy), while 12C ions of 382 keV/μm triggered the increase of 277-377 per cent compared to control (P<0.001) (Fig. 5).
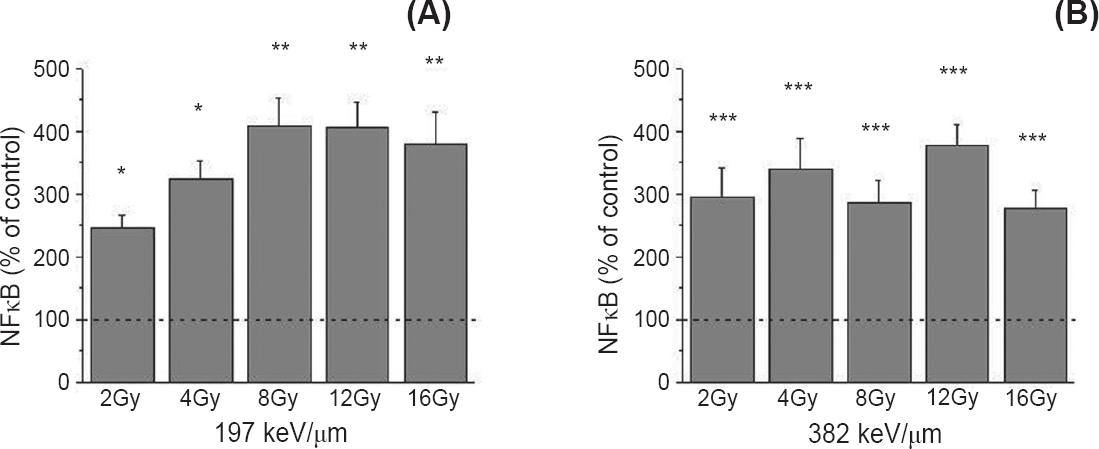
- Level of the nuclear factor kappa B (NFκB) protein 48 h after irradiation with 12C ions of 197 (A) and 382 keV/μm (B), obtained by Western blot. β-Actin was used as internal loading control. Irradiation doses were from 2 to 16 Gy. Mean values obtained from three experiments are presented as percentage of control ± SD. Control value was set as 100 per cent. P*<0.05, **<0.01, ***<0.001 compared to the control.
Except changes in expression on protein level, pro-apoptotic effects of 12C ions were also verified on the specific gene expression level. The Bax/Bcl-2 mRNA ratio increased in all irradiated samples. The more pronounced changes were detected for higher doses of 12C ions having LET of 197 keV/μm, reaching the value of 3.5 after irradiation with 16 Gy (Fig. 6). Unlike the expression of the NFκB protein, expression of the NFκB mRNA did not show significant change after irradiation with 12C ions having the two defined LET values (Fig. 7).
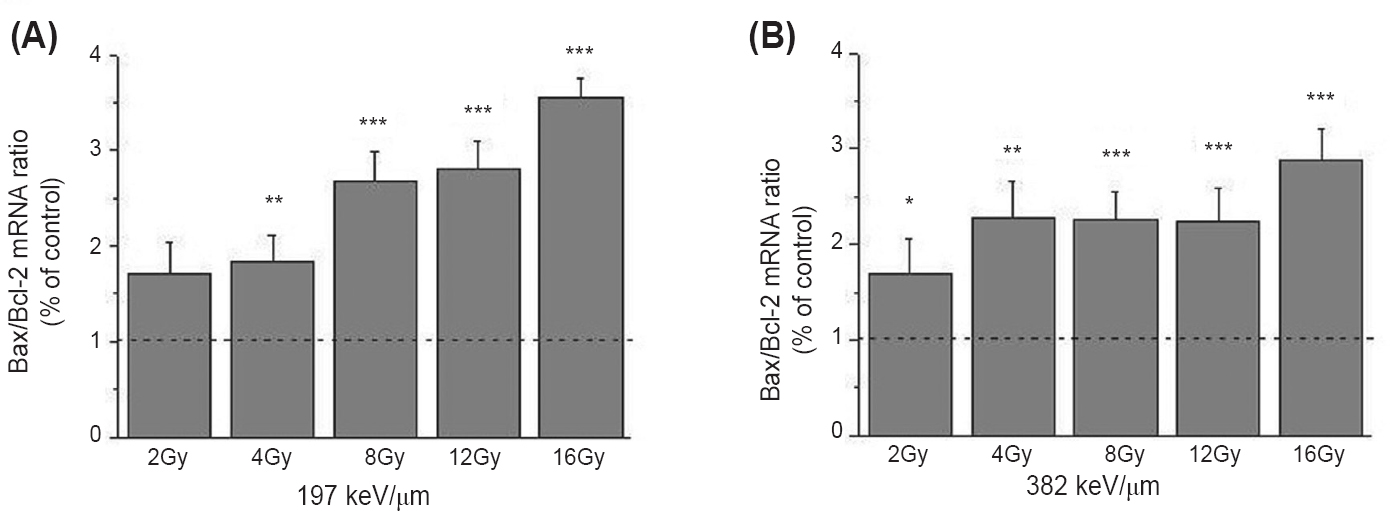
- The ratio of Bax/Bcl-2 mRNA in HTB140 human melanoma cells 48 h after irradiation with 12C ions of 197 (A) and 382 keV/μm (B). Irradiation doses were 2 - 16 Gy. RPL19 was used as internal control. Mean±SD values, obtained from three experiments, of Bax/Bcl-2 ratios for irradiated samples were normalized to Bax/Bcl-2 ratio for control sample which was set as 1. P*<0.05, **<0.01, ***<0.001 compared to the control.
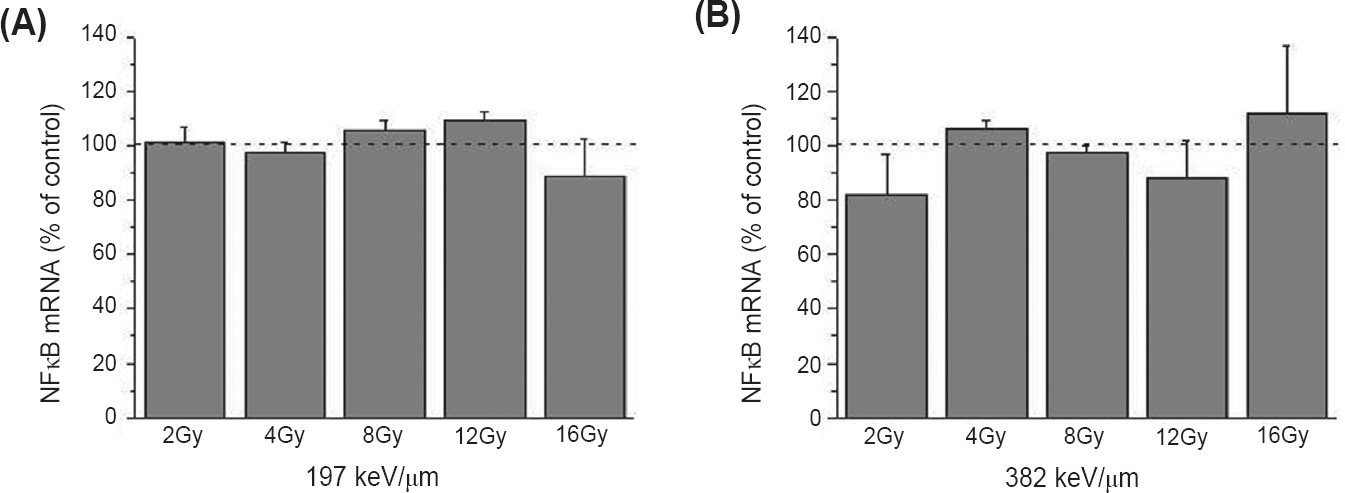
- Expression of NFκB mRNA in HTB140 human melanoma cells 48 h after irradiation with 12C ions of 197 (A) and 382 keV/μm (B). Irradiation doses were 2 - 16 Gy. RPL19 was used as internal control. Mean ± SD values obtained from three experiments are presented as percentage of control value, which was set as 100 per cent. P*<0.05, **<0.01, ***<0.001 compared to the control.
Discussion
Irradiation with protons and 12C ions has been shown to be effective in eliminating tumours resistant to conventional radiotherapy25. Carbon ions show LET-dependent anti-tumour effects with the maximum being at 200 keV/μm4. Further increase of LET value does not lead to better anti-tumour effects26. This could be attributed to the overkill effect27.
In this study the effects of 12C ions on cell viability were estimated seven days after irradiation. The data demonstrated that the 12C ions significantly decreased viability of HTB140 melanoma cells. Decrease of viability could be caused by the induction of apoptosis or cell cycle arrest. For the majority of cells, especially radio-resistant ones, apoptosis occurs after one or more cell divisions. Usually the maximum number of apoptotic cells in in vitro conditions can be found 48 h after irradiation28.
Estimation of cell cycle distribution 48 h after irradiation of HTB140 indicated significant increase of G2 fraction as compared to non-irradiated cells. The results are consistent with other studies suggesting that the 12C ion irradiated cells undergo prolonged G2 arrest. The longer G2 arrest may be attributed to the higher level of unrepaired DNA damages. These findings are in agreement with our previous results signifying induction of DSBs in HTB140 cells after exposure to 12C ions18.
Carbon ions are known to have lethal effects on radio-resistant tumours and can induce apoptosis effectively regardless of p53 gene status. In addition, even in cells having the p53, exposure to high LET radiation results in cell death with a higher apoptotic ratio than in cells exposed to conventional radiation5. Results obtained in this study indicated that the induction of apoptosis was caused by the increase of the p53 tumour suppressor protein. Similar results were reported for radio-resistant glioblastoma cells irradiated by 12C ions22. Anti-apoptotic factor Bcl-2, which negatively regulates apoptotic suicide machinery, is overexpressed in many cancers, including melanoma26. The impact of 12C ions on Bcl- 2 overexpressing tumours is yet to be characterized. Western blot analysis demonstrated expression of Bcl- 2 by HTB140 cells. This might be one of the reasons of their high radio-resistance. To determine whether apoptosis related molecules contribute to the inhibitory effects of 12C ions on HTB140 cells, the relative Bcl-2 and Bax expression levels were assessed. It was noted that 12C ions increased the ratio of Bax and Bcl-2 in a dose and LET-dependent manner, both on protein and gene expression levels. These findings imply that the treatments used are able to induce apoptosis through shifting the Bax/Bcl-2 ratio in favour of apoptosis.
Apoptotic cell death is confirmed by the cleavage of PARP protein which is apparent in samples irradiated with higher doses of 12C ions of 197 keV/μm. The detected percentage of apoptosis was in the range that was already reported for other radio-resistant cells irradiated with 12C ions28. Except for its important role in apoptosis, PARP protein has an influence on NFκB activation. However, its role in this process is still controversial29. This transcription factor is upregulated in different cancers, including melanoma30, and is involved in the regulation of cell survival, apoptosis, angiogenesis and tumour cell invasion30. Results obtained showed that NFκB transcription factor was expressed in HTB140 melanoma cells, with the increased expression level after irradiation with 12C ions. This increase was dose and LET-dependent. However, the level of NFκB mRNA was not significantly changed after the same treatment. These findings are in agreement with the data that suggest the involvement of NFκB in the modulation of survival response of cells irradiated by 12C ions31.
In summary, our results indicated significant anti-tumour effects of 12C ions. The intensity of the analyzed effects was LET-dependent. Carbon ions having LET of 382 keV/μm did not induce a higher level of HTB140 cell growth inhibition than that of 197 keV/μm. The results also suggest that 12C ions are promising for clinical use as an anti-cancer agent in treating radio-resistant tumours. However, molecular mechanisms involved in anti-tumour effects of these ions require further investigations.
Acknowledgment
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007- 2013 under Grant Agreement n° 262010 - ENSAR. This work was also financially supported by the Ministry of Education, Science and Technological Development of Serbia (grants 173046 and 171019) and by Istituto Nazionale di Fisica Nucleare, Laboratori Nazionali del Sud, Italy.
Conflicts of Interest: None.
References
- Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal. 2013;25:308-18.
- [Google Scholar]
- Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2009;75:1105-10.
- [Google Scholar]
- Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51-7.
- [Google Scholar]
- High LET radiation enhances apoptosis in mutated p53 cancer cells through Caspase-9 activation. Cancer Sci. 2008;99:1455-60.
- [Google Scholar]
- Radiation-induced apoptosis in human myeloma cell line increases BCL-2/BAX dimer formation and does not result in BAX/BAX homodimerization. Int J Cancer. 2001;92:651-60.
- [Google Scholar]
- Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther. 2004;3:985-92.
- [Google Scholar]
- High LET heavy ion radiation induces p53-independent apoptosis. J Radiat Res. 2009;50:37-42.
- [Google Scholar]
- DNA fragmentation induced in human fibroblasts by accelerated (56)fe ions of differing energies. Radiat Res. 2006;165:713-20.
- [Google Scholar]
- Optimizing normal tissue sparing in ion therapy using calculated isoeffective dose for ion selection. Int J Radiat Oncol Biol Phys. 2012;83:756-62.
- [Google Scholar]
- Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res. 2007;48(Suppl A):A1-13.
- [Google Scholar]
- Radiobiological analysis of human melanoma cells on the 62 MeV CATANA proton beam. Int J Radiat Biol. 2006;82:251-65.
- [Google Scholar]
- Response of a radioresistant human melanoma cell line along the proton spread-out Bragg peak. Int J Radiat Biol. 2010;86:742-51.
- [Google Scholar]
- International Atomic Energy Agency (IAEA). Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose to water. In: IAEA Technical Report Series. Vol 398. Vienna: IAEA; 2000. p. :135-50.
- [Google Scholar]
- A 62-MeV proton beam for the treatment of ocular melanoma at Laboratori Nazionali del Sud-INFN. IEEE Trans Nucl Sci. 2004;51:860-5.
- [Google Scholar]
- New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107-12.
- [Google Scholar]
- Carbon ions induce DNA double strand breaks and apoptosis in HTB140 melanoma cells. Nucl Technol Radiat. 2013;28:195-203.
- [Google Scholar]
- Characterization of the transporter B0AT3 (Slc6a17) in the rodent central nervous system. BMC Neurosci. 2013;14:54.
- [Google Scholar]
- Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncol Lett. 2012;4:1033-6.
- [Google Scholar]
- Quantitation of glucocorticoid receptor alpha and NF-κB pathway mRNA and its correlation with disease activity in rheumatoid arthritis patients. Genet Mol Res. 2010;9:2300-10.
- [Google Scholar]
- Irradiation with carbon ion beams induces apoptosis, autophagy, and cellular senescence in a human glioma-derived cell line. Int J Radiat Oncol Biol Phys. 2010;76:229-41.
- [Google Scholar]
- Melanoma cells along a carbon ion Bragg curve. LNS Activity Report 2010. Catania, Italy: National Institute for Nuclear Physics, Southern National Laboratory; 2012. p. :241-4.
- Effects of fotemustine or dacarbasine on a melanoma cell line pretreated with therapeutic proton irradiation. J Exp Clin Cancer Res. 2009;28:50.
- [Google Scholar]
- Apoptosis induced by heavy ion (carbon) irradiation of two human tumours with different radiosensitivities in vivo: relative biological effectiveness (RBE) of carbon beam. Anticancer Res. 1998;18:253-6.
- [Google Scholar]
- Energetic heavy ions overcome tumour radioresistance caused by overexpression of Bcl-2. Radiother Oncol. 2008;89:231-6.
- [Google Scholar]
- Exploration of “over kill effect” of high-LET Ar- and Fe-ions by evaluating the fraction of non-hit cell and interphase death. J Radiat Res. 2005;46:343-50.
- [Google Scholar]
- Reducing the radiation-induced G2 delay causes HeLa cells to undergo apoptosis instead of mitotic death. Int J Radiat Biol. 1996;69:575-84.
- [Google Scholar]
- Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene. 2009;28:832-42.
- [Google Scholar]
- Activation of the nuclear factor κB pathway by heavy ion beams of different linear energy transfer. Int J Radiat Biol. 2011;87:954-63.
- [Google Scholar]






