Translate this page into:
Association of organochlorine pesticides with the mRNA expression of tumour necrosis factor-alpha (TNF-α) & cyclooxygenase-2 (COX-2) genes in idiopathic preterm birth
Reprint requests: Dr B.D. Banerjee, Environmental Biochemistry & Molecular Biology Laboratory, Department of Biochemistry, University College of Medical Sciences & G.T.B. Hospital (University of Delhi), Dilshad Garden, Delhi 110 095, India e-mail: banerjeebd@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Preterm birth (PTB) is an important cause of prenatal death, neonatal morbidity and mortality and adult illness. Increased inflammation occurs in normal parturition, and inflammatory cytokines and oxidative stress are found to be higher in PTB cases. The present study was planned to investigate the association of organochlorine pesticides (OCPs) with mRNA expression of inflammatory pathway genes such as tumour necrosis factor-alpha (TNF-α) and cyclooxygenase-2 (COX-2) in preterm delivery (PTD) cases.
Methods:
Maternal blood samples of PTD (n=30) cases and equal number of term delivery (n=30) were collected at the time of labour. Women occupationally exposed to OCPs and other high risk factors such as anaemia, hypertension, bacterial vaginosis, renal and heart disease, diabetes, etc. were excluded. The OCP levels were estimated by gas chromatography, and mRNA expressions of TNF-α and COX-2 genes were analysed using real-time PCR (qPCR).
Results:
Significantly higher levels of β-HCH (beta-hexachlorocyclohexane, 95% CI=2.08-4.633, P=0.001), p’p’-DDE (para, para-dichlorodiphenyldichloroethylene, 95% CI=0.546-2.551, P=0.003), and o’p’-DDD (ortho, para-dichlorodiphenyldichloroethane, 95% CI=0.004-0.690, P=0.047) were observed in maternal blood of PTB cases as compared to term delivery. The mRNA expressions of COX-2 and TNF-α genes were 3.13 and 2.31 folds higher in PTB cases in comparison to term delivery. Linear positive correlations were observed between period of gestation (POG) and ΔCt of COX-2 and TNF-α genes.
Interpretation & conclusions:
Environmental factors such as OCPs may be associated with inflammatory events showing gene-environment interaction in PTB cases. Evaluating the molecular control of inflammation along with gene environment interaction may be used as a model to explore the aetiology of idiopathic PTB cases and may be considered for the prognosis of adverse reproductive outcomes.
Keywords
COX-2
gene-environment interaction
mRNA expression
organochlorine pesticides
preterm birth
TNF-α
Globally, 15 million neonates (11% of the total births) are born preterm every year1. Preterm infants face lifelong morbidity with increased risk of respiratory disease2, intellectual disability, cerebral palsy34 and vision impairment5. With a high risk of acquiring infection, preterm birth (PTB) is one of the major contributors to infant mortality. There are various known factors such as hormonal imbalance, oxidative stress, genetic disposition, gene environment interaction, low socio-economic status, etc. which may lead to PTB6.
Organochlorine pesticides (OCPs) are widely used in India under public health and agricultural programmes7. OCPs are xenoestrogenic in nature, slow in degradation and highly lipophilic. These pesticides tend to bio-accumulate in lipid-rich tissues because of their strong lipophilic nature and slow biodegradability8. Earlier studies, including those from our laboratory, have reported that OCPs may increase the risk of PTB7910. Further, we have also reported that polymorphisms in xenobiotic metabolizing genes along with more than 50th percentile of OCPs (≥ median value) may lead to a significant decrease in the period of gestation (POG)10.
An exaggerated inflammatory response is an emerging mechanism in conditions such as spontaneous abortion, PTB, preterm premature rupture of the membranes (PPROM), preeclampsia, and other “great obstetrical syndromes”11. Increased inflammation occurs in normal parturition, and inflammatory cytokines are comparatively higher in women who deliver preterm12. Tumour necrosis factor-alpha (TNF-α) is a pleiotropic cytokine that plays an important role in pregnancy as well as in the pathophysiology of inflammatory conditions. TNF-α is one of the several cytokines, which bears a potent regulatory effect on early preimplantation, development and pregnancy13. Blastocyst implantation, vascular permeability of the endometrium, and uterine deciduation are affected by cyclooxygenase (COX) gene expression, which is controlled by TNF-α14. The onset of labour, whether at term or preterm, involves upregulation of numerous inflammatory mediators like cytokines, prostaglandins (PGs), etc. TNF-α contributes to the process of parturition by stimulating uterine contractions in conjugation with other inflammatory cytokines, while the COX-2 enzyme acts as a link between inflammation and PTB through its involvement in the synthesis of prostaglandins15. High levels of TNF-α have been implicated in pregnancy complications such as infection and foetal growth retardation along with early and unexplained spontaneous abortions1617.
This study was undertaken to investigate the association of OCPs with mRNA expression of TNF-α gene, and gene-gene interaction between TNF-α and COX-2 genes in women who delivered preterm.
Material & Methods
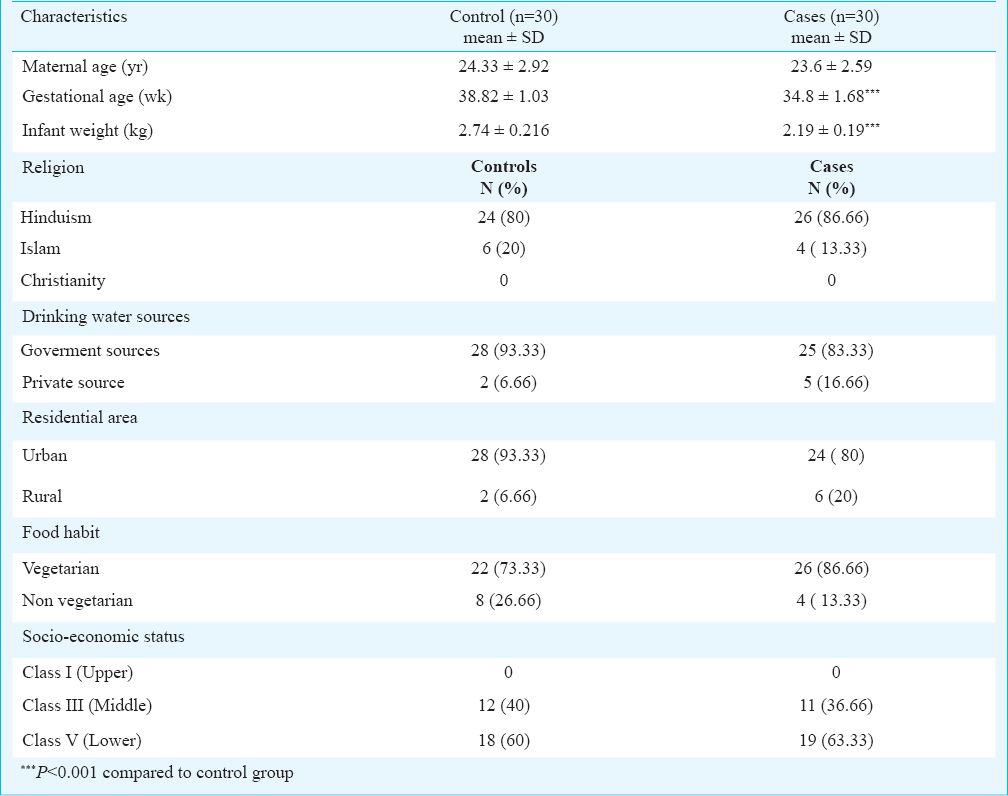
Subjects: In this age-matched, case-control study, blood samples from 30 women who had below 37 wk of gestation as cases and equal number of women having a gestation period of 37 wk or beyond as controls were collected at the time of spontaneous labour. The study was conducted in the departments of Obstetrics and Gynecology, and Biochemistry, Guru Teg Bahadur (GTB) Hospital associated with University College of Medical Sciences (UCMS), Delhi, India, from July 2012 to June 2013. All women went into labour spontaneously with intact membrane. A lifestyle survey of the study subjects was done to collect general demographic information to define the inclusion/exclusion criteria. Socio-economic status of cases and controls was decided by Kuppuswamy's scale18. Women with anaemia, hypertension, toxaemia of pregnancy, renal disease, heart disease, diabetes, urinary tract infections, bacterial vaginosis, metabolic disorders, tuberculosis, smoking, alcohol consumption or chronic drug intake and having complications during pregnancy and/or labour were excluded from the study. None of the women were occupationally and/or environmentally exposed to the agricultural pollutants. A written informed consent was taken from all the study participants. This study was approved by the institutional ethics committee for human research, UCMS and GTB hospital (University of Delhi), Delhi.
Two thousand two hundred fifty two (n=2252) women were screened during the study period from labour wards of the hospital over a period of one year. Of these, 1,832 delivered at term and 420 had preterm delivery. Among the 420 preterm deliveries, 35 women were included in the study as cases (sample of convenience). Of these, five samples were excluded: two during standardization procedure for RNA isolation and three due to transport delay. Forty five women with term delivery were included as controls initially, however, 15 samples were excluded: nine during standardization procedure for RNA isolation and six due to transport delay.
Sample collection and storage: Maternal blood (2 ml) was collected in EDTA containing vials at the time of spontaneous labour in Central Labour Room. A volume of 250 μl of whole blood sample was fixed in TRIzol LS (Ambion, USA) reagent and stored at -80°C and was processed within two to three days for RNA isolation. One ml blood was stored at 4°C for the pesticides residue analysis.
RNA isolation and complementary DNA (cDNA) synthesis: Total RNA was isolated from whole blood using TRIzol LS reagent19. Isolated total RNA was quantified using Nano drop (Thermo Fisher, USA) by measuring optical density value at 260/280 nm and concentration in ng/μl. Quality of isolated RNA was checked on denatured gel electrophoresis. Total RNA (1000 ng) was converted into first strand cDNA using maxima first strand cDNA synthesis kit (Fermentas, USA) according to manufacturer's protocol. For cDNA synthesis, RNA was first incubated for 10 min at 25°C followed by 15 min at 50°C; the reaction was terminated by heating at 85°C for five min. This cDNA was diluted four times in nuclease-free water and was used directly in qPCR or was stored at -80°C for longer storage.
Gene expression study: A qPCR experiment was conducted to measure expression of inflammatory genes that were differentially expressed in blood samples of women who delivered at preterm and term. The qPCR reactions were performed on CFX Connect™ Real-Time PCR Detection System (BioRad, USA). Briefly, the PCR amplification master mix of 45 μl contained 6.75 μl of diluted cDNA, 13 μl of evagreen master mix (BioRad, USA), 10 pmol of each forward and reverse specific primer pairs (Table I)2021, and nuclease-free water, and finally the master mix (20 μl) was dispensed into two PCR tubes. Each sample was run in duplicate along with no template control wells. Gene expression was normalized with reference to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, and the results were calculated using the comparative ΔΔCt method manually22, where threshold cycle (Ct) is the cycle number at which the fluorescence generated within a reaction crosses the fluorescence threshold, a fluorescent signal significantly above the background fluorescence, ΔCt = Ct(target gene)-Ct(reference gene) and ΔΔCt = ΔCt(case)- ΔCt(control).
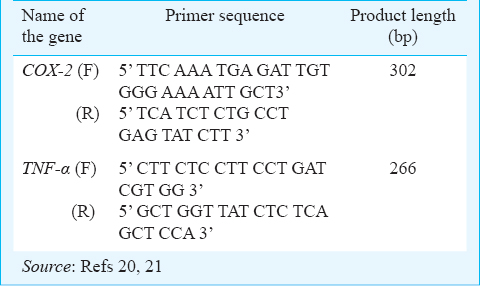
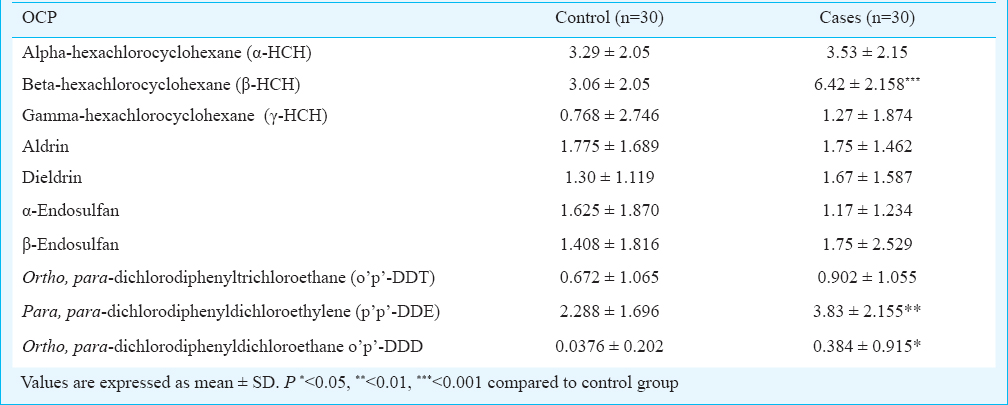
OCPs extraction and clean up: Extraction of OCPs was done using hexane and acetone (2:1 v/v) according to the standardized protocol from our laboratory10. Clean up of the samples was done by column chromatography following US environmental protection agency (USEPA) method23. Elutant was collected and hexane was evaporated to concentrate the sample using rotary evaporator (Shiwaki, India). The concentrated residues were dissolved using one ml of hexane for further analysis. Ten pesticides were estimated namely alpha-hexachlorocyclohexane (α-HCH), beta-hexachlorocyclohexane (β-HCH), gamma-hexachlorocyclohexane (γ-HCH), aldrin, dieldrin, α-endosulfan, β-endosulfan, ortho, para-dichlorodiphenyltrichloroethane (o’p’-DDT), para, para-dichlorodiphenyldichloroethylene (p’p’-DDE), and ortho, para-dichlorodiphenyldichloroethane (o’p’-DDD). OCP residue levels were estimated by a Perkin Elmer gas chromatography system equipped with a 63Ni selective electron capture detector (Perkin Elmer, USA). The detection limit was 0.05 pg for perchloroethylene and 4 pg/ml for each OCP. Quantitative analysis of OCP residues in each sample was done by comparing the peak areas with those obtained from a standard chromatogram of blended OCPs of known concentration. Five samples of maternal blood in triplicate each were spiked with a mixed standard of OCPs, 5 and 25 ng/ml, respectively. The average recovery of fortified samples exceeded 95 per cent. A quality check sample was always run for each set of sample for pesticide analysis to maintain accuracy.
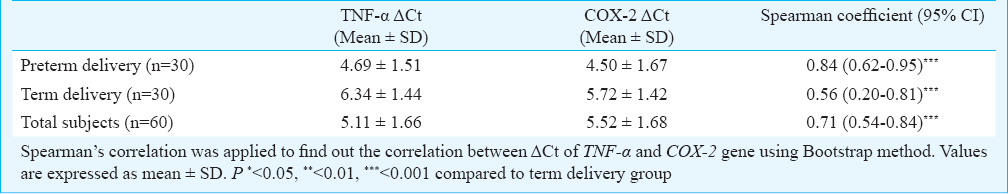
Statistical analysis: Statistical software SSPS for windows (version 16.0, Chicago, IL, USA) was used for statistical analysis. Chi-square/Fisher's exact tests was applied to compare all socio-demographic characteristics in preterm and term delivery cases according to data being quantitative or qualitative, respectively. As pesticides and gene expression data were not normally distributed, non-parametric Mann-Whitney U test was used to compare pesticide levels in cases and controls. Correlations were tested by Spearman's rank co-efficient of correlation. OCP levels and Ct of TNF-α and COX-2 genes were categorized as per the median values of control groups; <50 indicates value less than 50th percentile (median) and > 50 indicates value greater than 50th percentile (median). Binary logistic regression analysis was applied to analyze the odds ratio. Multivariable logistic regression was applied taking POG<37 wk (case=1) and POG>37 wk (control=0) and independent variables namely OCPs and TNF-α using backward elimination likelihood ratio method24; P<0.05 was used to enter the variable while P>0.10 was used to remove the variable from the model. Since, we analyzed DDT and its metabolites i.e. p’p’-DDE and o’p’-DDD separately, three different models were applied: model 1 for DDT along with other OCPs and TNF-α; model 2 for p’p’-DDE along with other OCPs and TNF-α; and model 3 for o’p’-DDD along with other OCPs and TNF-α. The classification of multivariable logistic regression was done considering the cut-off probability as 0.5 and percentage of correctly classified was determined. Nagelkerke R square was calculated for percentage of variable expanded by the variables in the models. Spearman's correlation was applied to find out the correlation between ΔCt of TNF-α and COX-2 genes using Bootstrap method (SPSS, ver. 21.0, Chicago, IL, USA).
Results
The demographic characteristics such as residential area, source of drinking water, socio-economic status and dietary habits were not significantly different in cases as compared to controls. However, cases were significantly (P<0.001) associated with the lower level of infant weight (Table II). Significantly higher levels of β-HCH (95% CI=2.08-4.63, P=0.001), p’p’-DDE (95% CI=0.546-2.55, P=0.003), and o’p’-DDD (95% CI=0.004-0.690, P=0.047) were observed in cases as compared to controls (Table III). The other OCPs levels, although higher but were not significantly different.
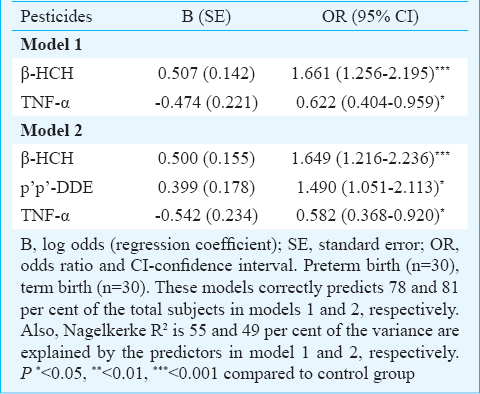
Multivariable logistic regression model of association between OCPs and ΔCt of TNF-α with PTB as outcome variable was applied. In model 1, a positive significant association was found between β-HCH and PTB (95% CI=1.256-2.195, P=0.001) and a significant negative association was found between ΔCt of TNF-α and PTB (95% CI=0.404-0.959, P=0.032). In model 2, β-HCH (95% CI=1.216-2.236, P=0.001) and p’p’-DDE (95% CI=1.051-2.113, P=0.025) were found to be significantly associated with PTB. A significant negative association was also found between ΔCt of TNF-α and PTB (95% CI=0.368-0.920, P=0.020) (Table IV). These models correctly predicted 78 and 81 per cent of the total subjects in models 1 and 2, respectively. Nagelkerke R2 values were 55 and 49 per cent of the variance as explained by the predictors in models 1 and 2, respectively. In model 3, no significant association was found (data not shown). It was found that in >50 β-HCH and <50 TNF-α, and >50 DDE and < 50TNF-α there was a significant reduction in POG also (Fig. 1a, b). This indicated that when both β-HCH levels and TNF-α gene expression as well as DDE levels and TNF-α gene expression were high in maternal blood, there was a significant reduction in POG.
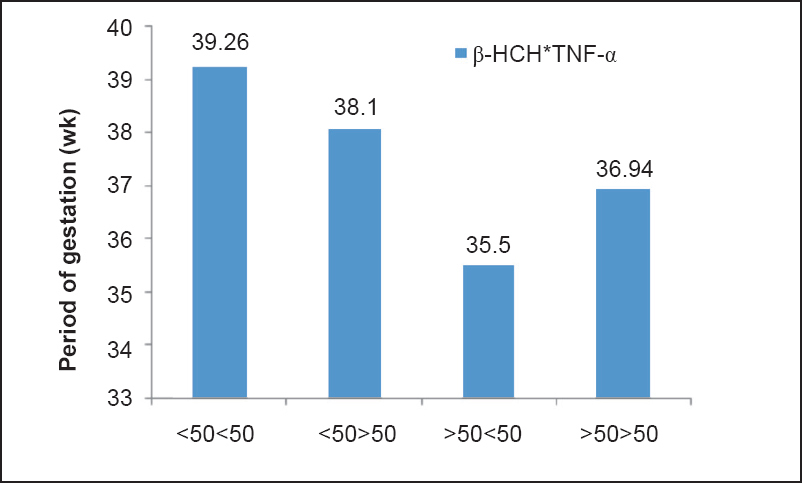
- Interactive effect of β-HCH and TNF-α on period of gestation (POG).
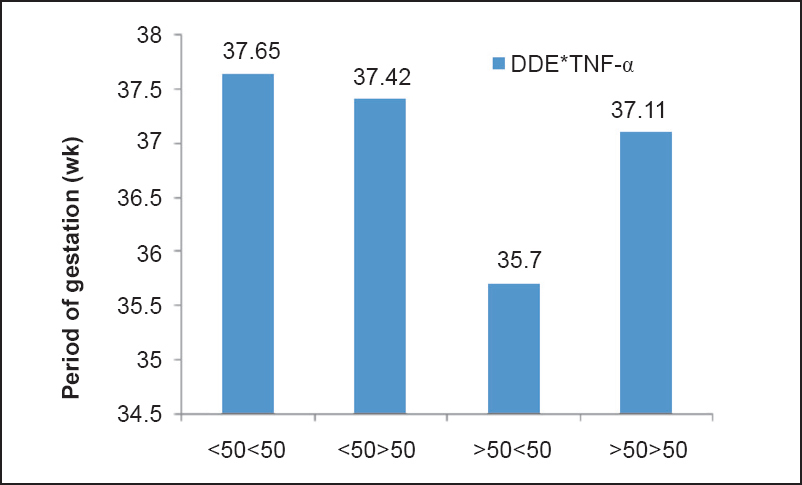
- Interactive effect of DDE and TNF-α on period of gestation (POG).
The mRNA expressions of COX-2 and TNF-α genes were 3.13 and 2.31 folds higher in PTB cases, respectively in comparison to term delivery. Significant positive correlations were observed between ΔCt of COX2 and TNF-α in PTB cases, term controls and total subjects (r=0.84, 0.56 and 0.71, respectively) (Table V). Linear mild positive correlations were observed between POG and ΔCt of COX-2 (r= 0.114) and TNF-α (r=0.1) (Figs. 2, 3) indicating that women having higher expression of COX-2 and TNF-α genes had reduction in POG leading to premature onset of labour.
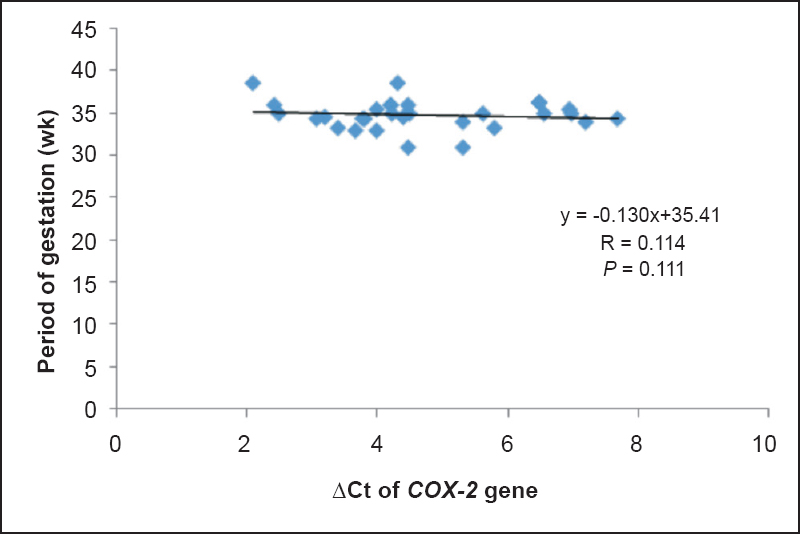
- Correlation between ΔCt of COX-2 and period of gestation (n=30).
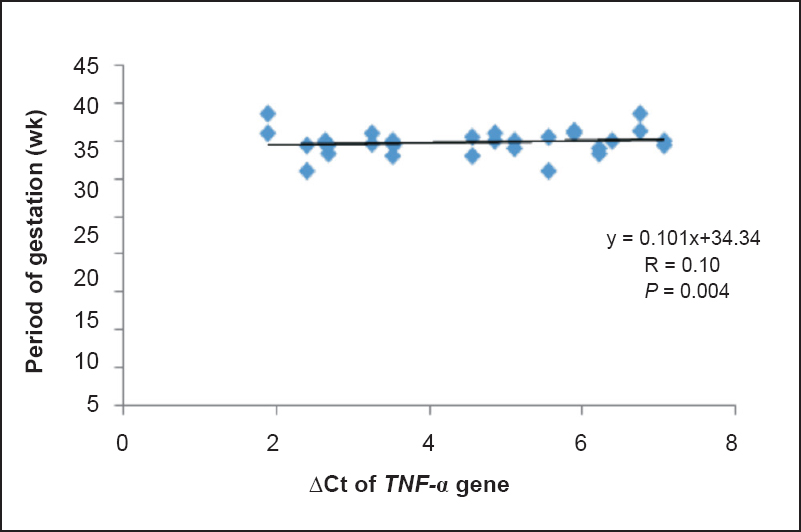
- Correlation between ΔCt of TNF-α and period of gestation (n=30).
Discussion
Despite the importance of PTB from a public health perspective, the aetiologies of PTB are not well understood, and no effective interventions are available. The present study was designed to understand the role of COX-2 and TNF-α genes expression, and their association with OCPs levels to find out possible gene-environment interaction in PTB. In the present study, the expressions of COX-2 and TNF-α genes were 3.13 and 2.31 folds higher in PTD cases as compared to term delivery group. The high expression of these genes may be influenced by various factors such as genetic, environmental factors, endogenus environment, infection, etc. Pollutants like pesticide, diesel particle may increase the expression of inflammatory genes2526.
It was observed that elevated maternal blood concentrations of β-HCH and p’p’-DDE were significantly associated with reduction in POG leading to PTB. Also, increased mRNA expression of TNF-α was found to be significantly associated with PTB. Frigo et al27 have demonstrated that xenobiotic exposure stimulates expression of the death ligand, TNF-α using qPCR and reporter gene assays. Studies have shown that exposure to environmental pollutants like o,p’-DDT and p’p’-DDE in in vitro model dose dependently increases the levels of COX-2 protein and mRNA and thereby increases the production of prostaglandins2528. Infection and inflammation pathways have been shown to be associated with a majority of PTBs, and especially with those that occur earlier in pregnancy29. Increased concentrations of inflammatory cytokines in amniotic fluid and cervical fluid have been considered as good biomarkers of PTB. Specifically, TNF-α, a pro-inflammatory cytokine has been found significantly associated with PTB3031.
We observed linear positive correlations between ΔCt of COX-2 and TNF-α. It may be possible that TNF-α increases the expression of COX-2 gene and thus prostaglandin secretion in PTB case. Although, COX-2 and TNF-α cannot be identified as direct biomarker for pesticide exposure, but these can be considered with other inflammatory markers and may contribute to the aetiology of PTB. In the present study, we observed a significant correlation between β-HCH level and reduction in POG.
Our study had certain limitations. Firstly, based on changes in mRNA level alone it is not possible to claim that biologically active TNF-α and prostaglandins are available to pursue downstream pathways resulting in early labour. Secondly, the sample size was small.
In conclusion, the expression of TNF-α and COX-2 genes was found to be associated with OCPs (β-HCH and p’p’-DDE) in maternal blood. The study of gene-environment interaction may be helpful for understanding the aetiology of idiopathic PTB. Further studies with larger sample size and other inflammatory genes as well as other environmental contaminants are required to validate the findings of the present study.
Acknowledgment
Authors thank the Indian Council of Medical Research, New Delhi, India, for financial support.
Conflicts of Interest: None.
References
- Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008;94:190-6.
- [Google Scholar]
- Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics. 2001;108:134-41.
- [Google Scholar]
- Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445-52.
- [Google Scholar]
- Genetic polymorphisms in Cytochrome P 4501B1 and susceptibility to idiopathic preterm labor in North Indian population. Clin Biochem. 2013;46:1812-5.
- [Google Scholar]
- Maternal and cord blood levels of organochlorine pesticides: association with preterm labor. Clin Biochem. 2009;42:746-9.
- [Google Scholar]
- Correlation of chlorinated pesticides concentration in semen with seminal vesicle and prostatic markers. Reprod Toxicol. 2004;19:209-14.
- [Google Scholar]
- Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110-4.
- [Google Scholar]
- Gene-environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol Hum Reprod. 2013;19:35-42.
- [Google Scholar]
- Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194-202.
- [Google Scholar]
- Cytokines modulate preimplantation development and pregnancy. Dev Biol. 1991;146:345-52.
- [Google Scholar]
- Tumour necrosis factor-α induces cyclo-oxygenase-2 gene expression in first trimester trophoblasts: suppression by glucocorticoids and NSAIDs. Placenta. 1997;18:521-6.
- [Google Scholar]
- The anti-inflammatory and antioxidative effects of nicotinamide, a vitamin B 3 derivative, are elicited by FoxO3 in human gestational tissues: implications for preterm birth. J Nutrl Biochem. 2011;22:1195-201.
- [Google Scholar]
- Tumor necrosis factor during pregnancy and at the onset of labor and spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 1999;83:77-9.
- [Google Scholar]
- Tumor necrosis factor receptor 1 expression and early spontaneous abortion. Int J Gynaecol Obstet. 2005;88:44-8.
- [Google Scholar]
- Kuppuswamy's socio-economic status scale-a revision of economic parameter for 2012. Int J Res Dev Health. 2013;1:2-4.
- [Google Scholar]
- Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-9.
- [Google Scholar]
- Alterations of metabolic activity in human osteoarthritic osteoblasts by lipid peroxidation end product 4-hydroxynonenal. Arthritis Res Ther. 2006;8:R159.
- [Google Scholar]
- Insulin up-regulates tumor necrosis factor-α production in macrophages through an extracellular-regulated kinase-dependent pathway. J Biol Chem. 2001;276:32531-7.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402-8.
- [Google Scholar]
- US Environmental Protection Agency (USEPA). Method 3620B: Florisil cleanup. Washington, DC: USEPA; 1996.
- Applied logistic regression (2nd ed). Canada: John Willey & Songs; 2004.
- o, p′-DDT induces cyclooxygenase-2 gene expression in murine macrophages: Role of AP-1 and CRE promoter elements and PI3-kinase/Akt/MAPK signaling pathways. Toxicol Appl Pharmacol. 2008;233:333-42.
- [Google Scholar]
- COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37:232-9.
- [Google Scholar]
- Xenobiotic-induced TNF-α expression and apoptosis through the p38 MAPK signaling pathway. Toxicol Lett. 2005;155:227-38.
- [Google Scholar]
- The effect of DDT and its metabolite (DDE) on prostaglandin secretion from epithelial cells and on contractions of the smooth muscle of the bovine oviduct in vitro. Toxicol Appl Pharmacol. 2012;259:152-9.
- [Google Scholar]
- Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161-7.
- [Google Scholar]
- The tumor necrosis factor α and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142-8.
- [Google Scholar]






