Translate this page into:
Association between statin use & risk of diffuse large B-cell lymphoma: A systematic review & meta-analysis
For correspondence: Dr Ben Ponvilawan, 2 Prannok Road, Bangkoknoi, Bangkok 10700, Thailand e-mail: ben.ponv@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Statin use has been shown to be associated with a decreased risk of several types of cancer, however, the data on diffuse large B-cell lymphoma (DLBCL) are still inconclusive. This study aimed to systematically summarize all available data on this association and conduct a meta-analysis on the same.
Methods:
A systematic review was performed using EMBASE and MEDLINE databases from inception upto October 2019 with a search strategy that included terms such as ‘statin’ and ‘DLBCL’. Eligible studies included either case–control or cohort studies that reported the association between statin use and the risk of DLBCL. Relative risk, odds ratio (OR), hazard: risk ratio or standardized incidence ratio of this association and standard error were extracted and combined for calculating the pooled effect estimate using random-effects, generic inverse variance method.
Results:
A total of 1139 articles were screened. Of these six studies satisfied the inclusion criteria and were included for the meta-analysis. Statin use was associated with a significantly reduced risk of DLBCL with the pooled OR of 0.70 (95% confidence interval, 0.56-0.88; I2=70%). The funnel plot (fairly symmetric) was not suggestive of the presence of a publication bias.
Interpretation & conclusions:
The present systematic review and meta-analysis found that statin use is associated with a 30 per cent reduced odds of DLBCL. However, the pooled analysis utilized data from observational studies so causation cannot be concluded upon. Hence, it suggested that randomized-controlled studies are still needed to confirm this potential benefit.
Keywords
Diffuse large B-cell lymphoma
lipid-lowering agent
meta-analysis
statin
Diffuse large B-cell lymphoma (DLBCL) is one of the most common aggressive subtype of non-Hodgkin lymphoma (NHL), responsible for around 30-40 per cent of all lymphomas1, with the annual incidence of 3-4 per 100,000 in Europe and 6.9 per 100,000 in the United States2. It is characterized by a growth pattern of diffused with medium-to-large B-lymphoid cells causing clinical symptoms of rapidly growing lymphadenopathy at nodal as well as extranodal sites, including the gastrointestinal tract, testes and brain3. About one-third of patients also experience constitutional symptoms (fever, weight loss and night sweats)3. Despite advancements in novel therapies, patients with DLBCL continue to have suboptimal prognosis with a five-year survival rate of only 60 per cent, which has not improved significantly over the past decade4. Prognosis varies to some extent across different subtypes of DLBCL, ranging from a five-year survival rate of 76-80 per cent in the germinal centre B-cell subtype to 45-56 per cent in the activated B-cell subtype. Approximately 20-50 per cent of patients are either refractory to the first-line regimen (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) or have relapse after achieving complete remission. These patients with a relapsed, refractory DLBCL have a poor prognosis with a median overall survival of only 6.3 months due to a poor prognosis5.
Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) inhibitors, are a class of cholesterol-lowering agents primarily used for primary and secondary atherosclerotic cardiovascular disease risk reduction6. Statins have some notable adverse effects, including elevated transaminase levels, myalgia, myositis and a slightly elevated risk of diabetes mellitus7. Multiple in vitro and in vivo studies have exhibited other pleiotropic effects of statins apart from lipid-lowering, including cell cycle modulation, apoptosis induction and angiogenesis inhibition8. In fact, a number of prior studies have suggested an association of statin use with a decreased risk of several types of cancer9,10, including haematologic malignancy11.
It is possible that statin use could also be associated with a decreased risk of DLBCL, although results from previous epidemiologic studies are still inconclusive12-17. This systematic review and meta-analysis aimed to summarize all available data on this association by comprehensively identifying all published studies so far.
Material & Methods
Search strategy: Published studies indexed in MEDLINE and EMBASE from inception upto October 2019 were independently reviewed by two investigators (B.P. and N.C.). keywords including ‘statins’ and ‘DLBCL’ were used as search terms for this systematic review. The comprehensive list of search terms and inclusion criteria of the studies are given in Supplementary Material. Two investigators independently determined the eligibility of each study, which was reviewed again by a senior investigator.
Inclusion & exclusion criteria: The Newcastle-Ottawa Quality Assessment Scale for case-control and cohort studies was used to evaluate the quality of each included study18.
Data extraction: A standardized data extraction form was devised and used for tabulating of the information including: last name of the first author, place of study conducted, study design, publication year, number of participants, sampling, ascertainment of statin use, definition of statin users and non-users, diagnosis of DLBCL, follow up duration (for cohort studies), average age of participants, percentage of male participants, comorbidities, variables adjusted in multivariate analysis and adjusted effect estimates with 95 per cent confidence interval (CI).
Statistical analysis: Data analysis was performed using the Review Manager 5.3 software (The Cochrane Collaboration, London, UK) and the Comprehensive Meta-analysis 3.0 software (Biostat Inc., Englewood, New Jersey, USA)19. The weighted point estimate in this study was pooled odds ratio (OR). It was calculated from OR of each case–control study and relative risk (RR), incidence rate ratio (IRR), hazard risk ratio (HR) or standardized incidence ratio (SIR) of each cohort study (RR, IRR, HR and SIR were used as an estimate for OR). The generic inverse variance method, as described by DerSimonian and Laird, was used as the analytical technique to give weight to each study for the calculation of pooled OR20. The random-effects model was used because the included studies had different designs, background populations and case definitions. Statistical heterogeneity was assessed using the Cochran’s Q test. Additonally τ2 and the I2 statistic were also determined as defined earlier21. A value of I2 of 0-40 per cent represents insignificant heterogeneity, 30-60 per cent moderate heterogeneity, 50-90 per cent substantial heterogeneity and 75-100 per cent high heterogeneity22. The presence of publication bias was assessed using the funnel plot along with Egger’s regression test. A sensitivity analysis was conducted by excluding each study at a time to assess if the pooled result would be significantly altered.
Results
Search results: Of the 1292 articles retrieved with the search terms from MEDLINE and EMBASE databases, 32 articles were put through a full length article review. Of these 26 articles were further excluded based on these reported the outcome of interest, were conducted in the population of interest or whether it these were not cases–control or cohort design. Finally, six studies (two retrospective cohort studies12,13, and four case–control studies14-17) satisfied the eligibility criteria and were included in the meta-analysis. The quality of the included studies was good as these had a Newcastle–Ottawa score of at least 723 (
- Study identification and literature review process.
| Characteristics | Jacobs et al12 | Desai et al13 | Zhang et al14 | Iwata et al15 | Fortuny et al16 | Ye et al17 |
|---|---|---|---|---|---|---|
| Study design | Retrospective cohort | Retrospective cohort | Case–control | Case–control | Case–control | Case–control |
| Total number of participants | Statin users: 28,950 Non-users: 104,305 | Statin users: 12,225 Non-users: 149,338 | Cases: N/A Controls: 717 | Cases: 66 Controls: 265 | Cases: 493 Controls: 2206 | Cases: 1126 Controls: 27,315 |
| Average age of participants (yr) | N/A | Statin users: 66.0 (median) Non-users: 63.0 (median) | N/A | N/A | N/A | N/A |
| Effect estimate | RR=0.79 | RR=0.62 | OR=0.50 | OR=2.10 | OR=0.69 | OR=0.77 |
| Weight in pooled analysis (per cent) | 22.8 | 15.4 | 22.2 | 4.5 | 10.7 | 24.5 |
N/A, not available; OR, odds ratio; RR, relative risk
| Characteristics | Jacobs et al.12 | Desai et al.13 | Zhang et al.14 | Iwata et al.15 | Fortuny et al.16 | Ye et al.17 |
|---|---|---|---|---|---|---|
| Country | USA | USA | USA | Japan | Czech Republic, France, Germany, Ireland, Italy and Spain | Canada |
| Study design | Retrospective cohort | Retrospective cohort | Case-control | Case-control | Case-control | Case-control |
| Year of publication | 2011 | 2017 | 2004 | 2006 | 2006 | 2018 |
| Total number of participants | Statin users: 28,950 Non-users: 104,305 | Statin users: 12,225 Non-users: 149,338 | Cases: N/A Controls: 717 | Cases: 66 Controls: 265 | Cases: 493 Controls: 2206 | Cases: 1126 Controls: 27,315 |
| Recruitment of participants | Participants were subjects of the prospective study of Cancer Prevention Study-II Nutrition Cohort who completed questionnaires which had questions on the use of cholesterol-lowering drugs in 1997 Participants with the diagnosis of cancer before the starting date were excluded | Participants were post-menopausal females enrolled in the Women’s Health Initiative Clinical Trial and Observational Study between 1 October 1993 and 31 December 1998 Participants with the diagnosis of lymphoma before the starting date were excluded | Cases: Cases were female aged 21-84 yr who were newly diagnosed with DLBCL between January 1996 and June 2000. Cases were identified from the Connecticut Tumor Registry Controls: Controls without DLBCL were residents of Connecticut who were randomly recruited using random-digit dialing methods through Connecticut addresses (<65 yr) and Lefts for Medicare and Medicaid Service files (>65 yr) Controls were matched to cases by age within five years | Cases: Cases were consecutive patients aged at least 40 yr who were hospitalized with DLBCL in the haematology ward of Toranomon Hospital, Tokyo, between April 1995 and March 2001 Controls: Controls without DLBCL were patients identified from the orthopaedic and eye-nose-throat ward of the same hospital Individuals with a history of any other type of malignancy were excluded from both the case and control groups Controls were matched 2:1 to cases by age, sex and year of admission | Cases: Cases were consecutive patients newly diagnosed with DLBCL who were prospectively recruited into the EPILYMPH multicentre case–control study from 1998 to 2004 Controls: Controls without DLBCL were sampled from the general population in Italy and Germany and were sampled from the same hospital as cases in the Czech Republic, France, Ireland and Spain In hospital-based studies, controls were excluded if they were hospitalized due to cancer, organ transplant or systemic infection at the time of recruitment Controls were matched to cases by age within five years, sex and study centre | Cases: Cases were patients with DLBCL aged at least 40 yr who were identified from the Manitoba Health database from 1999 to 2014 Controls: Controls without DLBCL were randomly selected from the same database Controls were matched 5:1 to cases by sex, region of residence, age within one year and length of the period of available prescription drug data |
| Ascertainment of statin use | From medical questionnaires which contain self-reported use of cholesterol-lowering drugs | From direct interviews at baseline and follow up visits (use of statin was considered a time-dependent variable) | From direct interview by trained study interviewers using standardized, structured questionnaire | From both inpatient and outpatient medical records | From self-administered medical questionnaires which contain self-reported use of statins | From pharmacy databases |
| Definition of statin user and non-user | Statin users were those who reported former or current use of cholesterol-lowering drugs in the questionnaires Non-users were those who reported no use of cholesterol-lowering drugs in the questionnaires | Statin users were those who reported the current use of any statins during each follow up visit Non-users were those who did report current use of any statins during each follow up visit | Statin users were those who reported the use of statins, at least once a day for a period of six months or more, at least one year before the diagnosis of DLBCL for cases or one year before interview for controls Non-users were those who did not fulfil the definition of statin users | Statin users were those who took prescribed statins of any type and dose at any time before admission Non-users were those with no history of statins | Statin users were those who reported any use of statins in the questionnaires Non-users were those who reported no use of statins in the questionnaires | Statin users were those who had at least one prescription for any statins Non-users were those who had no prescription for statins |
| Diagnosis of DLBCL | Self report via follow up questionnaires which were subsequently verified by review of medical records or through linkage with state cancer registries. Minority of cases were further identified through linkage with the National Death Index | Self report via direct interview during each follow up visit. Which were subsequently verified by review of medical records and pathology report | Verified by histopathology from two pathologists | Verified by histopathology according to the World Health Organization criteria by two experienced haematopathologists | Verified by histopathology, immunohistochemistry and flow cytometry | Verified by histopathology according to the 2008 World Health Organization classification of lymphoid neoplasms |
| Follow up | Until 2005 by which questionnaires were sent to participants every two years | Until planned termination in March 2005, and until September 2012 for participants providing reconsent | ||||
| Follow up duration (yr) | N/A | N/A | ||||
| Average age of participants (yr) | N/A | Statin users: 66.0 (median) Non-users: 63.0 (median) | N/A | Cases: N/A Controls: N/A | N/A | Cases: N/A Controls: N/A |
| Percentage of male | Statin users: 50.8 Non-users: 43.5 | Statin users: 0.0 Non-users: 0.0 | Cases: 0 Controls: 0 | Cases: N/A Controls: 38.7 | Cases: N/A Controls: 54.0 | Cases: N/A Controls: 55.1 |
| Comorbidities | Statin users Diabetes mellitus - 12.3% Hypertension - 54.5% Elevated cholesterol - 95.9% Heart disease - 32.8% Non-users Diabetes mellitus - 6.9% Hypertension - 36.2% Elevated cholesterol - 38.3 Heart disease - 8.2% | Statin users Current smoking - 6.1% Alcohol use - 65.3% Hormone therapy use - 54.1% History of lupus - 0.4% History of RA - 5.8% Non-users Current smoking - 7.1% Alcohol use - 70.6% Hormone therapy use - 56.3% History of lupus - 0.5% History of RA - 5.0% | ||||
| Variables adjusted in multivariate analysis | Age, sex, race, education, smoking, body mass index, physical activity level, non-steroidal anti-inflammatory drug use, hormone therapy, history of elevated cholesterol, heart disease, diabetes and hypertension | Age, current medical care provider, history of lupus and rheumatoid arthritis | Age, BMI, menopausal history and family history of NHL in first-degree relatives | Age, sex, year of visit and serological status of anti-HBsAg and anti-HCV Ab | Age, sex and study centre | Age, sex, area, cardiovascular disease (excluding hypertension), income quintiles and number of physician visits five years before index date, non-statin lipid-lowering drugs, non-aspirin NSAIDs and aspirin and derivatives |
| Effect estimate | RR=0.79 | RR=0.62 | OR=0.50 | OR=2.10 | OR=0.69 | OR=0.77 |
| Weight in pooled analysis (per cent) | 22.8 | 15.4 | 22.2 | 4.5 | 10.7 | 24.5 |
| Newcastle–Ottawa score | Selection: 3 Comparability: 2 Outcome: 2 | Selection: 3 Comparability: 2 Outcome: 2 | Selection: 3 Comaparability: 2 Expoure: 2 | Selection: 3 Comaparability: 2 Exposure: 2 | Selection: 4 Comparability: 2 Exposure: 2 | Selection: 4 Comparability: 2 Exposure: 3 |
Anti-HBsAg, anti-hepatitis B virus surface antigen; BMI, body mass index; DLBCL, diffuse large B-cell lymphoma; anti-HCV Ab, hepatitis C virus antibody; N/A, not available; NSAIDs, non-steroidal anti-inflammatory drugs; NHL, non-Hodgkin lymphoma; USA, United States of America; OR, odds ratio; RR, relative risk; RA, rheumatoid arthritis
Statin use and the risk of diffuse large B-cell lymphoma (DLBCL): The risk of DLBCL was significantly reduced among statin users compared to non-users with a pooled OR of 0.7 (95% CI, 0.56-0.88). This meta-analysis was substantially statistically heterogenous, with I2 of 70 per cent (Fig. 2). This 30 per cent reduced odds risk of developing DLBCL could have clinical importance as DLBCL has a fair prognosis.
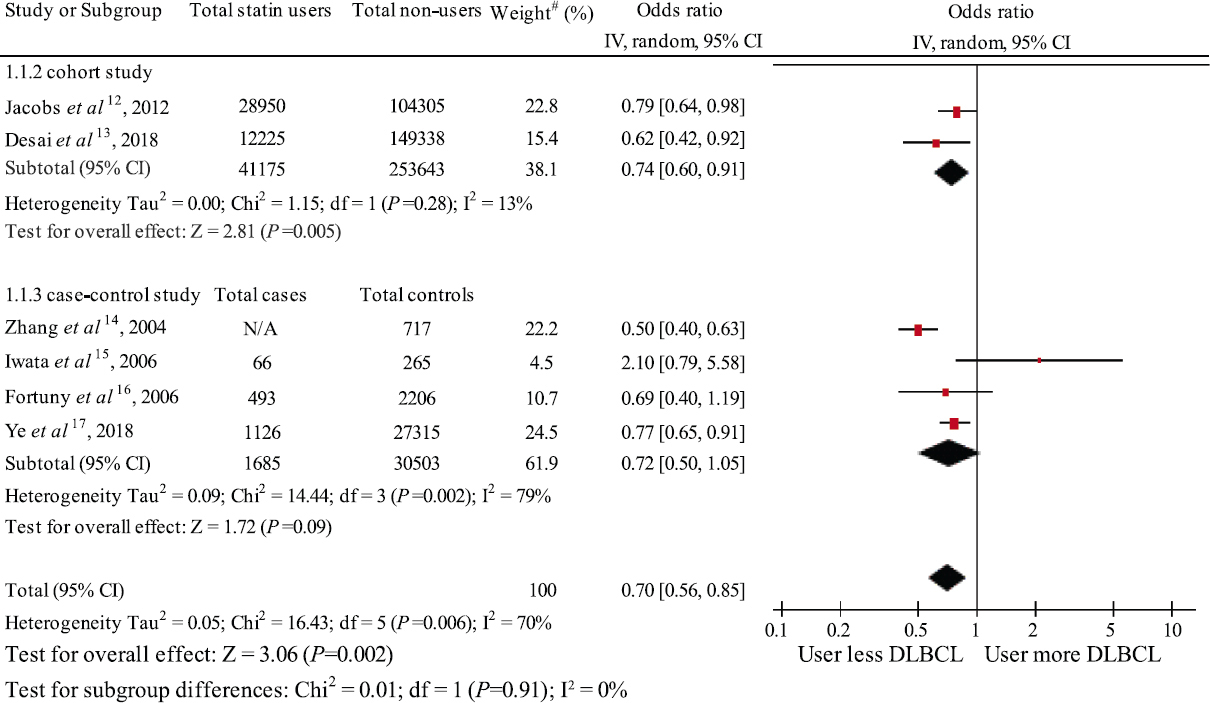
- Forest plot of the meta-analysis of the risk of DLBCL among statin users versus non-users. #weight of the study in met-analysis. DLBCL, diffuse large B-cell lymphoma; N/A, not applicable
Sensitivity analysis: A sensitivity analysis was performed by excluding each study from the pooled analysis due to the substantial heterogeneity. The exclusion of each study did not significantly change the pooled result and between-study heterogeneity (
Evaluation of publication bias: A funnel plot (Fig. 3) was used to assess the presence of publication bias. In this meta-analysis the funnel plot was fairly symmetric and did not provide any evidence for publication bias (P=0.57 by Egger’s regression test).
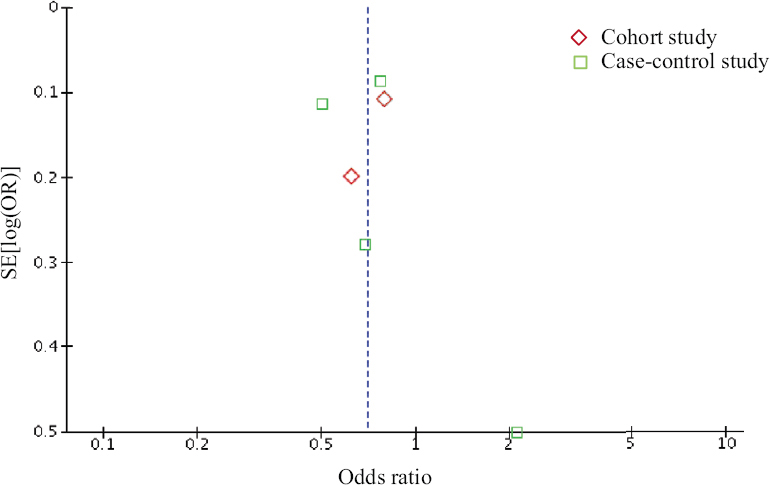
- Funnel plot of the meta-analysis of the risk of DLBCL among statin users versus non-users. DLBCL, diffuse large B-cell lymphoma
Quality of evidence using the Grading of Recommendations Assessment, Development & Evaluation (GRADE) approach: The quality of evidence generated by the current systematic review and meta-analysis was found to be low as it is a compilation of observational studies due to which there were some inconsistencies.
Discussion
This meta-analysis included cohort and case–control studies that investigated the relationship between statin use and the risk of DLBCL within the study period. By analyzing data from 327,006 participants, we found that statin users had a 30 per cent reduced odds risk of developing DLBCL compared to statin non-users. The prior systematic and meta-analysis that was published earlier in this context24 failed to demonstrate a significant risk reduction among statin users. However, two more studies have been published and were included in the current meta-analysis, which led to a significant result13,17,24. The mechanisms behind this protective effect of statins are not known with certainty, but there are some potential explanations based on data from in vitro and in vivo studies.
First, it is known that statins disrupt mevalonate synthesis and result in reduced production of isoprenoids, which impedes activation of multiple molecular pathways such as the phosphoinositide 3-kinase-Akt (PI3K-Akt) and p38 mitogen-activated protein kinase (p38 MAPK)25. It has also been shown that atorvastatin, fluvastatin and simvastatin activate p38 MAPK and inhibit PI3K-Akt and ERK pathways in lymphoma cells. Furthermore, the re-addition of HMG-CoA reductase products (mevalonate, farnesyl pyrophosphate and geranylgeranyl pyrophosphate) can reverse the aforementioned activation. Changes in these molecular cascades are reported to affect cancer cell proliferation, migration and invasion into the surrounding tissue25.
Second, studies have suggested that statins can directly induce apoptosis of lymphoma cells through multiple mechanisms26,27. It has been demonstrated that statins enhance DNA fragmentation and promote pro-apoptotic proteins such as caspase-3, PARP and Bax while they inhibit the anti-apoptotic protein Bcl-226. It has also been reported that mevastatin can disrupt lipid rafts in the cell membrane, inducing an extrinsic pathway of apoptosis via activation of Fas, Fas-associated death domain protein and caspase-827.
It is also possible that the negative association was not causal, particularly in developing countries. Since the incidence of premature death among HIV-infected patients in developing countries is still high and HIV infection is a well established risk factor for DLBCL, those HIV-infected patients with DLBCL may die at a young age before developing atherosclerotic cardiovascular disease, with a consequent lower use of statins.
It should be noted that there are some limitations in this study that may affect the validity of the results. Therefore, the role of statins in DLBCL prevention should be interpreted with caution. First, the ascertainment of statin use and diagnosis of DLBCL in some included studies may have limited accuracy as these depended on questionnaires that were reported by the patients or diagnostic codes retrieved from healthcare databases without further confirmation. Second, this study had substantial statistical heterogeneity, which could be a result of the difference in study methodology, background population and definition of statin use and diagnosis of DLBCL. Furthermore, most studies did not provide subgroup data and, therefore, subgroup analysis to look for the difference could not be conducted. Furthermore, also could not be meta-regression included <10 since studies were included. Finally, the interpretation of the funnel plot was also limited by the small number of included studies. Therefore, it is still possible that publication bias may have been present despite the relatively symmetric funnel plot.
Overall, this systematic review and meta-analysis found that statin use is associated with a 30 per cent reduced OR of DLBCL. This fairly big effect may, however, have clinical importance and warrant further randomized-controlled studies to confirm this benefit.
Financial support and sponsorship
None.
Conflicts of interest
The abstract of this study was previously presented and published in J Clin Oncol 2020 ASCO Annual Meeting (https://ascopubs.org/doi/10.1200/JCO.2020.38.15_suppl.e13585).
Supplementary Material
Supplementary Fig. 1
Supplementary Fig. 1 Histogram of each domain of NOS. NOS, Newcastle-Ottawa Scale.Supplementary Fig. 2
Supplementary Fig. 2 Sensitivity analysis.References
- Aggressive B-cell lymphomas-from morphology to molecular pathogenesis. Ann Lymphoma. 2019;3:1-22.
- [Google Scholar]
- 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443-59.
- [Google Scholar]
- SEER Cancer Statistics Review, 1975-2013. 2016. Bethesda, MD: National Cancer Institute. Available from:http://seer.cancer.gov/statfacts/html/mulmy.html
- [Google Scholar]
- Diffuse large B-cell lymphoma:2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94:604-16.
- [Google Scholar]
- Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-61.
- [Google Scholar]
- Can cardiovascular drugs support cancer treatment?The rationale for drug repurposing. Drug Discov Today. 2019;24:1059-65.
- [Google Scholar]
- Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology. 2020;71:2023-32.
- [Google Scholar]
- Statin use and site-specific risk of colorectal cancer in individuals with hypercholesterolemia from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) Nutr Metab Cardiovasc Dis. 2019;29:701-9.
- [Google Scholar]
- Previous exposure to statin may reduce the risk of subsequent non-Hodgkin lymphoma: A nationwide population-based case-control study. PLoS One. 2015;10:e0139289.
- [Google Scholar]
- Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71:1763-71.
- [Google Scholar]
- An analysis of the effect of statins on the risk of Non-Hodgkin's Lymphoma in the Women's Health Initiative cohort. Cancer Med. 2018;7:2121-30.
- [Google Scholar]
- Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15:419-28.
- [Google Scholar]
- Use of hydroxy-methyl-glutaryl coenzyme A reductase inhibitors is associated with risk of lymphoid malignancies. Cancer Sci. 2006;97:133-8.
- [Google Scholar]
- Statin use and risk of lymphoid neoplasms: Results from the European Case-Control Study EPILYMPH. Cancer Epidemiol Biomarkers Prev. 2006;15:921-5.
- [Google Scholar]
- Associations between statin use and risk of non-Hodgkin lymphomas by subtype. Int J Cancer. 2018;143:971-9.
- [Google Scholar]
- 2000. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Available from:http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5-18.
- [Google Scholar]
- 2018. Cochrane Consumers and Communication Group: Meta-Analysis Melbourne: La Trobe University. Available from: https://opal.latrobe.edu.au/articles/journal_contribution/Meta-analysis/6818885/1
- Newcastle-Ottawa Scale: Comparing reviewers'to authors'assessments. BMC Med Res Methodol. 2014;14:45.
- [Google Scholar]
- Associations between statin use and non-Hodgkin lymphoma (NHL) risk and survival: A meta-analysis. Hematol Oncol. 2017;35:206-14.
- [Google Scholar]
- HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis. 2013;4:e518.
- [Google Scholar]
- Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320:165-9.
- [Google Scholar]






