Translate this page into:
Burden of hepatitis B in asymptomatic blood donor population of India: A systematic review & meta-analysis
For correspondence: Dr Manisha Shrivastava, Department of Transfusion Medicine, ICMR - Bhopal Memorial Hospital and Research Centre Campus, Bhopal 462 038, Madhya Pradesh, India e-mail: manishasdr@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
India has been classified as an intermediate Hepatitis B Virus (HBV) endemic country, and the transmission is believed to mostly occur horizontally. However, community-based data on HBV prevalence among blood donors in India are limited. The burden of Hepatitis B Virus (HBV) is unknown in the asymptomatic blood donor population. We therefore conducted a meta-analysis to assess the prevalence of the HBV among the blood donor population in India.
Methods
We searched different databases for research articles on the prevalence of HBV in the blood donor population from India. Following the PRISMA guidelines, forty articles published between January 2013 and October 20, 2023, were selected for meta-analysis after removing duplicates and conducting a two-level screening process. Review Manager Version 5.3 (Rev Man 5.4) was used for statistical meta-analysis. The study has been registered with PROSPERO (number CRD42023487616).
Results
Forty articles were selected out of the 527 published manuscripts for meta-analysis, and a total of 22,22,736 blood donations were studied. Of these, 24,151 individuals (1.11%) were identified either as chronically infected with HBV or living with HBV infection. A pooled prevalence of approximately 1.11 per cent with a 95% confidence interval (CI) of (0.011; 0.0112) (common effect model) or 95% CI of (0.0079; 0.0116) (random effects model) was estimated. The included studies exhibited a high level of heterogeneity, probably due to different diagnostic approaches followed in different studies.
Interpretation & conclusions
The burden of hepatitis is profound, impacting public health, economies, and societies in India. The outcome of this study would help address such a burden and develop comprehensive strategies focused on prevention, early diagnosis, treatment, and necessary collaboration to achieve significant reductions in hepatitis-related morbidity and mortality.
Keywords
Blood donors
blood transfusion
hepatitis B virus
hepatitis C prevalence
disease burden
Hepatitis presents a significant burden in India, affecting millions of people and posing public health challenges across the country. Approximately 40 million people are chronically infected with hepatitis B virus (HBV), and an estimated 6-12 million with hepatitis C virus (HCV), in India1. HBV infection can lead to liver cirrhosis and hepatocellular carcinoma (HCC) if left untreated. Hepatitis viruses are mainly transmitted from mother to child during childbirth. Blood donors can be carriers of hepatitis viruses (especially hepatitis B and hepatitis C) if they have been exposed to these viruses through unsafe injection practices, unprotected sex, or other means of exposure to infected blood or bodily fluids. The prevalence of hepatitis viruses among blood donors can vary based on geographic location, socioeconomic factors, and prevalence rates of hepatitis in the general population. In India, where hepatitis is endemic in certain regions and populations, the risk among blood donors may be higher compared to countries with lower prevalence rates. Blood donation centres in India adhere to stringent screening protocols mandated by regulatory authorities to minimize the risk of transmitting hepatitis and other infectious diseases through blood transfusions. These protocols typically include, among other tests, screening for hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (anti-HCV). The national blood transfusion services and regulatory bodies in India play a crucial role in monitoring and regulating blood donation practices to ensure the safety of blood products. This includes implementing quality control measures and promoting voluntary blood donation, which is at lower risk of transmission of infections compared to the blood units obtained from the replacement donors.
According to a recent estimate, annually, 112 million blood donations occur across the globe, and blood donors constitute a critical resource in the healthcare landscape2. Ensuring the safety of donated blood by screening for hepatitis viruses is of paramount importance to prevent transmission to recipients and reduce the overall burden of hepatitis in the community. The National Viral Hepatitis Control Program (NVHCP) launched by the Government of India in 2018 addresses such key issues including the viral hepatitis prevention and control, and critical area of blood transfusion safety. Ensuring safe blood transfusion practices is essential in reducing the transmission of HBV and HCV. However, achieving zero risk of transfusion-transmitted hepatitis may not be fully attainable due to the inherent challenges. Therefore, continuous efforts to improve screening technologies, enhance blood banking infrastructure, educate donors, and implement stringent regulatory measures are crucial. Estimating the burden of HBV and HCV in a country at regular intervals is also essential. This research aimed to provide a systematic assessment of the prevalence of HBV in blood donors, quantify them through a meta-analysis, and provide a critical evaluation of the advantages and limitations of the different methodological approaches to address the disease burden in India.
Material & Methods
The research question guiding the present systematic review and meta-analysis was ‘What is the burden of hepatitis B in the asymptomatic blood donor population of India?’. We adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The search for relevant articles was conducted on PubMed, COCHRANE, SCOPUS, and Science Direct from January 2013 to October 20, 2023. Additionally, World Health Organization (WHO) databases were searched for related reports. Articles reporting the prevalence data of Hepatitis B in India were included. The selection process involved removal of duplicates and the screening of abstracts and titles to exclude unrelated articles. Table I provides an overview of the selection of studies.
| Database | Search |
|---|---|
| PubMed | ((“hepatitisb”[MeSHTerms]OR”hepatitisb”[AllFields])AND(“blooddonors”[MeSHTerms]OR(“blood”[AllFields]AND”donors”[AllFields])OR”blooddonors”[AllFields]OR(“blood”[AllFields]AND”donor”[AllFields])OR”blooddonor”[AllFields])AND(“india”[MeSHTerms]OR”india”[AllFields]OR”indias”[AllFields]OR”indias”[AllFields]))AND(1000/1/1:2023/10/20[pdat]) |
| COCHRANE | 3TrialsmatchinghepatitisBblooddonor IndiainTitleAbstractKeyword |
| SCOPUS | TITLE-ABS-KEY(hepatitisANDbANDblood ANDdonorANDIndia)AND(LIMIT-TO(DOCTYPE,”are”)) |
| Science Direct | Title, abstract, keywords: hepatitis B, blood donor, India |
Inclusion and exclusion criteria
This study focused on the burden of HBV in the blood donor population of India. Both nucleic acid amplification test (NAT) and ELISA-based (seroprevalence) methods were included for HBV screening. The following inclusion criteria were used: (a) studies published in last ten years (January 2013 to October 2023), (b) population as asymptomatic blood donors, (c) geographical location as India, and (d) full-text articles published in the English language.
The exclusion criteria included the studies focussing on other viruses, and different population (cornea donors, plasma exchange, liver disease, etc.). Additionally, extensive mutational molecular studies for HBV detection were also excluded. The articles were carefully screened for titles and abstracts, and irrelevant studies were excluded. If there was any uncertainty regarding assessing the suitability of a paper solely based on the abstract, we reviewed the full text. Manuscripts, without the availability of full text, could not be included.
Data collection
All the selected articles were compiled using Endnote software (version) and screened systematically. Search results were reviewed independently by two authors (AA and SM). To organize the information extracted from each reviewed study, a data extraction table with author name, year of publication, study region, study design, study duration, population size, prevalence of HBV, and the method employed for diagnosis, etc. was created (Table II)3-42.
| Author, yr | Region | Study type | Method applied | HBV | Sample size | Duration | TTI* | State/UT | Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al3, 2013 | New Delhi | Retrospective | ELISA | 779 | 73898 | 27 | 1104 | UT | 0.01 |
| Agarwal4, 2014 | Dehradun | Retrospective | ELISA | 225 | 48386 | 24 | 416 | Uttarakhand | 0.01 |
| Arcot et al5, 2022 | New Delhi | Prospective Study | CLIA & ELISA | 59 | 4843 | 14 | 148 | UT | 0.01 |
| Badhan & Cheema6, 2023 | Ambala | Retrospective | ELISA | 51 | 6505 | 14 | 160 | Haryana | 0.01 |
| Bhasker & Aluri7, 2021 | Hyderabad | Retrospective | ELISA | 106 | 17025 | 48 | 183 | Telangana | 0.01 |
| Bhaumik & Debnath8, 2014 | Tripura | Retrospective | ELISA | 2136 | 177302 | 96 | 2497 | Tripura | 0.01 |
| Chaithanya & Shivakumar9, 2020 | Mandya | Retrospective | ID NAT | 521 | 52417 | 72 | 667 | Karnataka | 0.01 |
| Chandra et al10, 2014 | Lucknow | Retrospective | ELISA | 3058 | 192348 | 48 | 4294 | Uttar Pradesh | 0.01 |
| Chaurasia et al11, 2016 | New Delhi | Retrospective | NAT | 124 | 10015 | 10 | 153 | UT | 0.01 |
| Chigurupati & Murthy12, 2015 | Rajahmundry | Retrospective | NAT | 330 | 15000 | 12 | 525 | Andhra Pradesh | 0.02 |
| Dara et al13, 2017 | Gurgaon | Cross Sectional Study | NAT | 747 | 106238 | 60 | 1776 | Haryana | 0.01 |
| Datta et al14, 2019 | New Delhi | Retrospective | NAT | 808 | 101411 | 72 | 1061 | UT | 0.01 |
| Dhiman et al15, 2019 | New Delhi | Retrospective | ELISA | 685 | 53740 | 36 | 1061 | UT | 0.01 |
| Hulinaykar & Krishna16, 2016 | Tumkur | Retrospective | ELISA | 17 | 3378 | 24 | 28 | Karnataka | 0.01 |
| Jadeja et al17, 2014 | Udaipur | Retrospective | ELISA | 75 | 5670 | 60 | 150 | Rajasthan | 0.01 |
| Karmakar et al18, 2014 | Kolkata | Retrospective | ELISA | 679 | 24320 | 12 | 679 | West Bengal | 0.02 |
| Kavitha et al19, 2023 | Egmore | Retrospective | ELISA | 215 | 23303 | 60 | 268 | Tamil Nadu | 0.01 |
| Keechilot et al20, 2016 | Cochin | Cross Sectional Study | NAT | 46 | 24338 | 18 | 124 | Kerala | 0.01 |
| Kumar et al21, 2015 | Ludhiana | Retrospective | NAT | 221 | 32978 | 12 | 589 | Punjab | 0.01 |
| Kumari22, 2020 | Patiala | Retrospective | ELISA | 151 | 15056 | 36 | 382 | Punjab | 0.01 |
| Makroo et al23, 2015 | New Delhi | Retrospective | ELISA | 2138 | 180477 | 96 | 3789 | UT | 0.01 |
| Mandal & Mondal24, 2016 | Darjeeling | Retrospective | ELISA | 353 | 28364 | 36 | 832 | West Bengal | 0.01 |
| Mukherjee et al25, 2014 | Kolkata | Retrospective | NAT | 206 | 27246 | 48 | 434 | West Bengal | 0.01 |
| Narayanasamy et al26, 2015 | Chennai | Retrospective | ELISA | 1494 | 152466 | 60 | 1571 | Tamil Nadu | 0.01 |
| Pandey et al27, 2015 | Noida | Prospective Study | NAT | 427 | 48441 | 27 | 1000 | Uttar Pradesh | 0.01 |
| Parveen et al28, 2015 | Srinagar | Retrospective | ELISA | 197 | 40616 | 130 | 283 | UT | 0.01 |
| Prasad et al29, 2021 | Burla | Retrospective | NAT | 88 | 83820 | 53 | 349 | Odisha | 0.001 |
| Ranganathan et al30, 2021 | Hyderabad | Retrospective | NAT | 699 | 80809 | 90 | 871 | Telangana | 0.01 |
| Rawat et al31, 2017 | New Delhi | Retrospective | ELISA | 3569 | 220482 | 72 | 9622 | UT | 0.01 |
| Saini et al32, 2017 | Indore | Observational Cross-Sectional Study | ELISA | 579 | 58998 | 60 | 674 | Madhya Pradesh | 0.01 |
| Sehgal et al33, 2017 | Andaman Nicobar Islands | Retrospective | ELISA | 128 | 12118 | 36 | 265 | UT | 0.01 |
| Shah et al34, 2013 | Ahmedabad | Retrospective | ELISA | 907 | 92778 | 91 | 1377 | Gujarat | 0.01 |
| Shrivastava et al35, 2023 | Bhopal | Retrospective | ELISA | 1061 | 57942 | 192 | 1614 | Madhya Pradesh | 0.01 |
| Sundaramoorthy et al36, 2018 | Madurai | Retrospective | CLIA | 38 | 9027 | 24 | 102 | Tamil Nadu | 0.01 |
| Thakur et al37, 2023 | New Delhi | Retrospective | ELISA | 188 | 16777 | 36 | 345 | UT | 0.01 |
| Tiwari et al38, 2018 | Chandigarh | Prospective Observational Study | CLIA & NAT | 318 | 52427 | 24 | 481 | UT | 0.01 |
| Tiwari et al39, 2020 | Gurgaon | Retrospective | ID-NAT | 55 | 10164 | 10 | 223 | Haryana | 0.01 |
| Tyagi & Tyagi40, 2013 | Noida | Retrospective | ELISA | 95 | 6000 | 48 | 209 | Uttar Pradesh | 0.01 |
| Varma et al41, 2019 | Madhya Pradesh | Retrospective | ELISA | 590 | 45704 | 48 | 658 | Madhya Pradesh | 0.01 |
| Yashovardhan et al42, 2015 | Tirupati | Retrospective | ELISA | 255 | 9909 | 18 | Andhra Pradesh | 0.02 |
Outcome measures
The burden of HBV in different studies was measured by several methods like rapid card test, serology test by ELISA (enzyme-linked immunosorbent assay) and molecular methods (NAT). In most of the studies, prevalence was estimated by serological assay. However, molecular method: NAT is considered as the gold standard for the diagnosis of HBV. NAT technology is highly sensitive and specific for detection of HBV DNA; it also detects the cases missed by serology. In the current study, data were analyzed to assess the burden of HBV in asymptomatic population of India from the reported literature. The Joanna Brigg’s Institute (JBI) critical appraisal checklist for studies reporting prevalence data tool was used to assess the quality of included studies43.
Statistical analysis
The R programming language with the ‘meta’ and ‘metafor’ packages were used to synthesize prevalence estimates among blood donors and perform meta-analysis. The calculation of Clopper-Pearson (CP) confidence intervals (CI) for individual studies using the ‘metafor’ package’s ‘confint()’ function with the ‘CP’ method was deemed appropriate for prevalence data. A random intercept logistic regression model was used to accommodate both within-study and between-study variabilities. A logit transformation of prevalence data was applied, and the maximum-likelihood estimator was used to estimate tau^2, providing a measure of between-study heterogeneity. The results were presented in a forest plot to show the individual study estimates with the pooled prevalence estimate and study heterogeneity. The chi-square statistic was used to evaluate heterogeneity among blood donor prevalence studies. The python programming language with the pandas, geopandas, and matplotlib, pyplotlibraries was used to show the information on blood donor prevalence across different regions.
Results
Study characteristics
A total of 527 records were identified through an electronic database search. After applying inclusion and exclusion criteria, 40 studies were included for quality assessment and meta-analysis (Fig. 1). These studies encompassed data generated from various regions of India, covering 29 States and eight Union Territories.
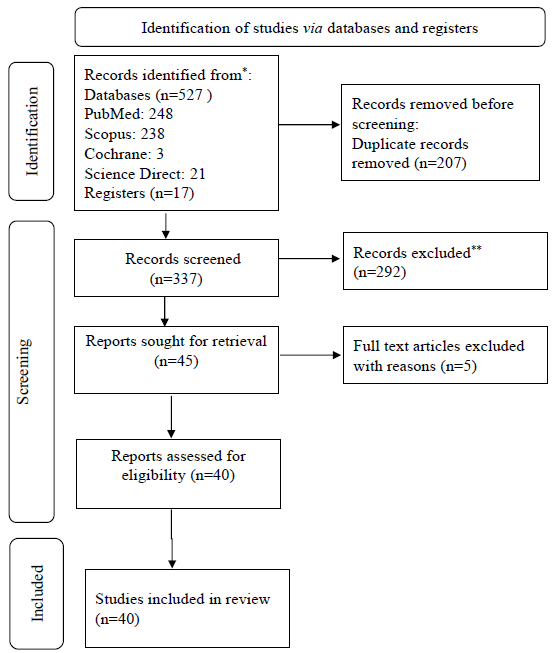
- PRISMA diagram showing details of selected studies.
Quality assessment
The quality assessment of the records was conducted using the JBI critical appraisal tool43. According to the tool, 90 per cent of the records clearly defined the inclusion criteria, 95 per cent provided detailed descriptions of the study participants setting, 82 per cent used appropriate statistical analysis, and 100 per cent applied standard criteria for measuring the condition (Table III)3-42. However, despite all records presenting prevalence data, none included an exposure measure.
| Author, yr | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Agarwal et al3, 2013 | Yes | No | NA | Yes | No | NA | Yes | No |
| Agarwal4, 2014 | Yes | Yes | NA | Yes | No | NA | Yes | No |
| Arcot et al5, 2022 | Yes | Yes | NA | Yes | No | NA | Yes | Yes |
| Badhan & Cheema6, 2023 | Yes | Yes | NA | Yes | Unclear | Unclear | Yes | Yes |
| Bhasker & Aluri7, 2021 | Yes | Yes | Yes | Yes | NA | NA | Yes | Yes |
| Bhaumik & Debnath8, 2014 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Chaithanya & Shivakumar9, 2020 | Yes | Yes | NA | Yes | Unclear | N0 | Yes | Yes |
| Chandra et al10, 2014 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Chaurasia et al11, 2016 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Chigurupati & Murthy12, 2015 | Yes | Yes | NA | Yes | Unclear | No | Yes | No |
| Dara et al13, 2017 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Datta et al14, 2019 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Dhiman et al15, 2019 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Hulinaykar & Krishna16, 2016 | Unclear | No | No | Yes | No | NA | Yes | No |
| Jadeja et al17, 2014 | Yes | Yes | No | Yes | No | Na | Yes | Yes |
| Karmakar et al18, 2014 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Kavitha et al19, 2023 | Yes | Yes | No | Yes | No | NA | Yes | No |
| Keechilot et al20, 2016 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Kumar et al21, 2015 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Kumari22, 2020 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Makroo et al23, 2015 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Mandal & Mondal24, 2016 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Mukherjee et al25, 2014 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Narayanasamy et al26, 2015 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Pandey et al27, 2015 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Parveen et al28, 2015 | Unclear | Yes | No | Yes | No | NA | Yes | No |
| Prasad et al29, 2021 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Ranganathan et al30, 2021 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Rawat et al31, 2017 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Saini et al32, 2017 | Unclear | Yes | No | Yes | No | NA | Yes | Yes |
| Sehgal et al33, 2017 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Shah et al34, 2013 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Shrivastava et al35, 2023 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Sundaramoorthy et al36, 2018 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Thakur et al37, 2023 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Tiwari et al38, 2018 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Tiwari et al39, 2020 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Tyagi & Tyagi40, 2013 | Unclear | Yes | No | Yes | No | NA | Yes | Unclear |
| Varma et al41, 2019 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
| Yashovardhan et al42, 2015 | Yes | Yes | No | Yes | No | NA | Yes | Yes |
1. Were the criteria for inclusion in the sample clearly defined?
2. Were the study subjects and the setting described in detail?
3. Was the exposure measured in a valid and reliable way?
4. Were objective, standard criteria used for measurement of the condition?
5. Were confounding factors identified?
6. Were strategies to deal with confounding factors stated?
7. Were the outcomes measured in a valid and reliable way?
8. Was appropriate statistical analysis used?
Meta-analysis results
The analysis incorporated data from forty studies assessing HBV prevalence in blood donors and included 22,22,736 individuals. Substantial screening occurred in Delhi (n=220,482), Uttar Pradesh (n=192,348), and Tripura (n=177,302), respectively. Under the common effect model, the pooled proportion was estimated at 0.0111, with a 95 per cent confidence interval (CI) of [0.011; 0.0112] across all included studies. In contrast, the random effects model yielded a slightly lower pooled proportion of 0.0095, with a 95 per cent CI of [0.0079; 0.0116] (Fig. 2). Heterogeneity details revealed Tau^2 (tau-squared) with substantial between-study variance at 0.3939. The standard deviation of true effects, Tau (τ), measured 0.6276. I^2 indicated high total variation due to heterogeneity at 98.9%. Cochran’s Q (H) was 9.69, indicating total heterogeneity across studies. The Q statistic produced significant results: Wald test - statistic 3756.84 with 40 degrees of freedom (d.f.), P < 0.001, and likelihood ratio test (LRT) - statistic 4751.78 with 40 d.f., P < 0.001 (Fig. 3).
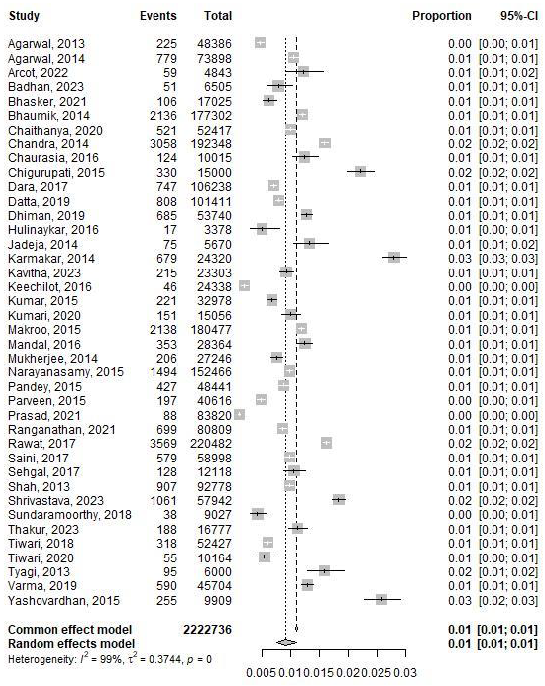
- Forest plot of studies assessing HBV prevalence in blood donors conducted in India between 2013 and October 2023.
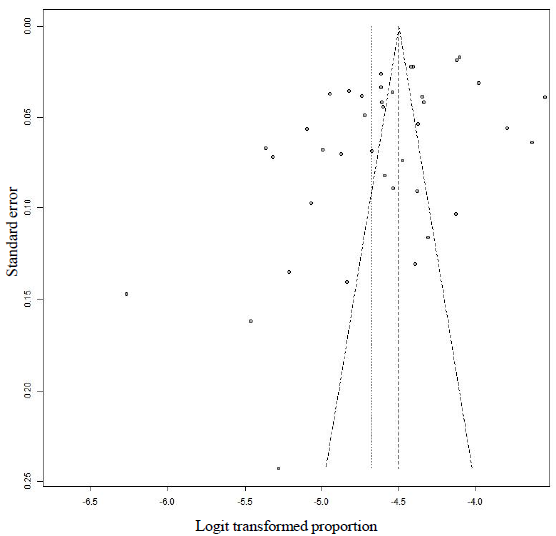
- Bias assessment plot (funnel plot) of reported studies HBV prevalence in blood donors conducted in India between 2013 and October 2023.
Correlation between sample size and positive test
Pooled estimates of HBV included all reported observations from different States of India since last 10 yr. However, the state wise proportion of the positive tests of HBV is likely to reflect the current situation of chronic HBV infection depending upon the total number of screened cases in a particular area. Regression model was applied to investigate the correlation between the positive tests among total no screened (Fig. 4).
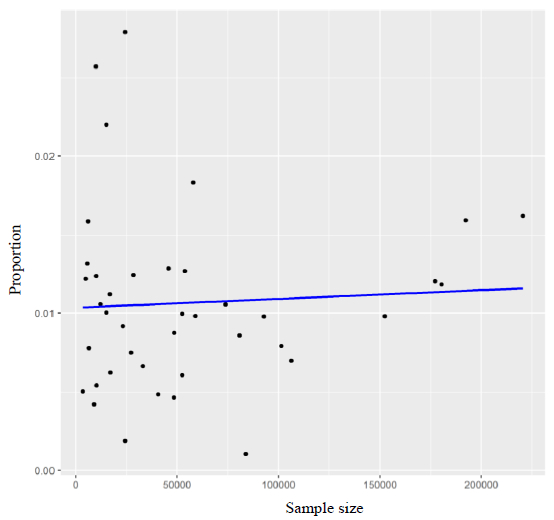
- Proportion of the positive tests of HBV prevalence.
Discussion
We report data combining various studies from individual States (with prevalence estimate) by a meta-analysis of peer-reviewed literature. This study reports HBV prevalence based on hepatitis B surface antigen (HBs-Ag) in the blood donor population across all States, for which epidemiologic data were available. We also estimated the number of people living with chronic HBV infection at the regional and national level and address changes over time. This systematic review revealed that approximately 24,151 individuals (n=22,22,736) from the nationwide asymptomatic population were either chronically infected with HBV or living with HBV infection. The pooled prevalence of HBV based on blood donation data was 1.01 per cent. Thus, the results of the meta-analysis aligned with the background understanding that HBV was of moderate prevalence in the asymptomatic population of India44. Preventing HBV is still the major objective for the Southeast Asian countries that needs to be addressed. Hence, the Government of India launched NVHCP in 2018, including both preventive measures (vaccination, blood safety) and early detection followed by linkage‐to‐care (screening at-risk population, provision of drugs, surveillance of chronic liver disease etc.) strategies with the aim of controlling and eliminating hepatitis as a public health problem by 20301. Since the implementation of NVHCP guidelines and the government’s commitment to eliminating viral hepatitis since 2018, the pooled prevalence of HBV has slightly decreased. This is in comparison with the WHO data fact sheet from 2016, which classified India as a country with intermediate HBV endemicity with a prevalence of 3 to 4.5 per cent45. It indicates toward successful execution of public health measures. A review on the impact and current status of the NVHCP showed that over 97 per cent of blood donations underwent quality assurance screening, with the goal of reaching 95 per cent screening by 2020 and 100 per cent screening by 2030. This contrasts with only 17 per cent (out of 4.5 million) eligible and diagnosed chronic hepatitis B cases being treated by the year 2016 against a global target of 80 per cent treatment coverage by 2030. The NVHCP program has also made significant alterations in the current infrastructure. Presently, every State and six union territories (UTs) have model treatment centres created. As part of the initiative, 301 treatment facilities have been established in 285 districts. Nine States—Bihar, Haryana, Jharkhand, Kerala, Maharashtra, Mizoram, Nagaland, Punjab, and Rajasthan—have operationalized treatment centres in every district. Almost 16 lakh tests have been performed to diagnose hepatitis B46.
There was a wide variation in the proportion of HBV prevalence between states, covering approximately 2.2 million blood donor populations. Due to population heterogeneity in India, the point prevalence of HBV in tribal areas, estimated at 15.9 per cent (95% CI: 11.4‐20.4%), is higher than in other areas, which is 2.4 per cent (95% CI: 2.2‐2.7%)4. This variation indicates the existence of geographic differences across India associated with economic, cultural, and socio-demographic constraints. Diverse and distinct cultural practices, beliefs, and lifestyles among tribal populations, including overcrowding and poor hygienic practices, might have contributed as risk factors for HBV disease burden47. In the year 2000, a study conducted by Mahapatra et al48. reported that 49 per cent of the participants used traditional measures of treatment mainly provided by local quacks and only 6 per cent exclusively used the allopathic system of treatment.
According to the recommendations of the Central Drugs Standard Control Organization (CDSCO), issued in 2013, any in-vitro diagnostic devices approved for diagnostic purposes can be utilized for screening of donated blood. This might have led to the heterogeneity in our study findings. In a recent study, the analytical sensitivity of rapid assays for HBsAg detection was 64.29 per cent with a specificity of 99.9–100 percent, while chemiluminescence immune assay (CLIA) showed low sensitivity and comparable specificity to rapid assays, 1.43 per cent and 97.77 per cent, respectively. The performance of CLIA as a screening assay was better as compared to the rapid assays. ELISA was gold standard and was better for batchwise testing of blood units and in a country with large number of carriers and possibility of detecting window period infections49. At times, there is a need to test donor samples with both serological and molecular assays. One of the studies mentioned that the presence of HBV DNA in large number of anti HBc positive samples called for introduction of better screening assay in order to detect occult HBV infection50. Considering such scenarios, the authors proposed that the most viable solution to enhance the safety of donated blood in India would be through the implementation of NAT testing, which would be capable of detecting the majority of potentially infectious blood units during window period donations and in the instances of seronegative infections50.
The present meta-analysis from India suggested a pooled prevalence estimate of approximately 1.11 per cent (common effect model) or 0.95 per cent (random effects model). The included studies exhibit high level of heterogeneity, and therefore, the random effect model appeared to be more reasonable. Linear correlation analysis for the proportion estimation of positive tests suggested that risk-based testing alone would not identify most individuals living with chronic HBV. Therefore, a universal screening pattern for certain populations (including blood donors, pregnant women, newly arrived refugees, persons initiating cytotoxic or immunosuppressive therapy, haemodialysis, healthcare personnel, perinatally exposed infants)51 would be appropriate that could guide vaccination strategies
Strengths and limitations
The key strength of this study rested in its comprehensiveness, covering various geographical regions and targeting a mixed population for HBV. A major limitation was the heterogeneity resulting from different diagnostic approaches followed in various geographical regions of India. A few studies included in this review reported the NAT-based prevalence of Hepatitis B, while most window period infections were missed by serological assays.
Conclusions
In India, a moderate level of HBV infections is detected among asymptomatic blood donors. Hence, the implementation of stringent donor screening policies is crucial to mitigate the risk of residual contamination. Estimates of burden of hepatitis B in asymptomatic blood donors in our study would play a vital role for guiding the expansion and targeting of hepatitis B vaccination programs, especially in areas with higher rates of asymptomatic carriers and support the NVHCP. Data from NVHCP on the burden of asymptomatic hepatitis among blood donors can contribute to global surveillance efforts and support the WHO’s goals for hepatitis elimination by 2030.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- National Viral Hepatitis Control Program. Available from: https://nvhcp.mohfw.gov.in/about_us, accessed on August 24, 2024.
- Factors contributing to the low number of blood donors among employed residents in Oshatumba village, Namibia. Afr J Prim Health Care Fam Med. 2023;15:e1-e8.
- [Google Scholar]
- Nucleic acid testing for blood banks: An experience from a tertiary care centre in New Delhi, India. Transfus Apher Sci. 2013;49:482-4.
- [Google Scholar]
- Response rate of blood donors in the Uttarakhand region of India after notification of reactive test results on their blood samples. Blood Transfus. 2014;12:s51-3.
- [Google Scholar]
- Comparative evaluation of ADVIA Centaur® XP chemiluminescence system for screening of HBV, HCV, HIV and syphilis in Indian blood donors. Transfus Apher Sci. 2022;61:103318.
- [Google Scholar]
- Seroprevalance of transfusion transmitted infections among blood donors at a tertiary care teaching institution in North India. Int J Life Sci Biotechnol Pharma Res. 2023;12:1217-23.
- [Google Scholar]
- Donor notification and counseling: Experiences and challenges from a private multi-specialty hospital in South India. Asian J Transfus Sci. 2021;15:166-71.
- [Google Scholar]
- Prevalence of blood-borne viral infections among blood donors of Tripura. Euroasian J Hepatogastroenterol. 2014;4:79-82.
- [Google Scholar]
- Evaluation of nucleic acid testing [NAT] of blood donors. Indian J Pathol Oncol. 2020;5:536-41.
- [Google Scholar]
- Decreasing prevalence of transfusion transmitted infection in Indian scenario. Sci World J. 2014;2014:173939.
- [Google Scholar]
- Comparison of Procleix Ultrio Elite and Procleix Ultrio NAT assays for screening of transfusion transmitted infections among blood donors in India. Int J Microbiol. 2016;2016:2543156.
- [Google Scholar]
- Automated nucleic acid amplification testing in blood banks: An additional layer of blood safety. Asian J Transfus Sci. 2015;9:9-11.
- [Google Scholar]
- Co-infection of blood borne viruses in blood donors: A cross-sectional study from North India. Transfus Apher Sci. 2017;56:367-70.
- [Google Scholar]
- Nucleic acid amplification test: Bridging the gap in blood safety & re-evaluation of blood screening for cryptic transfusion-transmitted infection among Indian donors. Indian J Med Res. 2019;149:389-95.
- [Google Scholar]
- Consecutive reactive results in screening of transfusion transmitted infections: Family history of blood donors is also important. Transfus Apher Sci. 2019;58:464-7.
- [Google Scholar]
- Seroprevalence of transfusion transmitted infections among blood donors in a tertiary care teaching hospital SIMSRH, Blood Bank Tumkur, Karnataka. Sch J Appl Med Sci. 2016;4:3702-6.
- [Google Scholar]
- Trend in seroprevalence of hepatitis b virus infection among blood donors at a tertiary care centre of Rajasthan, India. Nat J Med Res. 2014;4:205-7.
- [Google Scholar]
- Seroprevalence of transfusion transmissible infections among blood donors at the blood bank of a medical college of Kolkata. Indian J Public Health. 2014;58:61-4.
- [Google Scholar]
- Seroprevalence of transfusion transmitted infections among voluntary non-remunerated blood donors and replacement donors at a tertiary care maternity hospital in south India. Int J Life Sci Biotechnol Pharma Res. 2023;12:432-6.
- [Google Scholar]
- Detection of occult hepatitis B and window period infection among blood donors by individual donation nucleic acid testing in a tertiary care center in South India. Pathog Glob Health. 2016;110:287-91.
- [Google Scholar]
- Individual donor-nucleic acid testing for human immunodeficiency virus-1, hepatitis C virus and hepatitis B virus and its role in blood safety. Asian J Transfus Sci. 2015;9:199-202.
- [Google Scholar]
- Prevalence and trends of hepatitis B virus, Hepatitis C virus, Human immunodeficiency virus 1, 2 and syphilis infections among blood donors in a regional transfusion center in Punjab, India: A 3 years study. Indian J Sex Transm Dis AIDS. 2020;41:22-9.
- [Google Scholar]
- Seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north India. Indian J Med Res. 2015;142:317-22.
- [Google Scholar]
- Transfusion transmissible infections among blood donors from a sub-Himalayan rural tertiary care centre in Darjeeling, India. J Tradit Complement Med. 2015;6:224-9.
- [Google Scholar]
- Response to post-donation counseling is still a challenge in outdoor voluntary blood donation camps: A survey from a tertiary care regional blood center in Eastern India. Asian J Transfus Sci. 2014;8:80-3.
- [Google Scholar]
- Study of hepatitis B and C virus infection in urban and rural population of Tamil Nadu, India. Int J Curr Microbiol App Sci. 2015;4:443-51.
- [Google Scholar]
- A comprehensive serological and supplemental evaluation of hepatitis B “seroyield” blood donors: A cross-sectional study from a tertiary healthcare center in India. Asian J Transfus Sci. 2015;9:189-94.
- [Google Scholar]
- Seroprevalence of Hepatitis B virus (HBV), Hepatitis C virus (HCV) and HIV infection among replacement blood donors at a tertiary care hospital in Kashmir. J Clin Exp Hepatol. 2015;5:S27-S8.
- [Google Scholar]
- Implementation of nucleic acid testing for transfusion transmitted infection screening of blood donation in a tertiary care centre in Odisha. Panacea J Med Sci. 2021;11:561-4.
- [Google Scholar]
- Significance of adopting nucleic acid amplification technique for blood donor screening in a resource limited setting: A study from a single centre in south India. Indian J Hematol Blood Transfus. 2022;38:571-6.
- [Google Scholar]
- Seroprevalence & changing trends of transfusion-transmitted infections amongst blood donors in a regional blood transfusion centre in north India. Indian J Med Res. 2017;146:642-5.
- [Google Scholar]
- Hepatitis C virus: Unnoticed and on the rise in blood donor screening? A 5 years cross-sectional study on seroprevalence in voluntary blood donors from Central India. J Glob Infect Dis. 2017;9:51-5.
- [Google Scholar]
- Seroprevalence and trends of transfusion transmissible infections in blood donors in Andaman and Nicobar Islands- an institutional retrospective study. J Clin Diagn Res. 2017;11:EC21-24.
- [Google Scholar]
- Seroprevalence of HBV, HCV, HIV and syphilis among blood donors at a tertiary care teaching hospital in Western India. G M J. 2013;68:35-9.
- [Google Scholar]
- Time trend and prevalence analysis of transfusion transmitted infections among blood donors: A retrospective study from 2001 to 2016. Indian J Community Med. 2023;48:274-280.
- [Google Scholar]
- Seroprevalence of transfusion transmissible infections among blood donors by chemiluminescent assay in a tertiary care centre. J Infect Dev Ctries. 2018;12:31-6.
- [Google Scholar]
- Notification and counselling of hepatitis positive blood donors, their immediate emotional response, contact-testing and their follow-up: Study from a tertiary care hospital! Transfus Apher Sci. 2018;57:391-7.
- [Google Scholar]
- Head-to-head comparison of Enzyme Linked Immunosorbent Assay (ELISA) and Enhanced Chemiluminescence Immunoassay (ECLIA) for the detection of Transfusion Transmitted Disease (TTD) Markers; HIV, HCV and HBV in blood donors, in India. J Virol Methods. 2020;285:113962.
- [Google Scholar]
- Possible correlation of transfusion transmitted diseases with Rh type and ABO blood group system. J Clin Diagn Res. 2013;7:1930-1.
- [Google Scholar]
- Seroprevalence of transfusion transmissible infections among blood donors at a tertiary care teaching hospital in central India. Int J Contemp Med. 2019;6:L1-L4.
- [Google Scholar]
- Trend of Hepatitis B surface Antigen (HBsAg) among blood donors at the blood bank of a tertiary care referral teaching hospital in southern India. Int J Res Med Sci. 2015;3:1686-90.
- [Google Scholar]
- Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147-53.
- [Google Scholar]
- Chronic hepatitis B: Challenges and successes in India. Clin Liver Dis (Hoboken). 2021;18:111-16.
- [Google Scholar]
- Hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b, accessed on August 26, 2024.
- Viral hepatitis as a public health concern: A narrative review about the current scenario and the way forward. Cureus. 2022;14:e21907.
- [Google Scholar]
- Tribal population in India: A public health challenge and road to future. J Family Med Prim Care. 2020;9:508-12.
- [Google Scholar]
- Health seeking behaviour in a tribal setting. Health Popul Perspect Issues. 2000;23:160-9.
- [Google Scholar]
- Performance evaluation of blood donor screening assays for serological detection of hepatitis B surface antigen and antibodies to hepatitis C virus. Glob J Transfus Med. 2021;6:205-10.
- [Google Scholar]
- Significance of anti-HBc screening of blood donors and its association with occult hepatitis B virus infection: Implications for blood transfusion. Indian J Med Res. 2010;132:312-7.
- [Google Scholar]
- Screening and testing for hepatitis B virus infection: CDC recommendations - United States, 2023. MMWR Recomm Rep. 2023;72:1-25.
- [Google Scholar]






