Translate this page into:
Bacteriophages as therapeutic & disinfectant agents to tackle multidrug-resistant Acinetobacter baumannii
* For correspondence: Dr Rama Chaudhry, Department of Microbiology, All India Institute of Medical Sciences, New Delhi 110 029, India e-mail: drramach@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Multidrug-resistant (MDR) Acinetobacter baumannii is a serious threat for human health worldwide. The studies on agents targeting A. baumannii are imperative due to identified A. baumannii co-infections in COVID-19. Bacteriophages are promising antibacterial agents against drug-resistant bacteria. This study intended to isolate bacteriophages against MDR A. baumannii from the water of river Ganga, to be used potentially as therapeutic and disinfectant particles.
Methods:
Acinetobacter phages were isolated from the Ganga water collected from Kanpur and further tested on 50 MDR A. baumannii isolates to determine host range. The phages were morphologically characterized by transmission electron microscopy. The disinfectant property of the isolated phages was tested by spraying of bacteriophage cocktail on MDR A. baumannii contaminated plastic surface, analyzed by colony-forming unit (CFU) and bioluminescence assay (adenosine triphosphate monitoring).
Results:
A total of seven bacteriophages were isolated against MDR A. baumannii. The bacteriophages lysed three MDR A. baumannii isolates out of 50 tested, showing narrow host range. Electron microscopy revealed hexagonal heads and long tails of bacteriophages, belonging to order Caudovirales. The bacteriophage cocktail reduced the MDR A. baumannii load efficiently on plastic surface, evidenced by reduction in CFUs and bioluminescence.
Interpretation & conclusions:
The findings of this study suggest that the isolated bacteriophages are potential lytic agents for MDR A. baumannii clinical isolates, and may be used as potential therapeutic agents as well as disinfectant to combat MDR A. baumannii with due consideration to phage host specificity, with further characterization.
Keywords
Acinetobacter baumannii
antimicrobial
bacteriophage
disinfectant
multidrug resistant
Increasing antimicrobial resistance of pathogenic microorganisms is continually presenting a challenging scenario to human beings and creating a major financial burden on the healthcare system. Acinetobacter baumannii has been placed on the top priority by the WHO for research and development to discover novel antibiotics1 and has been grouped as an ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa and Enterobacter species) pathogen; a group of drug-resistant nosocomial microorganisms2. A. baumannii is a fast-growing aerobic bacterium causing a broad range of hospital-acquired infections, bacteraemia, endocarditis, meningitis, septicaemia, ventilator-associated pneumonia, urinary tract infections, wound sepsis, etc3. It poses a major concern due to the increasing prevalence of drug-resistant strains. Therefore, there is an immediate need to develop a new antibacterial agent to combat this troublesome pathogen.
Bacteriophages are the natural predator of bacteria and considered an attractive and promising class of antibacterial agents against drug-resistant bacterial infections4. Bacteriophages have been known as effective antibacterial agents for more than 100 years4 and can be used as a disinfectant in addition to therapeutic agents5,6. The bacterial infection can be controlled using a bidirectional approach by the application of bacteriophage as a therapeutic particle as well as a natural disinfectant. There are many reports suggesting the therapeutic and disinfectant potential of bacteriophages against pathogenic bacteria including A. baumannii worldwide7-9. However, limited studies on this exist from India.
Recent studies have identified A. baumannii co-infections10,11, in COVID-19 cases. Bacterial co-infection may deteriorate the disease condition, especially in ventilator-associated bacterial pneumonia in COVID-19 patients12, caused by many Gram-negative organisms including A. baumannii13. Therefore, studies on application of bacteriophage as therapeutic and disinfectant agents become more vital in the context of COVID-19 pandemic.
The present study aimed to, demonstrate the antimicrobial potential of the bacteriophages isolated from river Ganga against multidrug-resistant (MDR) A. baumannii as a therapeutic agent as well as a disinfectant on experimental plastic surfaces, for better patient care in the future.
Material & Methods
Antibiotic susceptibility testing: The isolation, identification and antibiotic susceptibility testing of the bacterial isolates were performed at the department of Microbiology (bacteriology section), All India Institute of Medical Sciences (AIIMS), New Delhi, where the samples including blood, pus, cerebrospinal fluid (CSF), bronchoalveolar lavage (BAL) and tracheal aspirates (TA) were obtained from different outpatient departments, wards and intensive care units (ICUs), between November 2017 to October 2019, after obaining approval by the Institutional Ethics Committee (Ref No: IEC-484/02.08.2019, RP-54/2019). The samples were processed for bacterial isolation and identification as per standard guidelines. Initially, the samples were inoculated on suitable culture media; blood, pus, ascitic fluid, bile and tissue biopsy on blood agar and MacConkey agar, CSF, sputum, BAL and TA on blood agar, MacConkey agar and chocolate agar, urine on cystine–lactose–electrolyte-deficient agar. The obtained pure culture was identified by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) spectrometry14.
For each bacterial isolate, the antibiotic susceptibility testing was performed on Mueller–Hinton agar (MHA) using Kirby–Bauer disc diffusion method as per the protocol suggested by Clinical and Laboratory Standards Institute guidelines 201815. The antibiotics used for susceptibility testing included cefotaxime, amikacin, ceftazidime, meropenem, imipenem, ciprofloxacin and piperacillin+tazobactam. The strain was identified as MDR if it was found to be resistant to more than three classes of antibiotics16. The bacterial isolates were preserved by making glycerol stocks and kept at −80°C, till further processing.
Collection of water samples & isolation of bacteriophages: Water samples were collected from five different ghats of River Ganga from Kanpur, Uttar Pradesh in sterile containers in December 2018 and transported to AIIMS, New Delhi, at 4°C. The samples were processed for isolation of bacteriophages against MDR A. baumannii AIIMS-17 with slight modification17. In brief, each water sample was treated with one per cent chloroform (vol/vol) and added to the bacterial culture (log phase) in 1:1 ratio and incubated overnight at 37°C. The suspension was further treated with one per cent chloroform; the supernatant was collected and tested for the presence of bacteriophages by spot assay.
Determination of the antibacterial activity of bacteriophages: To observe the lytic activity of bacteriophages, the spot assay was performed18. In brief, bacteriophage preparation (10 μl volume) was dropped onto the bacterial lawn of A. baumannii AIIMS-17 and incubated overnight to observe lytic activity. The bacteriophages were further subjected to plaque assay for quantification19 and presented as plaque-forming units/ml (PFU/ml). For scaling up, one plaque was cut from the plate, crushed and mixed with the log phase culture of their host and incubated overnight at 37°C. The bacteriophages were harvested after one per cent (v/v) chloroform treatment.
Electron microscopic analysis: The purified bacteriophage particles 1000 μl (107-108 PFU/ml) were filtered through membrane filters 0.22 μm pore size, and centrifuged at 20800 g for 75 min. The pellet was washed twice with 0.1M ammonium acetate (pH 7.0) and negatively stained using phosphotungstic acid (2%, pH 7.0)20. Briefly, 10 μl of bacteriophage preparation was added on the copper grid, allowed to absorb for 10 min, and further stained with two per cent phosphotungstic acid solution (staining solution) for 15 sec. The stained grid was visualized under transmission electron microscopy (Talos) at Sophisticated Analytical Instrumental Facility, AIIMS, New Delhi. Three measurements of phage head/tail lengths were done using Image J software.
Determination of lysis activity of bacteriophages and host range: To assess the lysis activity of each phage, the active log phage bacterial culture (107 colony-forming unit [CFU] /ml) of A. baumannii AIIMS-17 was mixed separately (107 PFU/ml) with phages. The mixture was incubated at 37°C and the bacterial culture optical density was observed at 600 nm using NanoQuant Infinite M200 Pro Tecan. The bacterial culture without any phage was used as a control. The experiment was done in triplicate.
To determine host range, each phage was subjected to spot assay against 50 MDR isolates of A. baumannii.
Thermal stability of bacteriophages: Thermal stability of all seven Acinetobacter phages was investigated at different temperatures. The phage aliquots were incubated at 4, 30, 40 and 60°C for 2 and 6 h, respectively, and enumerated by plaque assay.
Determination of antimicrobial property of bacteriophage cocktail on the plastic surface:
Colony-forming unit (CFU) analysis: To avoid any environmental inhibitory factor and observe the antibacterial property of bacteriophages on MDR A. baumannii AIIMS-17 (against which the bacteriophages were isolated) present on surface, the experiment was performed on the plastic surface, in 9 cm diameter petri-plates. The Acinetobacter phage cocktail of all seven isolated phages, AIIMS-Ab-A4, AIIMS-Ab-A5, AIIMS-Ab-A6, AIIMS-Ab-A7, AIIMS-Ab-A8-1, AIIMS-Ab-A8-2 and AIIMS-Ab-A8-3, was prepared by mixing each phage in equal proportion (107 PFU/ml).
Bacterial culture of MDR A. baumannii AIIMS-17 (200 μl of 1 × 107 CFU/ml) was spread in a petri-plate and allowed to partially dry. The plate was sprayed with 200 μl normal saline or Acinetobacter phage cocktail (107 PFU/ml) and covered with lid to avoid drying. At t=0, 2, 4 and 6 h, the swabs were collected (before collecting swab, the surface was allowed to dry) and suspended in 1 ml normal saline, 50 μl volume was spread over MHA plates for CFU analysis.
Adenosine triphosphate (ATP) monitoring on surface: To observe the adenosine triphosphate (ATP) level on the surface in the presence and absence of bacteriophage cocktail, the petri-plates were swabbed with MDR A. baumannii culture (200 μl of 1 × 107 CFU/ml), sprayed with normal saline or bacteriophage cocktail as mentioned for CFU analysis. The swabs were collected using UXL100 at different time points t=0, 2, 4 and 6 h, and ATP levels were monitored by using 3M™ Clean-Trace™ Hygiene ATP Monitoring system (3M Health Care, St Paul, MN, USA) as per the manufacturer’s instruction. The amount of ATP produced; directly proportional to the amount of light emitted by the bioluminescence assay was measured as relative light units (RLUs).
Statistical analysis: The bacteriophages’ head vs. head and tail vs.tail were compared using one-way analysis of variance (ANOVA.) The analysis of change in OD of bacterial culture for lysis assay, temperature stability of phages and CFU and ATP production (bioluminescence) on hard surface were statistically analyzed by GraphPad Prism (version 8.0.2) using repeated measure ANOVA.
Results
Bacterial isolation & identification: A total of 51 MDR clinical isolates of A. baumannii including A. baumannii complex were isolated from the clinical samples of the study participants. The clinical samples included blood, tracheal aspirate (TA), BAL, pus, CSF, mini BAL, ascitic fluid, bile, pleural fluid, sputum and tissue biopsy (Fig. 1A). The clinical isolates were numbered from AIIMS 1 to AIIMS 51. Each isolate was at least resistant to three classes of antibiotics, therefore, considered MDR. The drug-resistant rates of A. baumannii strains towards different classes of antibiotics are presented in Figure 1B. The A. baumannii isolates showed maximum resistance to amikacin (50/51), ceftazidime (50/51), piperacillin+tazobactam (50/51), cefotaxime (49/51), ciprofloxacin (49/51), imipenem (42/51) and meropenem (36/51).
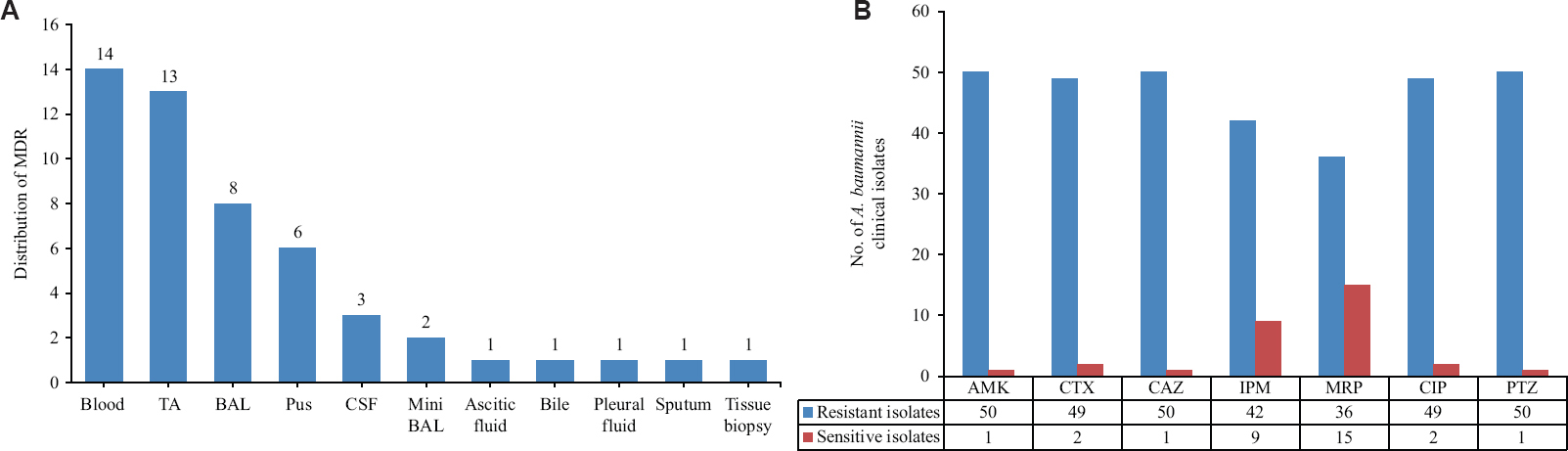
- (A) Distribution of 51 MDR A. baumannii clinical samples; blood, TA, BAL, Pus, CSF, mini BAL, ascitic fluid, pleural fluid, bile, sputum and tissue biopsy. (B) The drug-resistance pattern of 51 MDR A. baumannii clinical isolates for different antibiotics; AMK, CTX, CAZ IPM, MRP, CIP, PTZ. MDR, multidrug resistant; TA, tracheal aspirates; CSF, cerebrospinal fluid; AMK, amikacin; CTX, cefotaxime; CAZ, ceftazidime; IPM, imipenem; MRP, meropenem; CIP, ciprofloxacin; PTZ, piperacillin +Tazobactam
Isolation of bacteriophages & their lytic activity: A total of seven bacteriophages were isolated from five Ganga ghats including Parmat Ghat (A4), Bhairav Ghat (A5), Gola Ghat (A6), Sarsaiya Ghat (A7) and Bhagwatdas Ghat (A8) from Kanpur, Uttar Pradesh. The nomenclature of the phages was done as follows: AIIMS-two alphabets representing the genus and species of bacterium against which the phage was isolated, followed by the abbreviation of water samples and laboratory number. We could isolate seven bacteriophages: AIIMS-Ab-A4, AIIMS-Ab-A5, AIIMS-Ab-A6, AIIMS-Ab-A7, AIIMS-Ab-A8-1, AIIMS-Ab-A8-2 and AIIMS-Ab-A8-3 against one clinical isolate of A. baumannii designated as AIIMS-17. All isolated bacteriophages showed clear lytic activity on performing spot assay (Fig. 2A). The bacteriophages produced circular clear plaques of 1-2 mm in diameter (Fig. 2B).
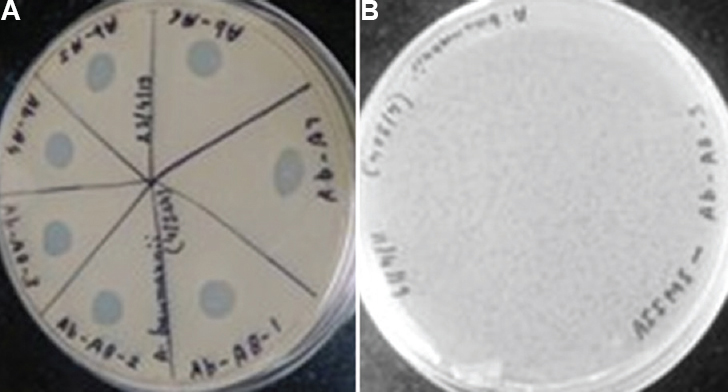
- (A) Spot assay showing lysis of A. baumannii by different isolated Acinetobacter phages; AIIMS-Ab-A4, AIIMS-Ab-A5, AIIMS-Ab-A6, AIIMS-Ab-A7, AIIMS-Ab-A8-1, AIIMS-Ab-A8-2, and AIIMS-Ab-A8-3. (B) A representative picture showing plaques of Acinetobacter phage AIIMS-Ab-A8-3.
Microscopic characterization of bacteriophages: The transmission electron microscopy revealed the hexagonal heads and long tail of bacteriophages; therefore, all phages belonged to order Caudovirales. The phages AIIMS-Ab-A4, AIIMS-Ab-A5, AIIMS-Ab-A8-1, AIIMS-Ab-A82 and AIIMS-Ab-A83 showed single morphology. The phage AIIMS-Ab-A6 showed a mixture of phages (presented as AIIMS-Ab-A6 (A), AIIMS-Ab-A6 (B) with almost similar head and varied tail size (contractile tail), presenting the characteristic feature of Myoviridae family of order Caudovirales. The morphologies of phages are presented in Figure 3 and measurements are listed in Table I. The head and tail size of phages was compared using one-way ANOVA (Table II) on considering coupled view of head as well as tail, the phages showed significant difference except AIIMS-Ab-A6 (B) and AIIMS-Ab-A8-3.

- The transmission electron microscopic view of bacteriophages showing hexagonal heads and long tails.
| Bacteriophage | Head size±SD | Tail length±SD |
|---|---|---|
| AIIMS-Ab-A4 | 57.66±1.11 | 151.74±1.15 |
| AIIMS-Ab-A5 | 64.08±1.21 | 192.43±2.21 |
| AIIMS-Ab-A6-(A) | 66.03±0.84 | 208.66±7.02 |
| AIIMS-Ab-A6-(B) | 67.36±1.18 | 154.63±0.53 |
| AIIMS-Ab-A7 | 64.51±0.69 | 146.58±2.42 |
| AIIMS-Ab-A8-1 | 58.94±0.76 | 142.46±1.83 |
| AIIMS-Ab-A8-2 | 73.67±0.43 | 144.92±0.56 |
| AIIMS-Ab-A8-3 | 69.83±0.52 | 147.78±2.51 |
SD, standard deviation; AIIMS, All India Institute of Medical Sciences
| Bacteriophage strain | AIIMS-Ab-A4 | AIIMS-Ab-A5 | AIIMS-Ab-A6 (A) | AIIMS-Ab-A6 (B) | AIIMS-Ab-A7 | AIIMS-Ab-A8-1 | AIIMS-Ab-A8-2 | AIIMS-Ab-A8-3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | T | H | T | H | T | H | T | H | T | H | T | H | T | H | T | |
| AIIMS-Ab-A4 | *** | *** | *** | *** | *** | NS | *** | NS | NS | * | *** | NS | *** | NS | ||
| AIIMS-Ab-A5 | NS | *** | ** | *** | NS | *** | *** | *** | *** | *** | *** | *** | ||||
| AIIMS-Ab-A6 (A) | NS | *** | NS | *** | *** | *** | *** | *** | ** | *** | ||||||
| AIIMS-Ab-A6 (B) | * | NS | *** | ** | *** | * | NS | NS | ||||||||
| AIIMS-Ab-A7 | *** | NS | *** | NS | *** | NS | ||||||||||
| AIIMS-Ab-A8-1 | *** | NS | *** | NS | ||||||||||||
| AIIMS-Ab-A8-2 | ** | NS | ||||||||||||||
P *<0.5; **<0.001; ***<0.001. NS, not significant; H, head of the phage; T, tail of the phage
The lysis curve: To assess the lysis activity of the phages on the host strain, the A. baumannii AIIMS-17 host was grown in LB broth and infected by all seven bacteriophages separately, blank served as bacterial control without any phage. The significant reduction in bacterial OD was observed on two hours, followed by a higher reduction up to six hours (Fig. 4) in comparison to a blank (P<0.01). Out of all seven used phages, the maximum bacterial growth reduction was observed by AIIMS-Ab-A7 and least by AIIMS-Ab-A8-2.
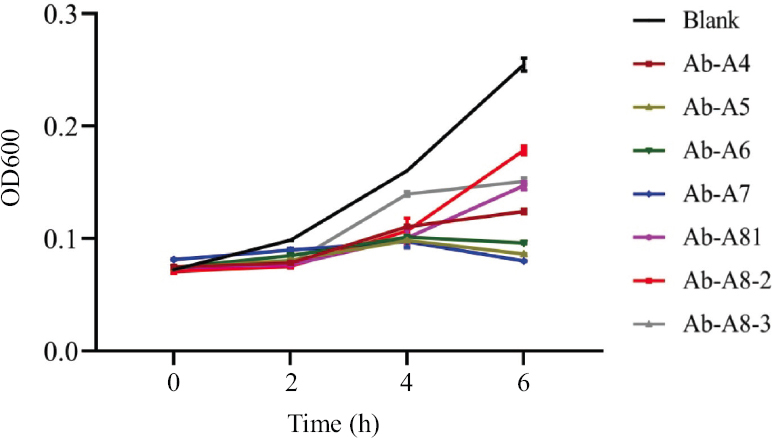
- The lysis curve of MDR Acinetobacter baumannii AIIMS-17 infected with different Acinetobacter phages, showing a significant reduction in OD in the presence of bacteriophage in comparison to uninfected bacterial culture (Blank). MDR, multidrug resistant
Host range analysis: In order to determine the host range of bacteriophages, the isolated bacteriophages were spotted on 51 isolates of MDR A. baumannii including the strain AIIMS-17 used as the host for isolation. The lytic activity was observed in only three out of the 50 tested isolates (Table III) in addition to the isolate against which the phages were isolated. The bacterial isolates lysed from the bacteriophages belonged to different clinical samples (Table IV).
| Bacterial isolate number | Bacteriophages | ||||||
|---|---|---|---|---|---|---|---|
| AIIMS-Ab-A4 | AIIMS-Ab-A5 | AIIMS-Ab-A6 | AIIMS-Ab-A7 | AIIMS-Ab-A8-1 | AIIMS-Ab-A8-2 | AIIMS-Ab-A8-3 | |
| AIIMS-1 to AIIMS-16 | − | − | − | − | − | − | − |
| AIIMS 17 | + | + | + | + | + | + | + |
| AIIMS-18 to AIIMS-30 | − | − | − | − | − | − | − |
| AIIMS-31 | + | + | + | + | + | + | + |
| AIIMS-32 | − | − | − | − | − | − | − |
| AIIMS-33 | + | + | + | + | + | + | + |
| AIIMS-34 to AIIMS 46 | − | − | − | − | − | − | − |
| AIIMS-47 | + | + | + | + | + | + | + |
| AIIMS-47 to AIIMS-51 | − | − | − | − | − | − | − |
All bacteriophages were isolated against A. baumannii AIIMS-17. + indicates lysis of bacteria in presence of phage; − indicates no lysis of bacteria in presence of phage
| Bacterial strain number | Sample type | Antibiotic resistance pattern |
|---|---|---|
| AIIMS-17 | Tracheal aspirate | AMK, CTX, CAZ, CIP, IPM, PTZ |
| AIIMS-31 | Pus (drain from cardiothoracic and vascular surgery) | AMK, CTX, CAZ, CIP, MRP, PTZ |
| AIIMS-33 | Bronchoalveolar lavage | AMK, CTX, CAZ, CIP, IPM, PTZ |
| AIIMS-43 | Endotracheal aspirate | AMK, CTX, CAZ, CIP, IPM, MRP, PTZ |
All bacteriophages have been isolated against A. baumannii AIIMS-17. AMK, Amikacin; CTX, Cefotaxime; CAZ, Ceftazidime; CIP, Ciprofloxacin; IPM, Imipenem; MRP, Meropenem; PTZ, Piperacillin + tazobactam
Bacteriophages’ thermal stability: All bacteriophages remain stable at 4°C for a 6 h exposure. While phage stability varied on temperature exposure of 30°C and 40°C, as presented in Figure 5. The AIIMS-Ab-A8-1 was observed as least stable and AIIMS-Ab-A8-3 showed higher stability at 30 and 40°C. However, at 60°C, no phage could survive even for two hours.
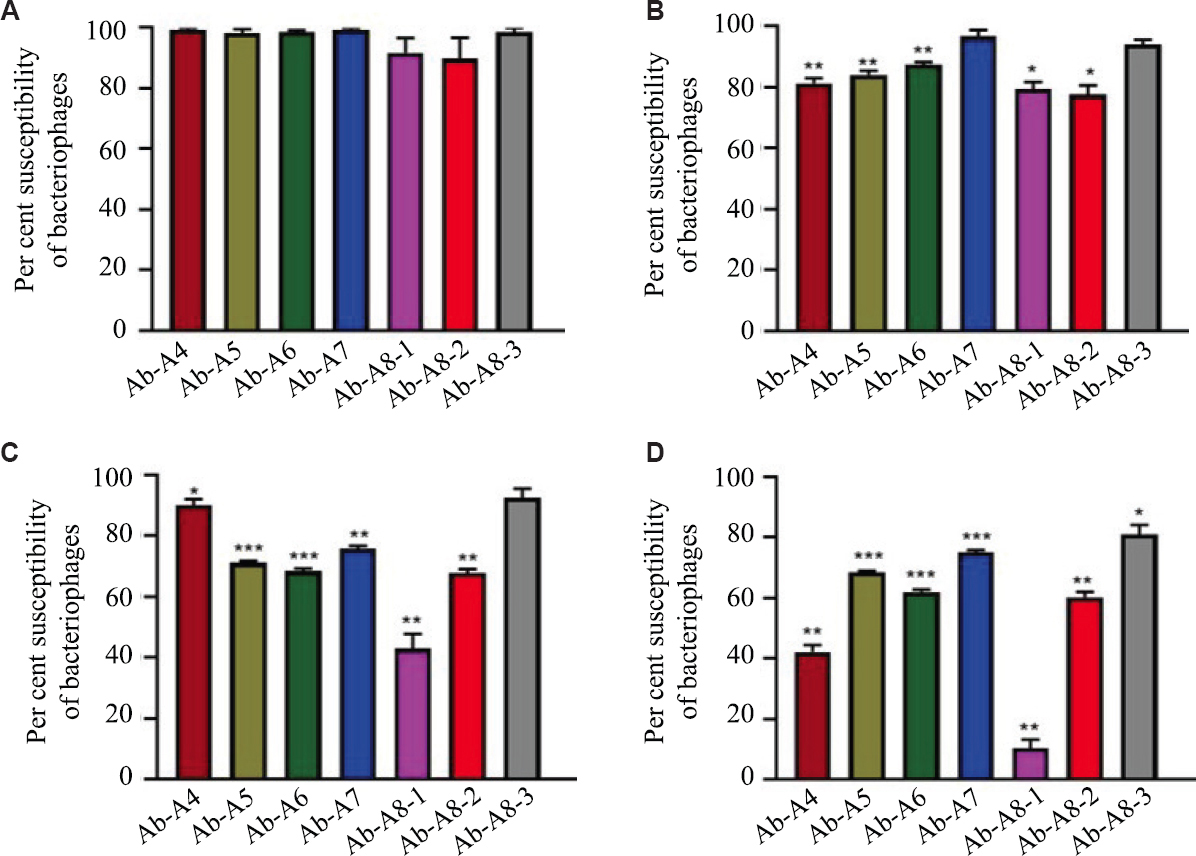
- Thermal stability of different bacteriophages exposed at (A) 30°C for two hours, (B) 30°C for six hours, (C) 40°C for two hours, and (D) 40°C for six hours.
Antibacterial activity of bacteriophage cocktail on the surface: The activity of Acinetobacter phage cocktail over bacterial culture was tested by CFU analysis as well as by the measurement of ATP in terms of RLUs. The CFU analysis showed a significant reduction in the presence of bacteriophages at each time point (P<0.001) (Table V). However, initially, RLUs levels increased, followed by a significant reduction in RLU, produced by phages to bacteria interaction after 4-6 h (Table VI). The initial enhancement in RLUs reflected active lysis of bacterial cells.
| Time point (h) | 105×CFU/ml CFU-Ab±SD | 105×CFU/ml CFU-AbP±SD | P CFU-Ab versus CFU-AbP |
|---|---|---|---|
| t=0 | 254±19.79 | 123±16.26 | <0.001 |
| t=2 | 530±14.14 | 56±3.53 | <0.001 |
| t=4 | 1187±9.8 | 33±1.41 | <0.001 |
| t=6 | 2306±7.7 | 25±1.41 | <0.001 |
CFU-Ab: t=0 versus t=2 P<0.001, t=2 versus t=4 P<0.001, t=4 versus t=6 P<0.001; CFU-AbP: t=0 versus t=2 P<0.001, t=2 versus t=4 P<0.05, t=4 versus t=6 P>0.05; CFU-Ab: CFUs of MDR Acinetobacter baumannii in presence of normal saline; CFU-AbP: CFUs of MDR A. baumannii in presence of Acinetobacter phage cocktail. SD, standard deviation; CFU, colony forming unit; MDR, multidrug-resistant
| Time point (h) | RLU-Ab±SD | RLU-AbP±SD | P RLU-Ab vs. RLU-AbP |
|---|---|---|---|
| t=0 | 2469±47.03 | 2692.218±17.38 | <0.05 |
| t=2 | 12817±394.62 | 17658.9±162.57 | <0.001 |
| t=4 | 56869±1623 | 46665.59±1858.51 | <0.01 |
| t=6 | 334299±4629 | 17525.01±216.38 | <0.001 |
RLU-Ab: RLU produced by MDR Acinetobacter baumannii on plastic surface sprayed with normal saline; RLU-AbP: RLU produced by MDR A. baumannii on plastic surface sprayed with Acinetobacter phage cocktail; RLU-Ab: t=0 vs. t=2 P<0.01, t=2 vs. t=4 P<0.01, t=4 vs. t=6 P<0.001; RLU-AbP: t=0 vs. t=2 P<0.001, t=2 vs. t=4 P<0.01, t=4 vs. t=6 P<0.01. SD, standard deviation; RLU, relative light units
Discussion
The MDR A. baumannii has become a serious threat to human health worldwide in the recent years1. Phage therapy is emerging as a promising approach to treat drug-resistant bacterial infections21. Recently, many reports have suggested the potential impact of phage as a therapeutic agent against MDR A. baumannii22,23, as well as a disinfectant8,9. The advantage to apply bacteriophages in place of other antimicrobial agents includes; no harm to normal microbiota, auto-dosing of bacteriophages24. The safe application of bacteriophages protecting normal microbiota is attributed due to the host specificity of bacteriophages, while the replication ability of phages on its host provides them this auto-dosing property. For therapeutic applications, the phage dose should be maintained for repeated application due to the clearance of phages from the immune system. However, upon application as a disinfectant, auto-dosing plays a role for a better effect24.
In the present study, seven bacteriophages were isolated, active against MDR A. baumannii from the water of the river Ganga. The phages showed a narrow host range on MDR A. baumannii clinical isolates. The phages showed effective lysis of the host in liquid culture but varied in their thermal stability. The bacteriophage cocktail effectively lysed the A. baumannii on a plastic surface demonstrated by CFU analysis and bioluminescence assay. The TEM showed hexagonal heads and the long tail of all phages.
Bacteriophages are ubiquitous in nature. These are present in rivers, lakes, ponds, sewage, soil, sea and hydrothermal vents25-27. The presence of bacteriophages in the Indian rivers Ganga and Yamuna was first discovered in 1896 by Hankin28. A recent report29 also suggested the presence of bacteriophages in the Ganga water against putrefying and pathogenic bacteria. The present study further supports the presence of bacteriophages against MDR A. baumannii, attributing the antimicrobial nature of the river Ganga water. The isolated bacteriophages produced clear spots and plaques on MDR A. baumannii lawns, indicating their lytic action, which is in accordance with previous findings30,31, with a narrow host range of the isolated bacteriophages as reported earlier32-34. This phage specificity and narrow host range are probably due to the attachment of the bacteriophages to the specific receptor binding proteins present on host bacterial surface35.
The disinfectant nature of A. baumannii bacteriophages has been successfully demonstrated by others8,9. Chen et al8 showed the disinfectant potential of phages on A. baumannii on the glass surface. Another study9 presented the decreased rates of infection caused by carbapenem-resistant A. baumannii across ICUs in a teaching hospital upon application of daily cleaning practices added with a bacteriophage-containing aerosol against Carbapenem-resistant A. baumannii. In this study too, a significant reduction in bacterial load on the application of bacteriophage cocktail over MDR A. baumannii (against which, the phages were isolated) was observed. The lysis was also documented by ATP monitoring. The initial interaction of bacteria to phage produced an increase in bioluminescence; indicating the active lysis of bacterial cells36, followed by reduced bioluminescence in the presence of phage cocktail suggesting a lower bacterial load in comparison to control (bacteria without phage).
The phage characterization including parameters such as morphology, molecular features, pharmacology and immunological aspects are the key components to study and apply them for human benefits37. Morphological features are considered an important aspect for bacteriophage classification and characterization. On performing TEM, all bacteriophages showed hexagonal heads and long tails. Therefore, the isolated phages belonged to order Caudovirales.
There is growing evidence suggesting the potential of bacteriophage therapy against drug-resistant pathogens. In addition, the bacteriophages may be used as surface disinfectant6. The isolation of bacteriophages against A. baumannii have been reported earlier for their therapeutic applications. The bacteriophages have been found as an effective antibacterial agent against A. baumannii in Galleria mellonella larvae as well as the mouse model of acute pneumonia38. A successful treatment using bacteriophage was demonstrated against MDR A. baumannii for a 68 yr old diabetic patient having necrotizing pancreatitis22. In another study, a 77 yr old patient having post-operative MDR A. baumannii infection with cerebritis, subdural and epidural empyema was cured by bacteriophage therapy23.
Recently, A. baumannii co-infections have been reported with SARS-CoV-210,11, strengthen the urgency of effective and natural antimicrobials against A. baumannii. The bacterial viral co-infections may cause miscommunication between the innate and adaptive immunity leading to indirect death in COVID-19 patients. Initially, the innate immune system against high viral load may produce too aggressive response and secretion of inflammatory material into the lungs. The cell debris may nourish the bacterial cells thus causing bacterial co-infections39. Bacterial cells may aggravate further innate immune system to add more inflammatory fluid to the lungs and causing severe damage, sepsis and death40. On the other hand, the immunosenescence can cause late antibody production, thus delay in recovery in elderly patients41. The bacteriophages may be directly applied as aerosol to lyse bacterial co-infections as well as to produce artificial antibodies by phage display42. The bacteriophages may also be used to maintain hospital environmental hygiene9. Thus, multidirectional application of bacteriophages may lead to rapid recovery and better management of COVID-19 patients.
This is a proof of concept study that bacteriophages are innovative methods to deploy to lyse-specific MDR A. baumannii. The present study suggests that the strategy to use bacteriophages as a disinfectant may be promising to eliminate specific host with knowledge of the strains present on the surface to be disinfected and its susceptibility to phage collection due to their narrow host range. Therefore, we conclude that the isolated bacteriophages are promising therapeutic and disinfectant agents for specific MDR A. baumannii. However, further investigations are required.
Financial support and sponsorship
This study was financially supported by National Mission of Clean Ganga (NMCG), Ministry of Water Resources and River Rejuvenation (MoWR), Government of India, New Delhi (GKC-01/2016-17/212/NMCG-Research).
Conflicts of interest
None.
Acknowledgment:
Authors acknowledge Drs Geetika Yadav, Indian Council of Medical Research, New Delhi; Krishna Khairnar, Environmental Virology Cell, CSIR-National Environmental Engineering Research Institute, Nagpur, for coordinating the study; Gopal Nath, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi for providing training for bacteriophage isolation.
References
- Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: WHO Press; 2017. p. :1-7.
- [Google Scholar]
- Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis. 2008;197:1079-81.
- [Google Scholar]
- Analysis of penicillin-binding proteins (PBPs) in carbapenem resistant Acinetobacter baumannii . Indian J Med Res. 2011;133:332-8.
- [Google Scholar]
- Bacteriophage prehistory: Is or is not Hankin, 1896, a phage reference? Bacteriophage. 2011;1:174-8.
- [Google Scholar]
- Bacteriophages as weapons against bacterial biofilms in the food industry. Front Microbiol. 2016;7:825.
- [Google Scholar]
- Efficient removal of hospital pathogens from hard surfaces by a combined use of bacteriophages and probiotics: Potential as sanitizing agents. Infect Drug Resist. 2018;11:1015-26.
- [Google Scholar]
- A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin Infect Dis. 2019;69:167-78.
- [Google Scholar]
- Potential of bacteriophage ΦAB2 as an environmental biocontrol agent for the control of multidrug-resistant Acinetobacter baumannii . BMC Microbiol. 2013;13:154.
- [Google Scholar]
- Application of bacteriophage-containing aerosol against nosocomial transmission of carbapenem-resistant Acinetobacter baumannii in an Intensive Care Unit. PLoS One. 2016;11:e0168380.
- [Google Scholar]
- Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622-9.
- [Google Scholar]
- Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol Spectr. 2021;9:e00163-21.
- [Google Scholar]
- COVID-19: An alert to ventilator-associated bacterial pneumonia. Infect Dis Ther. 2020;9:417-20.
- [Google Scholar]
- Microbial etiology and prognostic factors of ventilator-associated pneumonia: A multicenter retrospective study in Shanghai. Clin Infect Dis. 2018;67:S146-52.
- [Google Scholar]
- Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543-51.
- [Google Scholar]
- Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI Supplement M100. Wayne, PA: CLSI; 2018.
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-81.
- [Google Scholar]
- Phage host range and efficiency of plating. In: In: Bacteriophages. New York, NY, USA: Humana Press; 2009. p. :141-9.
- [Google Scholar]
- Isolation and genome sequence characterization of bacteriophage vB_SalM_PM10, a Cba120virus, concurrently infecting Salmonella enterica serovars typhimurium, typhi, and enteritidis. Curr Microbiol. 2019;76:86-94.
- [Google Scholar]
- Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointes Pharmacol Ther. 2017;8:162-74.
- [Google Scholar]
- Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61:e00954-17.
- [Google Scholar]
- Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis. 2018;5:ofy064.
- [Google Scholar]
- Deep-sea hydrothermal vent viruses compensate for microbial metabolism in virus-host interactions. mBio. 2017;8:e00893-17.
- [Google Scholar]
- The bactericidal action of the waters of the Jumna and the Ganges on the cholera vibrio. Ann Inst Pasteur. 1896;10:511-23.
- [Google Scholar]
- Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii . BMC Microbiol. 2010;10:131.
- [Google Scholar]
- Novel lytic bacteriophage AB7-IBB1 of Acinetobacter baumannii: Isolation, characterization and its effect on biofilm. Arch Virol. 2012;157:1441-50.
- [Google Scholar]
- Isolation and characterization of phi AB2: A novel bacteriophage of Acinetobacter baumannii . Res Microbiol. 2010;161:308-14.
- [Google Scholar]
- The tail associated protein of Acinetobacter baumannii Phage ΦAB6 is the host specificity determinant possessing exopolysaccharide depolymerase activity. PLoS One. 2016;11:e0153361.
- [Google Scholar]
- Isolation of bacteriophages against multidrug resistant Acinetobacter baumannii . Res Pharm Sci. 2017;12:373-80.
- [Google Scholar]
- Reprogramming bacteriophage host range: Design principles and strategies for engineering receptor binding proteins. Curr Opin Biotechnol. 2021;68:272-81.
- [Google Scholar]
- Specific assays for bacteria using phage mediated release of adenylate kinase. J Appl Microbiol. 1998;84:661-6.
- [Google Scholar]
- Pharmacological and immunological aspects of phage therapy. Infect Microbes Dis. 2019;1:34-42.
- [Google Scholar]
- Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019;19:70.
- [Google Scholar]
- The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-74.
- [Google Scholar]
- Together forever: Bacterial-viral interactions in infection and immunity. Viruses. 2018;10:122.
- [Google Scholar]
- Immunosenescence in aging: Between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21.
- [Google Scholar]
- Bacteriophages could be a potential game changer in the trajectory of coronavirus disease (COVID-19) Phage (New Rochelle). 2020;1:60-5.
- [Google Scholar]






