Translate this page into:
Association between rs9930506 polymorphism of the fat mass & obesity-associated (FTO) gene & onset of obesity in Polish adults
Reprint requests: Dr Małgorzata Wrzosek, Department of Pharmacogenomics, Division of Biochemistry & Clinical Chemistry, Faculty of Pharmacy, Medical University of Warsaw, Banacha St. 1, 02-097 Warsaw, Poland e-mail: malgorzata.wrzosek@wum.edu.pl
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The fat mass and obesity-associated (FTO) gene is known to be associated with obesity. However, no data are available on the relation between FTO rs9930506 polymorphism and obesity in Polish population. The aim of this study was to evaluate an association between rs9930506 variants of the FTO gene and obesity in Polish adults.
Methods:
The study group consisted of 442 adults, aged 33.9 ±12.7 yr, with mean BMI 27.2 ± 5.4 kg/m2. The following variables were determined for each subject: fasting blood glucose, total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides. Real-time PCR was used to detect the A/G alleles of the rs9939506 polymorphism in the FTO gene. An association between the rs9930506 polymorphism and obesity was determined using codominant, dominant, and recessive models. The odds ratio (OR) was calculated to determine the risk of obesity associated with this polymorphism.
Results:
It was observed that the presence of FTO rs9939506 G allele was associated with increased risk for obesity and this association was found significant in both recessive (OR = 1.72, P = 0.014) and co-dominant (OR = 1.36, P = 0.031) models of inheritance. The FTO rs9939506 GG homozygotes had a significantly higher BMI than those with other genotypes.
Interpretation & conclusions:
This study shows that FTO rs9939506 GG genotype is related to higher BMI and is associated with obesity in Polish adults.
Keywords
Adults
BMI
FTO gene
obese
obesity
polymorphism
Obesity has become a serious public health issue and its prevalence is increasing in both developed and developing countries1. The World Health Organization (WHO) has defined obesity as a condition with excessive fat accumulation in the body, corresponding to a body mass index (BMI) ≥of 30 kg/m2 in Caucasians2. The main adverse consequences of obesity are cardiovascular disease, type 2 diabetes, and several cancers3. Environmental factors including excessive energy intake and lack of physical activity are known to play a key role in obesity development. However, person-to-person variations seen in response to an obesogenic environment suggest the existence of a genetic predisposition to the excessive accumulation of adipose tissue4. The twin and family studies suggest that genetic factors may have a strong effect on the variations seen in body mass index (BMI) and body fat percentage567. Genetic susceptibility to obesity was also revealed by genome-wide association studies (GWAS)89. The fat mass and obesity-associated (FTO) gene is recognized as associated with enhanced adiposity and seems to influence the risk of obesity in various populations910.
The FTO gene is located in chromosome region 16q12.211 and encodes the nucleic acid demethylase. Recombinant human nucleic acid demethylase can demethylate 3-methylthymine in single-stranded DNA and 3-methyluracil in RNA1213. However, the exact mechanism by which FTO variants influence metabolism and lead to obesity is still unknown. The catalytic activity of FTO may regulate the transcription of genes involved in metabolism by nucleic acid demethylation. It was found that FTO-dependent demethylation of specific mRNAs in vivo relates to the control of the dopaminergic signaling pathway14. This is important because reward behaviour and the motivation behind feeding behaviour seem to be mediated by dopamine neurons located in the midbrain15. Previous studies have reported an association between variations in the FTO gene and obesity phenotypes, and have highlighted the role of FTO rs9939609 single nucleotide polymorphism (SNPs)1617. A meta-analysis of 17.037 white European individuals revealed associations between FTO variants not only with BMI, but also with fasting insulin, glucose, triglycerides, and HDL-cholesterol concentrations18. Some studies, however, did not confirm the importance of the FTO gene as a genetic candidate for higher BMI1920. The influence of ethnic variation is often cited as the cause of these differences212223. Other polymorphisms like rs9930506 have also been observed to lead to the increased risk of obesity924. There are no data regarding the frequency of genetic variations in rs9930506 polymorphism of the FTO gene, nor its relation to BMI and the occurrence of obesity in the Polish population. Therefore, the aim of our study was to assess the frequency of genotypes and alleles of the rs9930506 polymorphism of the FTO gene and to investigate the association between this polymorphism and the onset of obesity.
Material & Methods
Obese and non-obese unrelated individuals were consecutively recruited on the basis of clinical investigation, between September 2012 and December 2013 from patients who had been directed to the Outpatient Clinic at the National Food and Nutrition Institute, Warsaw, Poland, due to obesity or for a routine general health screening. The individuals included in the study had no signs or symptoms of thyroid or other endocrine diseases, renal and hepatic disorders, as well as diabetes or history of hypoglycaemic treatment, were free from any psychotropic medication, did not receive medications known to influence plasma lipid levels and body mass, and women did not use hormonal therapy. They were asked not to take part in weight loss programmes and not to successfully lower their body mass. Demographic and clinical variables were recorded: age, weight, height, BMI= weight/hight2 (kg/m2), blood pressure. Obesity was defined as BMI ≥30 kg/m2 according to WHO classification2. Obese subjects were consecutively selected from patients attending the Outpatients clinic. Non-obese subjects were age- and sex matched subjects who came for annual medical checkup. Eleven obese and 19 non-obese individuals did not give consent, therefore, they were not qualified for the study.
All participants underwent a comprehensive medical evaluation including clinical history, physical examination, anthropometric parameters and blood pressure (BP) measurements. They completed a questionnaire concerning smoking habits, physical activity, medications and dietary supplements.
All individuals provided written informed consent prior to inclusion in the study. The study protocol was approved by the local research ethics committee (KB/127/2012, Medical University of Warsaw, N151923 Grant National Food and Nutrition Institute in Warsaw).
Peripheral fasting blood samples (5 ml) were collected in commercially available vacuum tubes. The plasma was separated by low speed centrifugation and used for glucose and lipid analyses. Fasting plasma glucose (FPG) was determined by the glucose oxidase method25. Enzymatic methods were used to determine concentrations of total cholesterol (Chol) and triglycerides (TG)26. HDL-cholesterol was measured after precipitation of apolipoprotein B containing lipoproteins, and LDL-cholesterol level was calculated using Fridewald formula27.
Genomic DNA was extracted from peripheral whole blood (1ml) using the Blood Mini genomic DNA purification kit (A&A Biotechnology, Poland) according to the manufacturer's instructions. DNA concentration and purity were determined with UV spectrophotometry, measuring absorbance ratios of 260/280 nm. High quality DNA was considered to have an A260/A280 ratio of 1.85 - 2.10. All genomic DNA was diluted to a final concentration of 20 ng/µl. Genotyping of polymorphism rs9930506 of FTO gene was performed by TaqMan allelic discrimination real-time PCR28. Validated TaqMan SNP genotyping assays were obtained from Life Technologies (Thermo Fisher Scientific, USA). The initial step of the allelic discrimination genotyping assay protocol included: 95°C for 10 min, 40 cycles of 15 sec each at 95°C and 60°C for 1 min. More than 50 per cent of the 442 genotypes were determined twice, and genotyping was 100 per cent concordant.
Statistical analysis: The data were analyzed using Statistica Software, version 10.0 (StatSoft Inc, Tulsa, Oklahoma, USA). Allelic frequencies were calculated by gene counting. Quantitative variables were expressed as mean ± standard error (SE). Baseline characteristics and the differences between obese and non-obese groups were assessed using Student t test.
A link between the polymorphism rs9930506 in the FTO gene and obesity was determined by using codominant (genotype test), recessive (increased risk in GG vs. AG + AA) and dominant (increased risk in GG or AG vs. AA) models of inheritance29. Differences in minor allele frequencies and genotype distributions among obese and non-obese patients with corresponding odds ratios (OR) and the 95 % confidence interval (CI) were analyzed by likelihood ratio tests with calculation of the P value by Chi-square (χ2) approximation to its distribution using Web-Assotest program (http://www.ekstroem.com). P values for a model fit (Pfit) were calculated and Pfit < 0.05 indicated that given model of inheritance should be rejected.
Analysis of variance (ANOVA), Tukey post-hoc analysis and the t test were applied to test the differences in BMI and studied parameters (FPG, Chol, LDL, HDL, TG) across the genotypes and alleles of rs9930506 polymorphism.
Results
Altogether, 163 obese subjects and 279 non-obese subjects matched for age and sex participated in the study. The obese participants with class I/II obesity had mean BMI = 33.2 ± 2.6 kg/m2. The group of non-obese had mean BMI 23.7 ± 3.2 kg/m2. No difference in body height between obese and non-obese participants was observed. Obese compared to non-obese participants had significantly (P<0.001) higher systolic and diastolic blood pressure, fasting blood glucose concentrations, plasma triglycerides (P<0.05) and significantly (P<0.001) lower HDL-cholesterol concentrations (Table I). All these differences are commonly related to differences in BMI and body fatness.
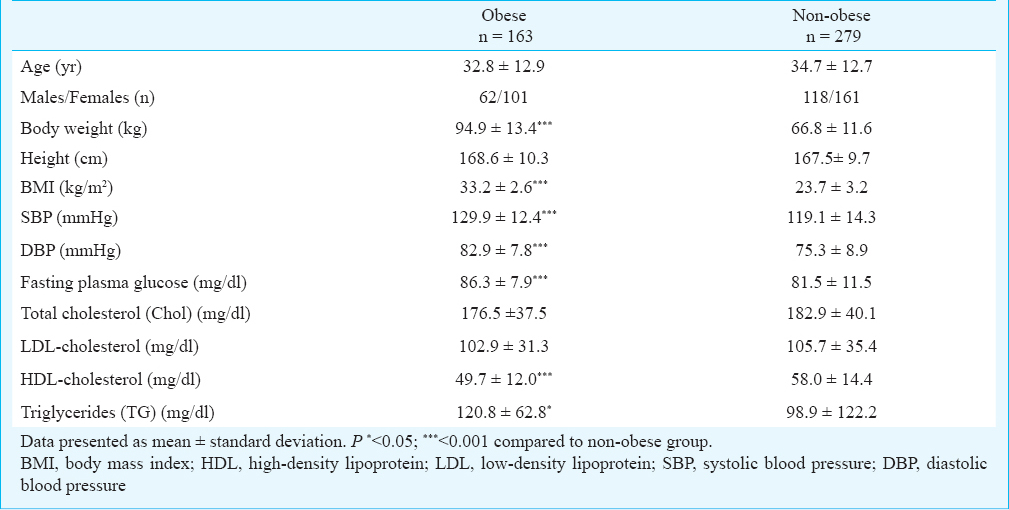
Distribution of the FTO rs9930506 genotypes, presented in Table II, did not deviate from Hardy-Weinberg equilibrium in both obese (p = 0.60, χ2 = 0.27, df = 1) and non-obese (p = 0.21, χ2 = 1.61, df = 1) groups. The frequency of rs9930506 G allele among obese (56%) was higher than among non-obese (49%, OR = 1.34, P = 0.033). The statistical analysis revealed a significant association between polymorphism rs9930506 and obesity in a recessive (OR = 1.72, P = 0.014) and a co-dominant models of inheritance (OR = 1.36, P = 0.031). The dominant model was sufficiently different from the general model (Pfit = 0.025) as it did not produce a good fit and could be rejected (Table II). Our study could detect with power of 78.9% (α = 0.05) the genotypic association conferring OR= 1.72.
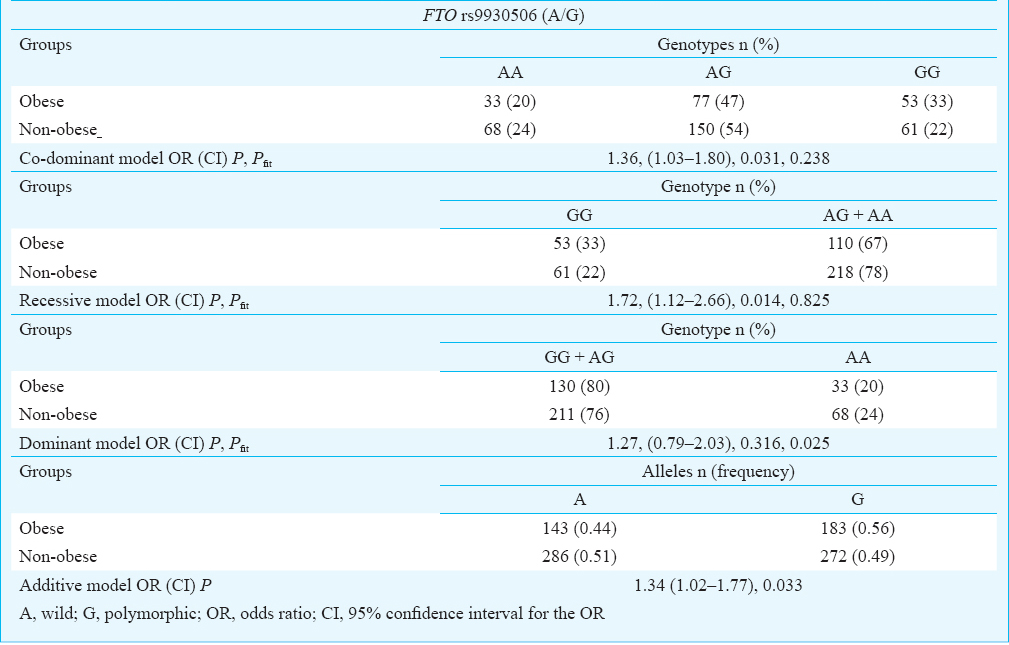
Mean BMI levels were found to be significantly higher (P = 0.012) in subjects with the GG genotype than in carriers of other genotypes (AA and AG). Homozygotes for the G allele of the FTO rs9930506 polymorphism differed from carriers of the A allele by, on average, about 1.5 BMI units (kg/m2) (Table III).
There were no significant differences between the three genotypes of FTO rs9930506 polymorphism among the entire group of 442 subjects in concentrations of fasting plasma glucose, total cholesterol, LDL-cholesterol, HDL-cholesterol, and plasma triglycerides. There was also no association between the FTO rs9930506 G allele and the biochemical parameters tested (FPG, Chol, LDL, HDL, TG).
Discussion
The FTO as the susceptibility gene recognized by genome-wide association studies has attracted much attention in obesity research. The mechanism underlying the increased risk of obesity in presence of a specific allele remains unclear. It is suggested that the risk variant influences ghrelin mRNA expression and the levels of circulating ghrelin, and the failure to suppress hunger is related to loss of control over eating and to the selection of energy dense food3031. Among the various SNPs in the FTO gene reported to be associated with obesity, polymorphism rs9939609 has been of particular interest1617. However, under specific conditions, both environmental and ethnic, particular genetic variants may have different degrees of influence on body fatness and BMI measurements. In the Polish population, the presence of AA genotype of rs9939609 polymorphism on the FTO gene was reported to be associated with higher BMI in both children and adults3233, however, no data on polymorphismrs9930506 have been presented previously in the Polish population. Studies on Sardinian and Italian samples924 have revealed significant associations of rs9930506 polymorphism of the FTO gene with BMI and obesity. Our study showed an association between this rs9930506 A/G polymorphism, and BMI and obesity among Polish adults. In the present study, the G allele of rs9930506 was found to be associated with higher BMI, and a 1.5 kg/m2 increase in BMI per this allele copy was recognized. A similar association between rs9930506 FTO polymorphismand BMI was reported in Italian subjects, where the mean difference in BMI level between the AA genotype and other genotypes was 1.4 kg/m2,24 and in Sardinians subjects, where the two homozygotes (AA vs. GG) differed, on average, by 1.5 BMI units9.
The frequency of the G allele in the obese participants was significantly higher compared to non-obese group. The allele frequency observed in the Polish adults was similar to that observed in Italian and Sardinian population924. In contrast, in the Asian population, a lower frequency of the G allele of the rs9930506 polymorphism of the FTO gene was found: a G allele frequency of 0.20 in a Chinese Han Population23 and 0.23 in a Beijing population34, and no association between FTO genetic variants and BMI and obesity was revealed23.
In our study, carriers of the GG genotype had an increased risk of obesity compared to other genotypes. A similar association was reported by Sentinelli et al24. In the Italian individuals, where the G allele of FTO rs9930506 was significantly associated with class I/II obesity. No significant differences were observed in concentrations of fasting plasma glucose, total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides between carriers of the A allele and GG homozygotes in our study, while higher BMI was observed in GG homozygotes. Similar results were reported for the Italian subjects by Sentinelli et al24. The possible explanation for these observations may be the relatively young age of the participants in both studies, and, as suggested by Sentinelli et al24, these subjects may develop metabolic abnormalities in the future. It indicates that differences in biochemical parameters between obese and non-obese subjects are caused by enhanced adipose tissue accumulation, rather than the occurrence of specific variants in FTO gene, which interact with obesity-promoting environmental factors and influence the risk for obesity.
In conclusion, our study reported significant association between FTO rs9939506 GG genotype and BMI and obesity in the Polish population. In favour of the role of FTO gene in obesity, Smemo et al35 showed that FTO was functionally connected with regulation of IRX3 gene expression. IRX3 encodes a transcription fact or highly expressed in brain and is an important determinant of body mass and metabolism. Our results indicate that parts of the Polish population are carriers of a genetic variant which, in an obesogenic environment, may significantly enhance the risk of developing obesity. This is an additional argument indicating the need to make continuous and intensive effort to promote changes in lifestyle and dietary habits to stop the epidemic of obesity.
Acknowledgment
This work was supported by Medical University of Warsaw Grants FW113/NM2/13, and FW113/NM1/14 and by the Polish National Science Centre Grant N151923.
Conflicts of Interest: None.
References
- Introduction: obesity and lifestyle issues in women. Clin Obstet Gynecol. 2014;57:433-45.
- [Google Scholar]
- World Health Organization (WHO). Obesity: preventing and managing the global epidemic. Report of a WHO consultation, No. 894. In: WHO Tech Rep Ser. Geneva: WHO; 2000. p. :1-253.
- [Google Scholar]
- Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497-538.
- [Google Scholar]
- The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71:478-87.
- [Google Scholar]
- Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 2003;6:409-21.
- [Google Scholar]
- The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes (Lond). 2010;34:29-40.
- [Google Scholar]
- Increased genetic variance of BMI with a higher prevalence of obesity. PLoS One. 2011;6:e20816.
- [Google Scholar]
- Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes. 2010;59:2980-8.
- [Google Scholar]
- Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115.
- [Google Scholar]
- Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr. 2013;97:1395-402.
- [Google Scholar]
- A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889-94.
- [Google Scholar]
- The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469-72.
- [Google Scholar]
- Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313-9.
- [Google Scholar]
- The fat mass and obesity associated gene (FTO) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042-8.
- [Google Scholar]
- Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375-81.
- [Google Scholar]
- Variant rs9939609 in the FTO gene is associated with body mass index among Chinese children. BMC Med Genet. 2010;11:136.
- [Google Scholar]
- Association of the FTO and ADRB2 genes with body composition and fat distribution in obese women. Maturitas. 2013;76:165-71.
- [Google Scholar]
- Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419-26.
- [Google Scholar]
- A systematic mapping approach of 16q12.2/FTO and BMI in more than 20,000 African Americans narrows in on the underlying functional variation: results from the Population Architecture using Genomics and Epidemiology (PAGE) study. PLoS Genet. 2013;9:e1003171.
- [Google Scholar]
- No association of obesity gene FTO with body composition at the age of 6 months. The Generation R Study. J Endocrinol Invest. 2011;34:16-20.
- [Google Scholar]
- An FTO variant is associated with Type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet Med. 2011;28:673-80.
- [Google Scholar]
- Genetics of obesity and type 2 diabetes in African Americans. J Obes 2013 2013:1-7.
- [Google Scholar]
- Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes. 2008;57:264-8.
- [Google Scholar]
- Association of FTO polymorphisms with early age of obesity in obese Italian subjects. Exp Diabetes Res 2012 2012:1-7.
- [Google Scholar]
- Comparison of the glucose oxidase method for glucose determination by manual assay and automated analyzer. J Pharmacol Toxicol Methods. 2000;44:543-6.
- [Google Scholar]
- Lipids in serum of patients with malignant ovarian neoplasms. Int J Gynaecol Obstet. 1997;57:287-93.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- Detection and genotyping of varicella-zoster virus by TaqMan allelic discrimination real-time PCR. J Clin Microbiol. 2004;42:1409-13.
- [Google Scholar]
- Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet Epidemiol. 2007;31:358-62.
- [Google Scholar]
- A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123:3539-51.
- [Google Scholar]
- The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009;90:1483-8.
- [Google Scholar]
- The association of the FTO rs9939609 polymorphism with obesity and metabolic risk factors for cardiovascular diseases in Polish children. J Physiol Pharmacol. 2012;63:241-8.
- [Google Scholar]
- Inverse association of the obesity predisposing FTO rs9939609 genotype with alcohol consumption and risk for alcohol dependence. Addiction. 2011;106:739-48.
- [Google Scholar]
- The application of a high resolution melting-based genotyping method in studying the association between FTO rs9930506 polymorphism and metabolic syndrome in Beijing population. Zhonghua Nei Ke Za Zhi. 2012;51:8-12.
- [Google Scholar]
- Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371-5.
- [Google Scholar]






