Translate this page into:
Association of reduced count of interleukin-13-producing cells in breast milk with atopic dermatitis in infancy
For correspondence: Dr Mohammad Mahdi Mohammadi, Department of Immunology, Physiology Research Center, Institute of Basic and Clinical Physiology, Kerman University of Medical Sciences, P.O. Box 444, Kerman 76169-14115, Iran e-mail: mmmohamadi@razi.tums.ac.ir
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Atopic dermatitis (AD) is one of the most common pathologic conditions of skin in children. The effect of breastfeeding on the risk of AD remains controversial. The aim of this study was to determine the counts of cytokine-producing cells in the mothers' breast milk of infants with and without AD to assess association, if any.
Methods:
Breast milk samples (10 ml) were obtained from mothers of 25 infants with AD and of 26 healthy infants as a control group. The number of cytokine-producing cells including interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), interleukin-13 (IL-13) and IL-4 in the milk samples was determined using an enzyme-linked immunospot assay technique.
Results:
The mean of IL-13-producing cells in milk was significantly lower in mothers of AD-affected infants in comparison with mothers of normal infants (324.91±255.45 vs. 538.93±465.39, P<0.05). There were no significant differences between mothers of infants with and without AD regarding milk count of IFN-γ-, TNF-α- and IL-4-producing cells.
Interpretation & conclusions:
Our results showed lower number of IL-13-producing cells in milk of mothers of infants with AD. Therefore, lower count of IL-13-producing cells in mothers' milk may confer a susceptibility to AD. Further studies with a large number of samples need to be done to confirm our findings.
Keywords
Atopic dermatitis
breastfeeding
cytokine-producing cells
infants
interleukin-13
milk
Atopic dermatitis (AD) is a common dermatological disorder in infants. The age of onset for nearly 45 per cent of all cases is the first six months of life1. The prevalence of AD varies from two per cent in Iran to 20 per cent in Australia, the UK and Scandinavian countries2. AD is identified to be a complex of genetic predisposition, environmental factors, inflammatory skin mechanisms and some immunologic reactions3. Two mechanisms contribute to the development of AD: immunoglobulin (Ig)E-mediated sensitization and/or dysfunction of the skin barrier4. Clinically, AD may present as acute, sub-acute or in chronic phase. Acute type applies to a rapidly developing red rash which may be blistered and swollen. Chronic form refers to a longstanding irritable area. It is often darker than the encircling skin, thickened (lichenified) and much scraped5. In acute phase, helper T-type 2 (Th2) cells initiate and maintain local inflammation through secretion of interleukin-4 (IL-4) and IL-13 which in turn stimulate IgE production and through IL-5 which mediates eosinophil differentiation. The cytokine IL-4 induces IgE and IgG1 secretion from B-cells6. IL-13 also plays an important role in cutaneous Th2-related immune response7 and can directly induce IL-5 expression and eosinophil infiltration8. However, a shift from Th2 to Th1/Th0 cell-related immune responses was determined in acute-chronic transition9. Interferon gamma (IFN-γ) induces Th1 cells10 and inhibits Th2 cell-related cytokine production11. Tumour necrosis factor-alpha (TNF-α) is a mediator of both Th1 and Th2 responses11, which induces the production of chemotactic agents in skin. Human milk is the preferable way for infant nutrition12. The breast milk is important for immune development, tolerance and regulation of inflammation in infants13. However, its contribution to the prevention of allergic disorders is controversial12. While some reported its protective effect14 and increasing the risk of disease15, others showed no association16.
There is no information regarding the relation of the cytokine-producing cells in the breast milk and AD in infancy. Therefore, this study was undertaken to determine the number of cytokine-producing cells [including Th1 cell-related cytokines (IFN-γ and TNF-α) and Th2-related cytokines (IL-13 and IL-4)] in the mother's breast milk of infants with and without AD to assess the association.
Material & Methods
This study was conducted in the department of Immunology, Kerman University of Medical Sciences, Kerman, Iran, during March to September 2013. Breast milk samples were obtained from mothers of 25 infants (age 10.02±5.82 months) with AD during seven months from March to September 2013 in Afzalipour Hospital of Kerman (a city located in the southeast of Iran). An expert dermatologist and an allergy specialist confirmed the presence of AD, according to the well-known Hanifin and Rajka diagnostic criteria17. The women were chosen based on convenience criteria. A questionnaire including age of breastfed infants and their mothers, type of allergy, drug(s) taken, gender, parents' consanguineous relationship and current diet was filled. The infants who received any immunomodulating and immunosuppressive agents such as antihistamine and local and systemic steroid for two weeks were excluded from the study. Perseverance of mothers for exclusive breastfeeding and abstinence of them from prevalent allergenic foods was considered as inclusion criteria. In all, mothers of 60 infants with AD visited the hospital during the study, of whom 35 women were excluded based on the exclusion criteria. This study was approved by the Ethical Committee of Kerman University of Medical Sciences, Kerman, Iran, and written informed consent was obtained from mothers if they agreed for breast milk sampling.
The control group consisted of 26 mothers of age-matched (9.84±5.50 months) healthy infants, with no history of AD disease and other dermatologic diseases. Age-matching was on the basis of group matching (not individual matching). The mothers of healthy infants visited the health centres for vaccination and monitoring of height and weight of the breastfed infants. All mothers were healthy, with no acute or chronic illnesses. The mothers with a history of recurrent infections, asthma, allergy and atopic diseases, any suspected immunological disorders, cigarette smoking and use of any drugs were excluded from the study. Other exclusion criteria were malignancy, surgery and major trauma in previous months. None of the mothers received any immunomodulating treatment within six months before milk collection.
A breast milk sample (10 ml) was obtained from all mothers included in the study, and the water layer was separated and stored at 4°C until the time of analysis. The number of cells producing cytokines including IFN-γ, TNF-α, IL-13 and IL-4 in milk samples was determined using the commercial ELISpot kits (Mabtech, Stockholm, Sweden) according to the manufacturer's guidelines.
Briefly, fresh milk samples were centrifuged at 2000 ×g for 10 min. After elimination of the fat layer, the pellet was washed with Hank's balanced salt solution and the viable-floating cells (epithelial, mononuclear cells) were counted by a standard method using a haemocytometer slid and trypan blue staining (Merck, Germany). ELISpot 96-well flat-bottomed plates were coated overnight at 4-8°C with 100 μl/well of specific capture antibody including anti-TNF-α and anti-IL-13 [15 and 10 μg/ml in phosphate-buffered saline (PBS), 100 μl/well, respectively]. The coated plates of IFN-γ and IL-4 were prepared. Then, the wells washed with PBS and subsequently blocked with a complete culture medium (Gibco, Invitrogen Ltd, Paisley, UK) containing 10 per cent heat-inactivated foetal calf serum (FCS) (Gibco). The milk cells (2.5×106/well) were plated to a final volume of 200 μl/well of complete culture medium and incubated for 24 h at 37°C with 5 per cent CO 2. After washing, the cytokine-producing cells were visualized using detection antibodies; 100 μl/well [(anti-TNF-α, 0.5 μg/ml), (anti-IL-13, 1 μg/ml), (IFN-γ, 7-B6-ALP diluted 1:200) and (IL-4, IL4-Π-ALP diluted 1:300)]. Streptavidin-conjugated alkaline phosphatase diluted 1:1000 (v/v) in PBS/0.5 per cent FCS (for TNF-α and IL-13) was added and kept for one hour at room temperature (the washing were repeated) and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt and nitroblue tetrazolium chloride substrate incubated for 10-30 min at room temperature in the dark successively (was washed with distilled water). The wells were observed using a stereomicroscope (Macro-Eye, Gordak Instruments, China). The number of specific cytokine-producing cells [spot-forming cells (SFCs)] was calculated by subtracting the number of spots in negative control wells from experimental ones18.
Statistical analysis: Comparison between demographic characteristics of breastfed infants in the two groups was made by Chi-square test. Differences in variables were analyzed using unpaired t test as appropriate. The unadjusted and adjusted odds ratios (ORs) and 95 per cent confidence intervals (CIs) of the risk factors for AD were assayed by binary logistic regression model. The data were analyzed by SPSS statistical software (version 18, Chicago, IL, USA).
Results
Demographic characteristics of mothers and their infants are summarized in Table I. In the present study, the age range of infants was 1-21 months [9.93±5.60 (mean±SD)] (n=51). The age of the mothers of infants with AD was significantly higher than those mothers with infants without AD. A significant difference was also observed between infants with and without AD in case of their sex ratio (M/F) (18/7 in case and 10/16 in control, respectively) (P<0.05) and also the parents' consanguineous relationship (P<0.05).
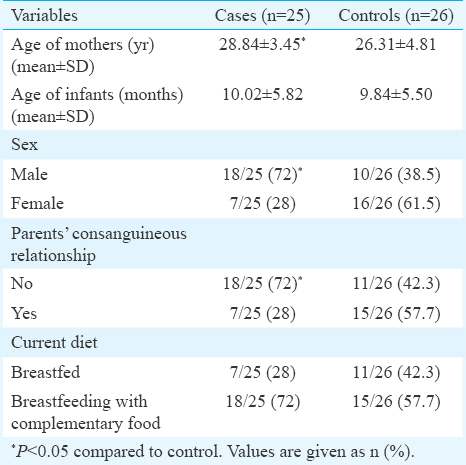
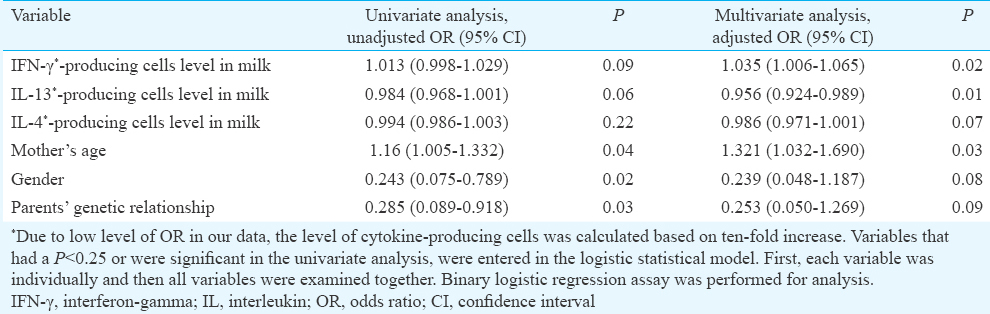
The ORs and 95 per cent CIs of the risk factors of AD are given in Table II. Both unadjusted and adjusted ORs were also calculated. In the adjusted model, between demographic variables, parents' consanguineous relationship and the gender had no significant association with AD, when these variables were matched between the two groups, whereas mother's age has significant association with AD (P=0.03). Moreover, in AD-breastfed infants, one unit increase in age of mother was associated with 32 per cent increase in odds of getting the disease in comparison with healthy breastfed infants.
The quantity of Th1 cell-related cytokine-producing cells in milk is summarized in Table III. The number of IFN-γ-producing cells was higher in the milk of mothers with AD infants compared to mothers with non-AD infants, but the difference was not statistically significant (P=0.08). No significant difference was also observed between mothers with and without AD infants with respect to the TNF-α-producing cells; however, this parameter was found to be augmented in mothers with AD infants.
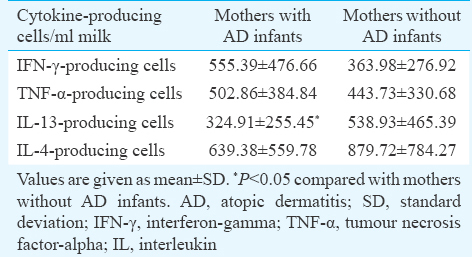
The mean count of IL-13-producing cells was significantly lower in mothers of infants with AD in comparison to mothers of healthy infants (P<0.05). Furthermore, the risk of the disease was decreased to 4 per cent based on ten-fold increase in the milk level of IL-13-producing cells in allergic infants in comparison with non-allergic infants (Table II). No significant variance was observed between mothers with and without AD infants with respect to the IL-4-producing cells count. This parameter, however, was found to be lower in mothers with AD infants. For evaluating the accuracy of our results, the relation of the mean production level of each SFC was analyzed. There was no significant association between the mean count of IL-13- (434.02) with the IL-4- (761.90) and IFN-γ-producing cells (457.81) (P=0.47 and P=0.18, respectively). Conversely, there was a direct correlation between the mean count of IL-4- (761.90) with the IFN-γ mean count of cells (457.81) (P=0.004) (r=0.398) (not shown). Our study also indicated that most cytokine-producing cells were mononuclear and the viability percentage of cells was from 50 to 100 per cent.
Discussion
In this study cytokine-producing cells were measured in the breast milk of mothers feeding their infants with and without AD. In general, the role of Th2 and Th1 as pro-inflammatory and anti-inflammatory cytokines in allergic disorders is agreed. However, the results of the present study showed that the count of IL-13-producing cells was notably less in the milk of mothers of AD infants compared to healthy group. The multivariate analysis also showed that the determination of IL-13-secreting cells might be useful to predict the risk of AD. Moreover, the count of IL-4 producing cells was lower in the milk of mothers with AD infants compared to mothers with non-AD infants, although the difference was not significant.
The precise mechanisms by which the lower count of Th2-cytokine-producing cells may contribute to the development of AD in infancy are not clear. It is known that atopic diseases are caused by Th2 cytokine response to allergens19 and increased levels of IL-4 and IL-13 have been shown in such conditions320. The role of IL-13 in prognosis of AD appears to be complex. This cytokine may function as a pro-inflammatory and an anti-inflammatory cytokine21. Since AD is caused by Th2 cytokine response dominance in acute phase and on the other hand, normal pregnancy is associated with the production of IL-13; based on these findings, infants at risk of atopic disease exhibit defective IL-13 production at birth. This may depict an inherent immaturity in the development of T cell-cytokine responses or could be an outcome of downregulation of responses by other factors22. Furthermore, IL-13 response significantly decreases during the first year of life23.
The reduction in number of the two types of Th2-related cytokine-producing cells may contribute in the development of AD in infancy through impairment of mucosal immunity in the newborn intestine which leads to the local infections, and the infectious agents may have a role in the AD development. The association of a number of infections with AD development at infancy has been reported24.
In accordance with our results, elevated levels of IFN-γ and TNF-α have been reported in allergic patients2526, however, there is a disagreement between our findings and other studies27. The discrepancy may be because of difference in sample type (milk vs. blood) and phase of disease (early in infancy vs. elder age).
It should also be noted that both Th1 and Th2 cells are involved in the pathogenesis of AD. Actually, a biphasic immune response with a predominance of Th2 cell-related response during the acute phase and a dominant Th1 cell-related response in the chronic phase of AD has been reported28. Accordingly, reduced number of mothers' milk count of Th2 cell-related cytokines may result in an imbalance in the Th1/Th2 cell-associated immune responses with tendency toward the Th2 responses that may result in AD development and subsequent dominance of Th1-related pathways. However, modulation of the Th1 and Th2 cell-related immune responses is required for the control of AD29.
Although we found no significant association between type of nutrition and AD, the impact of breast milk in preventing allergy has been reported controversially12141516. These discrepancies may result from differences in study methods or disregarding the patients' genetic dissimilarities and complex immunologic properties of breast milk12.
Our study also showed that most cytokine-producing cells were mononuclear, and this was compatible with the predominance of these cells in breast milk30. On the other hand, viability percentage of cells was from 50 to 100 per cent. Determination of viability percentage is of importance because high level of dead cells (30-50% and more) can represent excessive background staining and lack of special spots in the test7.
This study had several limitations. The sample size was small to draw valid conclusions. Resolving limitations of such studies (e.g. small sample size, deficiency of random selection, short-term period of breastfeeding, absence of planning a single-blind study) and designing more interventional studies to elucidate efficacy or lack of efficacy of breastfeeding on allergic disorders can be beneficial.
In conclusion, our findings showed that small number of IL-13-producing cells in breast milk might be a potential biomarker for predicting AD in infancy. More research is necessary to validate the prediction models for AD in infancy based on evaluation of other cytokines originated from cells existing in the breast milk.
Acknowledgment
Authors thank Ms Arezoo Khosravi and Fatemeh Ezzatkhah, department of Immunology, Kerman University of Medical Sciences, Kerman, Iran, for their help and cooperation with laboratory techniques to carry out this study.
Financial support & sponsorship: This study was financially supported by the Cardiovascular & PhysiologyResearch Center, Institute of basic and clinical Physiology, Kerman University of Medical Sciences, Kerman, Iran (grants number: 90/129).
Conflicts of Interest: None.
References
- International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-54.e15.
- [Google Scholar]
- Association between copy-number variations of the human histamine H4 receptor gene and atopic dermatitis in a Chinese population. Clin Exp Dermatol. 2013;38:295-300.
- [Google Scholar]
- All about the skin. Dermatitis. Available from: https://www.dermnetnz.org/topics/dermatitis/
- Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58-66.
- [Google Scholar]
- Atopic dermatitis. In: Marcdante KJ, Kliegman RM, Jenson HB, Behrman RE, eds. Nelson essentials of pediatrics (6th ed). Philadelphia, PA: Saunders Elsevier; 2011. p. :324-7.
- [Google Scholar]
- Comparison of the Th1, IFN-γ secreting cells and foxP3 expression between patients with stable graft function and acute rejection post kidney transplantation. Iran J Allergy Asthma Immunol. 2013;12:262-8.
- [Google Scholar]
- Intranasal, liposome-adjuvanted cockroach allergy vaccines made of refined major allergen and whole-body extract of Periplaneta americana. Int Arch Allergy Immunol. 2013;161:351-62.
- [Google Scholar]
- The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115:1238-48.
- [Google Scholar]
- The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135:1-4.
- [Google Scholar]
- American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183-91.
- [Google Scholar]
- Effect of prolonged breast-feeding on risk of atopic dermatitis in early childhood. Allergy Asthma Proc. 2014;35:66-70.
- [Google Scholar]
- A multinational study to compare prevalence of atopic dermatitis in the first year of life. Pediatr Allergy Immunol. 2015;26:359-66.
- [Google Scholar]
- Defining ELISpot cut-offs from unreplicated test and control wells. J Immunol Methods. 2013;392:57-62.
- [Google Scholar]
- Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin Exp Dermatol. 2014;39:48-53.
- [Google Scholar]
- IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:298-310.
- [Google Scholar]
- Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103-11.
- [Google Scholar]
- Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol. 2000;105:951-9.
- [Google Scholar]
- Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol. 2003;112:740-6.
- [Google Scholar]
- Evaluation of the concentration of proinflammatory/Pro Th1 cytokines IFN-γ and TNF-α and anti inflammatory/Pro Th2 Cytokines IL-13 and IL-4 in breast milk and their relationship to atopic dermatitis. Tehran Univ Med J. 2013;70:640-51.
- [Google Scholar]
- Antigen specific cytokine response in pediatric patients with atopic dermatitis. Pediatr Allergy Immunol. 2005;16:113-20.
- [Google Scholar]
- Reduced IFN-gamma- and enhanced IL-4-producing CD4+ cord blood T cells are associated with a higher risk for atopic dermatitis during the first 2 yr of life. Pediatr Allergy Immunol. 2010;21:5-13.
- [Google Scholar]
- Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-5.
- [Google Scholar]
- The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr Probl Dermatol. 2011;41:80-92.
- [Google Scholar]
- Cytokines in human milk: Properties and potential effects upon the mammary gland and the neonate. J Mammary Gland Biol Neoplasia. 1996;1:251-8.
- [Google Scholar]






