Translate this page into:
Baseline characteristics of HIV & hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India
Reprint requests: Dr Subhasish Kamal Guha, Calcutta School of Tropical Medicine, 108 Chittaranjan Avenue Road, Kolkata 700 073, West Bengal, India e-mail: drskguha@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Hepatitis B virus (HBV) and HIV co-infection has variable prevalence worldwide. In comparison to HBV mono-infection, the course of chronic HBV infection is accelerated in HIV/HBV co-infected patients. The present study was carried out to analyse the baseline characteristics (clinical, biochemical, serological and virological) of treatment naïve HIV/HBV co-infected and HIV mono-infected patients.
Methods:
Between July 2011 and January 2013, a total number of 1331 HIV-seropositive treatment naïve individuals, enrolled in the ART Centre of Calcutta School of Tropical Medicine, Kolkata, India, were screened for hepatitis B surface antigen (HBsAg). A total of 1253 HIV mono-infected and 78 HIV/HBV co-infected patients were characterized. The co-infected patients were evaluated for HBeAg and anti-HBe antibody by ELISA. HIV RNA was quantified for all co-infected patients. HBV DNA was detected and quantified by real time-PCR amplification followed by HBV genotype determination.
Results:
HIV/HBV co-infected patients had proportionately more advanced HIV disease (WHO clinical stage 3 and 4) than HIV mono-infected individuals (37.1 vs. 19.9%). The co-infected patients had significantly higher serum bilirubin, alanine aminotransferase (ALT), alkaline phosphatase and ALT/platelet ratio index (APRI). CD4 count was non-significantly lower in co-infected patients. Majority (61.5%) were HBeAg positive with higher HIV RNA (P<0.05), HBV DNA (P<0.001) and APRI (P<0.05) compared to those who were HBeAg negative. HBV/D was the predominant genotype (73.2%) and D2 (43.7%) was the commonest subgenotype.
Interpretation & conclusions:
HIV/HBV co-infected patients had significantly higher serum bilirubin, ALT, alkaline phosphatase and lower platelet count. HBeAg positive co-infected patients had higher HIV RNA and HBV DNA compared to HBeAg negative co-infected patients. Prior to initiation of antiretroviral treatment (ART) all patients should be screened for HBsAg to initiate appropriate ART regimen.
Keywords
Antiretroviral therapy
HBeAg
HBsAg
HIV/HBV co-infection
tuberculosis
Approximately 5-10 per cent of HIV infected people suffer from chronic co-infection with hepatitis B virus (HBV) because of shared mode of transmission1. In co-infection, HIV significantly modifies the natural course of HBV infection23. Compared with the HBV mono-infected individuals, the course of chronic HBV infection in HIV co-infected patients is more aggressive with lower transaminase elevation, higher HBV DNA levels, lower inflammatory activity and a higher prevalence of cirrhosis and hepatocellular carcinoma4. According to geographic regions and exposure risk, there is considerable variation in prevalence of HIV/HBV co-infection in India56789.
It has been observed that antiretroviral therapy (ART) having lamivudine as the sole HBV active agent in the regimen results in development of HBV resistance against lamivudine in 50 per cent patients by two years and in 90 per cent by four years10. So it is recommended to use lamivudine or emtricitabine along with another HBV active agent like tenofovir as part of antiretroviral therapy11121314. The WHO recommends initiation of ART in HIV/HBV co-infected patients with CD4 count < 500 cells/μl and in patients with severe chronic liver disease, irrespective of CD4 count15. It advocates the use of tenofovir plus lamivudine or emtricitabine and efavirenz as the preferred first-line regimen for all HIV/HBV co-infected patients. As per the guidelines of the National AIDS Control Organization (NACO)16, ART should be initiated in HIV/HBV co-infection with CD4 count < 350 cells/μl and in patients with severe chronic active hepatitis, irrespective of CD4 count. Despite the widespread use of nucleoside analogues with dual activity against HIV and HBV, research gaps exist about the impact of ART on liver-related outcomes in HIV/HBV co-infected people in resource-limited settings. We undertook this study to analyse baseline characteristics (clinical, biochemical, serological, virological) HIV/HBV co-infected and HIV mono-infected patients before initiating ART.
Material & Methods
A total number of 2099 patients were enrolled in the HIV Care Register at the ART centre of Calcutta School of Tropical Medicine, Kolkata, West Bengal, India, from July 2011 to January 2013 and as per NACO guidelines15 1331 eligible patients were initiated on antiretroviral therapy. All patients, before starting ART, were subjected to baseline mandatory laboratory testing [CD4 count, complete haemogram, liver function tests (LFT), fasting blood sugar, blood urea, serum creatinine, venereal disease research laboratory (VDRL), hepatitis B surface antigen (HBsAg), routine stool and urine analysis and chest X-ray [posterior anterior (PA) view]. Additional tests like sputum for acid fast bacilli (AFB), ultrasonogram of whole abdomen, study of body fluids (CSF, pleural fluid, and ascitic fluid), aspiration cytology/ biopsy of lymph node, etc. Were carried out depending on the clinical presentation of patients for diagnosing opportunistic infection and neoplasm. Seventy eight consecutive HBsAg reactive patients were enrolled in this study after obtaining written, informed consent. This research protocol was approved by the Institutional Ethics Committee of Calcutta School of Tropical Medicine, Kolkata.
Laboratory methods: In addition to the mandatory and symptom and signs directed laboratory investigations, all the HIV/HBV co-infected patients enrolled in the study were subjected to hepatitis B e antigen (HBeAg), anti-HBe antibody, quantitative HBV DNA estimation, HBV genotyping and prothrombin time estimation. Co-infection with hepatitis A, C and E virus was excluded by qualitative anti-HAV [Dia. Pro Diagnostic Bioprobes Srl, Sesto San Giovanni (MI)- Italy], anti-HCV (Transasia, Bio-Medical Ltd., Daman, India) and anti-HEV ELISA [Dia. Pro Diagnostic Bioprobes Srl, Sesto San Giovanni (MI), Italy] tests, respectively.
The diagnosis of HIV infection was established at the Integrated Counselling and Testing Centre (ICTC) of Calcutta School of Tropical Medicine as per the NACO guidelines15. Briefly, rapid card tests were used in an algorithm of parallel testing. Kits that were used sequentially were SD Bioline for HIV 1/2 3.0 (Standard Diagnostics Inc., India). Samples that were reactive by this method were re-checked by two other rapid tests-HIV Triline Rapid HIV 1/2 (Bhat Bio-Tech India Pvt. Ltd. Bengaluru, India) and HIV Trispot Rapid HIV 1 and 2 (Bhat Bio-Tech India Pvt. Ltd). HBV specific enzyme-linked immunosorbent assay (ELISA) kits were used for the detection of HBsAg, HBeAg and anti-HBe (Diasorin, S.P.A, Saluggia, Italy). Qualitative anti-HAV, anti-HCV and anti-HEV ELISA (Dia. Pro, Italy and Biorad, France) tests were performed. All the serological assays were performed according to the manufacturer's instruction. DNA was extracted from HBsAg positive samples using QIAamp DNA Blood kit (Qiagen, Germany) according to manufacturer's protocol. HBV DNA was detected by real time PCR technique17. HBV genotype was determined by a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method, as previously described18. The results of PCR-RFLP were further confirmed by direct sequencing with Prism Big Dye kit (Applied Biosystems, USA) and ABI 3130xl Genetic Analyzer (Applied Biosystems, USA).
Liver function tests [serum bilirubin (conjugated and unconjugated), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total protein, albumin, globulin] and complete haemogram including platelet count, were routinely performed. AST to platelet ratio-index (APRI) was calculated for all patients. Hepatitis specific markers (HBsAg, HBeAg and anti-HBe), hepatitis B DNA, quantitative PCR and HBV genotyping were done.
All patients registered at the ART centre were evaluated for exclusion of active tuberculosis before ART initiation as per Revised National Tuberculosis Control Programme (RNTCP) guideline17.
Statistical analysis: Comparison of baseline characteristics of HIV, mono-infected and HIV/HBV co-infected patients was done. For 78 HIV/HBV co-infected patients, 312 (78 × 4) controls were randomly selected from 1253 HIV mono-infected individuals by computer generated random numbers. Similarly, for 13 HIV/HBV/TB co-infected patients 52 (13x4) controls were selected from 103 HIV/TB co-infected patients. Mann-Whitney U test and unpaired Student t test were performed for comparisons of continuous variables between the groups using the Graph pad Prism (version 4.0.3, San Diego, CA, USA). Categorical variables were analyzed using the chi-square test or Fisher exact test, as appropriate.
Results
A total number of 1331 HIV seropositive treatment naïve patients were enrolled at the ART centre during the study period, of whom 78 were HIV/HBV co-infected (5.9%, 95% CI: 1.04-1.07). Among the 312 [age: median (range) 32 (18-72) yr] HIV mono-infected patients, 218 (70%) were males and 94 (30%) were females with meadian age 35 yr (21-62 yr). According to the self-reported risk factors, the most probable routes of transmission for HIV/HBV co-infection were heterosexual route (250/312; 80%), blood transfusion (13/312; 4%) and injection drug use (15/312; 5%). Alcohol use was noted among 162 (52%) patients. These patients were categorized in WHO clinical stage of 1 (221/312; 71%), 2 (27/312; 8.5%), 3 (25/312; 8%) and 4 (39/312; 12.5%). At baseline, 19 (59/312) and 3.2 per cent (10/312) of HIV mono-infected patients had ALT elevation of grade 1 (1.25-2.5 upper limit of normal, ULN) and 2 (2.5-5 ULN), respectively.
Among the HBV/HIV co-infected patients 78.3 (61/78) and 21.7 per cent (17/78) were males and females, respectively with median age of 35 yr (18-70 yr). According to the self-reported risk factors, the most probable routes of transmission for HIV/HBV co-infection were heterosexual route (39/78; 50%), blood transfusion (20/78; 25.6%) and injection drug use (8/78; 10.3%). Alcohol use was noted among 47 (60.3%) patients. The patients were distributed in WHO clinical stage of 1 (38/78; 48.7%), 2 (11/78; 14.1%), 3 (13/78; 16.6%) and 4 (16/78; 20.5%). At baseline, 19.2 (15/78) and 6.4 per cent (5/78) of HIV/HBV co-infected patients had ALT elevation of grade 1 (1.25-2.5 ULN), and 2 (2.5-5 ULN), respectively. The differences of median haemoglobin (11.5 vs. 11.3 g/dl) and platelet count (169 × 103 vs. 189 × 103/µl) were non-significant among HIV/HBV co-infected and HIV mono-infected patients, respectively (Table I). The median serum bilirubin (0.61 vs. 0.4 mg/dl; P<0.05), ALT (41 vs. 32 U/l; P<0.01) and alkaline phosphatase (215 vs. 146 U/l; P<0.01) were significantly higher among HIV/HBV co-infected patients than HIV mono-infected ones. However, the difference in serum AST was not significant (Table I). The APRI was significantly higher in HIV/HBV co-infected subjects (0.8 vs. 0.5; P<0.001) as compared to HIV mono-infected patients.
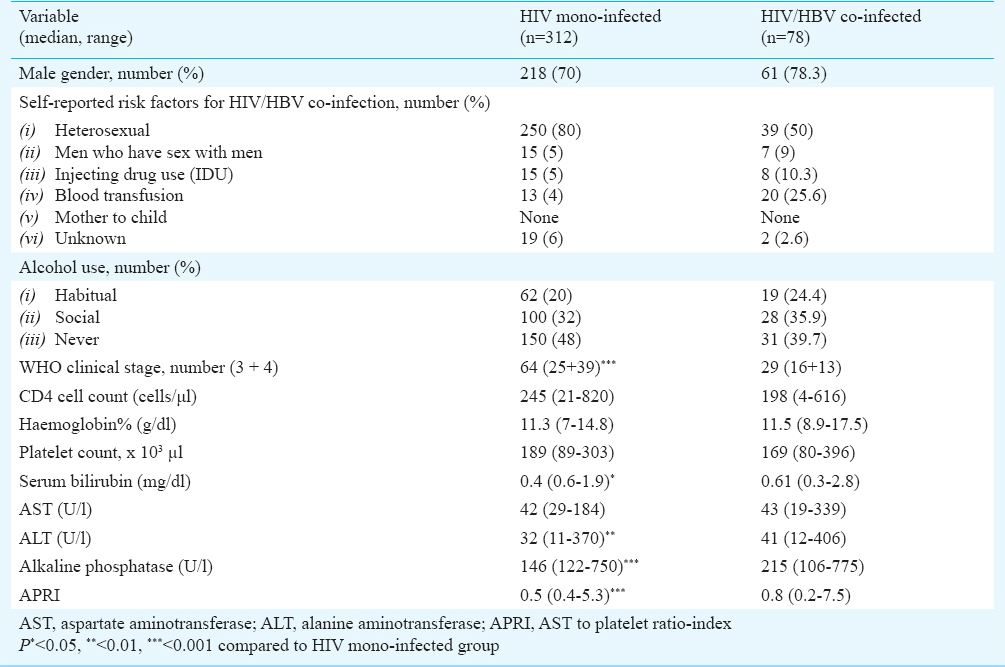
Association of CD4 T-cell count with HIV infection and HIV/HBV co-infection: The difference in median baseline CD4 cell count was not significant between HIV mono-infected (245; 21-820 cells/μl) and HIV/HBV co-infected (198; 4-616 cells/μl) patients (Table I). Although proportionately more of the HIV/HBV co-infected patients had CD4 count < 200 cells/ μl (38/78; 48.7%) as compared to HIV mono-infected patients (78/312; 25%) but the difference was not significant. Among HIV/HBV co-infected individuals with CD4 count < 200 cells/μl median serum albumin (P<0.05) and haemoglobin (P<0.001) levels were lower and prothrombin time prolonged (P<0.01) as compared to those with CD4 >200 cells/μl (data not shown).
HBeAg status in HBV/HIV co-infected patients: Of the 78 HIV/HBV co-infected patients, 48 (61.5%) were HBeAg positive and 30 (38.5%) were HBeAg negative. HBeAg positive co-infected patients had higher median HIV RNA (P<0.05), HBV DNA (P<0.001), AST (P<0.05) and AST to platelet ratio index (P<0.05) than HBeAg negative individuals (Table II). However, the CD4 cell count, serum ALT and albumin were not significantly different. Among HBeAg positive co-infected patients with CD4 <200 cells/μl HBV DNA (P<0.01) and ALT (P<0.01) were significantly higher in comparison with HBeAg negative co-infected patients (data not shown).
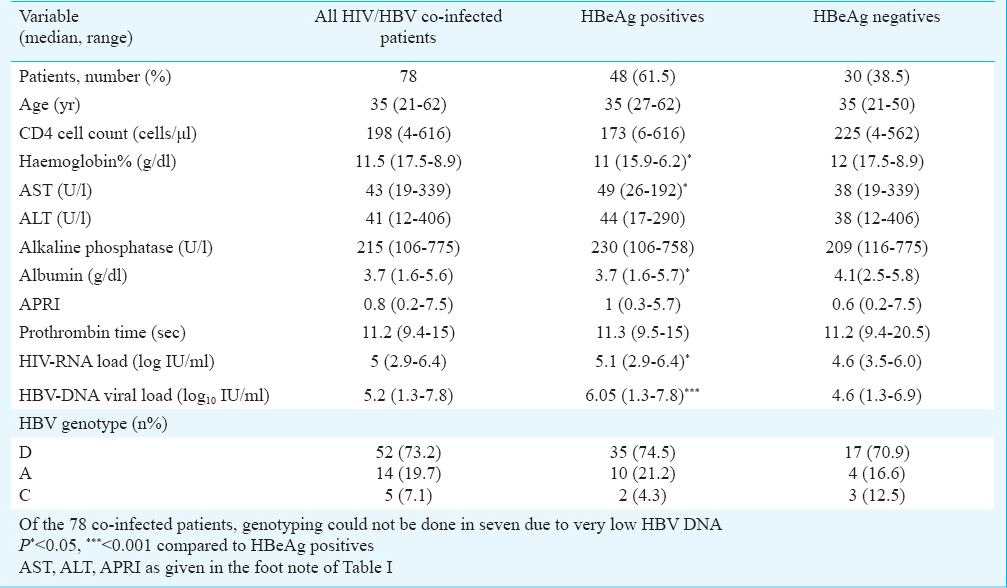
HBV genotyping of HIV/HBV co-infected patients: Genotype HBV/D (73.2%) was the predominant genotype followed by HBV/A (19.7%) and HBV/C (7.1%) among the HIV/HBV co-infected patients (Table II). Subgenotyping revealed preponderance of D2 (31/71; 43.7%).
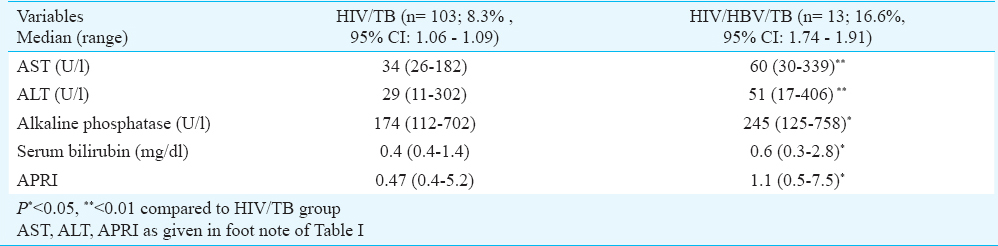
Association of tuberculosis (TB) with HIV infected and HIV/HBV co-infected patients: Tuberculosis was significantly (P<0.05) associated with HIV/HBV co-infected (13/78, 16.6%) patients as compared to HIV mono-infected (103/1253, 8.3%) individuals. HIV/HBV and tuberculosis co-infected patients had significantly higher serum bilirubin (P<0.05), ALT (P<0.01), AST (P<0.01), alkaline phosphatase (P<0.05) and APRI (P<0.05) than HIV/TB co-infected patients without HBV infection (Table III). HIV/HBV co-infected patients with TB (n=13) had significantly lower CD4 count (213 vs. 185 cells/μl; P<0.01) than those without TB. However, the HIV RNA (5 vs. 4.9 log IU/ml) and HBV DNA (7.5×105 vs. 3.5 × 105 IU/ml) did not differ significantly (data not shown).
Discussion
The risk of HIV and HBV co-infection depends on the individual's age at the time of exposure to both the viruses20. In the United States and Western Europe, HBV is typically acquired during adolescence or early adulthood through sexual activity21. Although, there is a high rate of spontaneous clearance of HBV (>90%) in immunocompetent adults, chronic infection develops in 20 per cent of adults with HIV infection after exposure to HBV21. In the areas of Asia and sub-Saharan Africa vertical and perinatal transmission of HBV is common, chronic HBV infection develops in more than 90 per cent of infants exposed to HBV22. In this study about 50 per cent individuals had heterosexual risk behaviour for contracting the HIV/HBV co-infection. Majority of individuals attending the ICTC belonged to high risk behaviour group. Provision of hepatitis B and C screening at ICTCs might help in identifying undiagnosed hepatitis B and C mono-infection as well as co-infection with HIV.
In HIV/HBV co-infected patients the risk of cirrhosis, end-stage liver disease, and death from liver disease, especially in patients with low CD4 cell count or concomitant alcohol use is increased23. In a Nigerian study24, the association of HIV/HBV co-infection with lower CD4 T-cell counts was demonstrated. One possible mechanism for this association is the immune activation, which increases CD4 T-cell apoptosis due to chronic hepatitis B infection. In our study the median CD4 counts of HIV/HBV co-infected patients were lower than that of HIV mono-infected individuals, but it was not significant.
In our study, tuberculosis was significantly associated with HIV/HBV co-infection as compared to HIV mono-infection. Such HIV/HBV and tuberculosis co-infected patients had significantly higher baseline serum bilirubin, ALT, AST, alkaline phosphatase and APRI than HIV/TB co-infected patients without concomitant HBV infection. Thus, these HBV and TB co-infected HIV patients are at increased risk for developing hepatotoxicity following anti-tuberculosis treatment.
Significantly raised serum alkaline phosphatase levels were observed in HIV/HBV co-infected patients in the present study. Co-infected patients had significantly increased serum ALT, bilirubin and APRI at baseline compared to HIV mono-infected patients, and they deserve close monitoring of these parameters following initiation of antiretroviral therapy.
In this study, HBeAg positive co-infected patients had higher median HIV RNA, HBV DNA, AST and APRI than HBeAg negative patients. This may lead to rapid progression of both HIV and HBV disease, in addition to transmission of both the viruses. It is well known that HBeAg positivity is associated with higher HBV DNA25. Higher HIV-1 RNA among HBeAg positive co-infected patients could be due to increased HBV X protein which serves as a transactivator of HIV transcription26. Moreover, enhanced T cells activation due to HBV infection can also lead to increase in HIV-1 RNA. Like many other studies from India27282930, HBV/D was the predominant genotype in our study also. Similarly, the subgenotype D2 was the commonest one25.
In conclusion, this study shows that HIV and HBV co-infection is an important public health issues in West Bengal, India. Major strength of this study was that it captured the data of treatment naïve HIV/HBV co-infected patients, and carefully characterized the different HBV parameters at baseline. Ruling out of concomitant HBV infection is necessary before initiation of antiretroviral therapy among HIV infected patients. Majority of the co-infected patients were HBeAg positive, with median CD4 count less than 200 cells/μl and had high HBV DNA requiring antiretroviral therapy. Considering that the patients co-infected with HIV, HBV and tuberculosis have significant baseline liver dysfunction predisposing them to increased risk of toxicity to anti-tuberculosis drugs, physicians treating such cases should be more vigilant for monitoring of hepatotoxicity.
The present study had some limitations. The patients were selected from the ART centre where routine viral load estimation was not done before ART initiation in treatment naïve patients. Hence, HIV RNA could not be quantified for HIV mono-infected individuals. Though, HBsAg screening was done to identify HBV infection in all HIV seropositive individuals, abdominal ultrasonographic examination was not done for HIV/HBV co-infected patients. Liver stiffness measurement by transient elastography and liver biopsy were also not performed in HIV/HBV co-infected patients.
Acknowledgment
Authors thank the staff of Anti Retroviral Therapy Centre, Calcutta School of Tropical Medicine and ICMR Virus Unit, Kolkata, and acknowledge the Indian Council of Medical Research, New Delhi, for financial support. Authors also thank Dr B.B. Rewari, National Programme Officer, National AIDS Control Organization, Government of India for administrative support and Dr Nandita Basu, Director, School of Tropical Medicine, Kolkata for providing the facilities to conduct this research work.
Conflicts of Interest: None.
References
- Prevalence of markers for HIV, hepatitis B and hepatitis C infection in UK military recruits. Epidemiol Infect. 2011;139:1166-71.
- [Google Scholar]
- Hepatitis B and/or C co-infection in HIV infected patients: A study in a tertiary care centre from south India. Indian J Med Res. 2013;138:950-4.
- [Google Scholar]
- Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23:125-36.
- [Google Scholar]
- Hepatitis viruses and human immunodeficiency virus co-infection: pathogenesis and treatment. J Hepatol. 2004;41:156-66.
- [Google Scholar]
- HIV-HBV co infection among individuals attending the ICTC of a tertiary care hospital in West Bengal, India. ISRN Virology 2013 2013 Article ID180150, 3 pages
- [Google Scholar]
- Characterization of treatment-naive HIV/HBV co-infected patients attending ART clinic of a tertiary healthcare centre in eastern India. PLoS One. 2013;8:e73613.
- [Google Scholar]
- Seroprevalence of anti HCV and hepatitis B surface antigen in HIV infected patients. Indian J Med Microbiol. 2003;21:268-70.
- [Google Scholar]
- Low prevalence of hepatitis B virus and hepatitis C virus co infection in patients with human immunodeficiency virus in Northern India. J Assoc Physicians India. 2007;55:429-31.
- [Google Scholar]
- Co-infetion of hepatitis B & hepatitis C in HIV infected patient. World J Gastroenterol. 2007;13:5015-20.
- [Google Scholar]
- Hepatitis B virus (HBV) mutations associated with resistance to lamivudine in patients co-infected with HBV and human immunodeficiency virus. J Clin Microbiol. 1999;37:3013-6.
- [Google Scholar]
- Long term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302-6.
- [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available from: http://aidsinfo.nih.gov/ContentFiles/Adultand AdolescentGL.pdf
- [Google Scholar]
- Hepatitis B in HIV patients: what is the current treatment and what are the challenges? J HIV Ther. 2009;14:13-8.
- [Google Scholar]
- ART guidelines for HIV-infected adults and adolescents, May 2013. available from: www.naco.gov.in
- [Google Scholar]
- 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; Available from: http://www.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf
- Variations in the functional domain of basal core promoter of hepatitis B virus among eastern Indian patients with prevalence of genotypes A, C, and D among the same ethnic population. J Med Virol. 2011;83:253-60.
- [Google Scholar]
- Frequency and distribution of hepatitis B virus genotypes among eastern Indian voluntary blood donors: Association with precore and basal core promoter mutations. Hepatol Res. 2009;39:53-9.
- [Google Scholar]
- Central Tuberculosis Division. 2005. Technical and operational guidelines for tuberculosis control. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; Available from: http://www.tbcindia.org/pdfs/Technical%20&%20Operational%20guidelines%20for%20TB%20Control.pdf
- [Google Scholar]
- Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991;163:454-9.
- [Google Scholar]
- HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921-6.
- [Google Scholar]
- Hepatitis B virus co-infection impacts baseline HIV parameters and HAART-related hepatotoxicity risk in an HIV infected Nigerian cohort. Clin Infect Dis. 2009;49:1268-73.
- [Google Scholar]
- Influence of HIV-associated degree of immune suppression on molecular heterogeneity of hepatitis B virus among HIV co-infected patients. Virology. 2013;436:134-42.
- [Google Scholar]
- Idendification of a region within the human immunodeficiency virus type 1 long terminal repeat that is essential for transactivation by the hepatitis B virus gene X. J Virol. 1989;63:2857-60.
- [Google Scholar]
- Anti-hepatitis B core antigen testing with detection and characterization of occult hepatitis B virus by an in-house nucleic acid testing among blood donors in Behrampur, Ganjam, Orissa in southeastern India: implications for transfusion. Virol J. 2010;7:204.
- [Google Scholar]
- Distribution of hepatitis B virus genotypes: phylogenetic analysis and virological characteristics of genotype C circulating among HBV carriers in Kolkata, Eastern India. World J Gastroenterol. 2006;12:5964-71.
- [Google Scholar]
- Hepatitis B virus genotypes and serotypes in western India: lack of clinical significance. J Med Virol. 2003;69:324-30.
- [Google Scholar]
- Distribution of hepatitis B virus genotypes in blood donors and chronically infected patients in a tertiary care hospital in southern India. Clin Infect Dis. 2004;38:e81-6.
- [Google Scholar]






