Translate this page into:
Aspirin & clopidogrel non-responsiveness & its association with genetic polymorphisms in patients with myocardial infarction
For correspondence: Dr Madhu Dikshit, THSTI National Chair Translational Health Science and Technology Institute, NCR Biotech Science Cluster, Faridabad 121 001, Haryana, India e-mail: drmadhudikshit@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Cytochrome P450, P2Y12, cyclooxygenase-1 (COX1) and glycoprotein V1 (GPVI) gene polymorphisms are known to affect patient responsiveness towards aspirin and clopidogrel dual antiplatelet therapy (DAPT). The present study was undertaken to identify aspirin and clopidogrel non-responsiveness and its association with genetic polymorphism in patients with myocardial infarction (MI).
Methods:
A total of 207 MI patients who were on DAPT, were included. The DAPT non-responsiveness was determined by light transmittance aggregometry using arachidonic acid and adenosine diphosphate and high platelet reactivity by collagen. Platelet activation biomarkers, thromboxane B2 (TxB2) and soluble CD40 ligand (sCD40L) were measured in plasma. Patient compliance was checked by estimating drug and its metabolite levels (aspirin and clopidogrel) in plasma using liquid chromatography-mass spectrometry/mass spectrometry. Genomic DNA was extracted, amplified by polymerase chain reaction and subsequently sequenced to identify CYP450, P2Y12, COX1 and GPVI gene polymorphisms.
Results:
Of the 207 patients, 32 were non-responders. The DAPT non-responsiveness was found in 15.5 per cent patients. The non-responsiveness showed a significant and an independent association with gender [odds ratio (OR)=0.18, 95% confidence interval (CI)=0.01-0.78, P=0.023], TxB2 (OR=1.00, 95% CI=1.00-1.01, P=0.013), CYP2C19*2 G>A (OR=3.33, 95% CI=1.04-10.69, P=0.044) and GPVI T>C (OR=0.23, 95% CI=0.08-0.67, P=0.007) after adjusting the demographic, clinical and genetic confounding factors when assessed between non-responder and responder compliant patients.
Interpretation & conclusions:
The study showed a significant association of genetic polymorphisms (CYP2C19*2 G>A and GPVI T>C) with DAPT non-responsiveness in MI patients. The findings of this study need further validation in a large cohort of patients with clinical follow up.
Keywords
Aspirin
clopidogrel
drug compliance
myocardial infarction
non-responders
platelet activation
platelet aggregation
responders
single-nucleotide polymorphism
Aspirin and clopidogrel are widely used anti-platelet drugs for preventing adverse thrombotic episodes in patients with cardiovascular disease1. Aspirin inhibits the synthesis of thromboxane A2 by acetylating cyclooxygenase 1 (COX-1) enzyme in platelets1. The thienopyridine clopidogrel is a pro-drug which utilizes hepatic cytochrome P450 enzyme system to get converted into an active thiol metabolite. The active metabolite blocks G-protein-coupled receptor P2Y12 on platelet surface. Although dual antiplatelet therapy (DAPT) is globally successful, it has shown inter-individual variation in patient responsiveness1.
Antiplatelet drug non-responsiveness can be defined as failure of an antiplatelet drug to block its specific target2. The DAPT non-responsiveness in cardiovascular disease patients may be caused by clinical factors such as platelet hyperactivity, poor patient compliance, drug-drug interactions and poor absorption3. Majority of these clinical pharmacokinetic variables such as intestinal absorption and hepatic metabolism of drugs are directly regulated by genetic polymorphisms of drug-metabolizing enzymes cytochrome P4504. Amongst CYP450 enzymes, the CYP2C19 and CYP3A4 genetic variants have been extensively investigated and shown direct association with reduced platelet responsiveness. The molecular mechanism of CYP2C19 and CYP3A4 has also been reviewed in detail5. The single-nucleotide polymorphisms (SNPs) disrupt the metabolic functionality of CYP450 enzymes, thereby reducing the plasma levels of pharmacologically active clopidogrel metabolite leading to minimal or no platelet inhibition. The high platelet reactivity despite the drug intake may induce major adverse cardiac events such as secondary myocardial infarction (MI), cerebral stroke or death. Moreover, genetic variants of platelet surface receptors (P2Y12 and GPVI) and COX1 enzyme independently affect platelet responsiveness towards aspirin and clopidogrel, thereby resulting in compromised platelet inhibition6. The platelet glycoprotein VI (GPVI) receptor binds subendothelial collagen and induces a complex downstream signalling, and genetic variations in GPVI have been reported to alter platelet function and the risk of cardiovascular disease7. However, the precise mechanisms are not completely understood.
The present study was undertaken to identify the presence of aspirin and clopidogrel non-responsiveness and its association with genetic polymorphisms in patients with MI. Platelet aggregation was measured to identify DAPT responders and non-responders and levels of thromboxane B2 (TxB2) and soluble CD40 ligand (sCD40L) were assessed as platelet activation markers in patient's plasma. The probable association of genetic polymorphisms (CYP2C19*2 G>A, CYP2C19*3 G>A, CYP3A4 G>A, P2RY12 T>C, COX1 A>G and GPVI T>C) with DAPT non-responsiveness was also evaluated.
Material & Methods
A total of 207 MI patients who were on DAPT reporting for follow up at the Outpatient Department of Cardiology, King George's Medical University (KGMU), Lucknow, India, over a period of six years (December 2009 to December 2015) were recruited. A total of 108 healthy individuals were included to serve as controls (Fig. 1). The controls were research scholars, faculty members and voluntary blood donors, and were not taking any non-steroidal anti-inflammatory drugs (NSAIDs). The contribution of controls was limited to provide the maximal platelet aggregation response for the assessment of non-responsiveness in patients. The clinical details such as age, sex, smoking, hypertension, diabetes mellitus and concomitant drug use were noted.
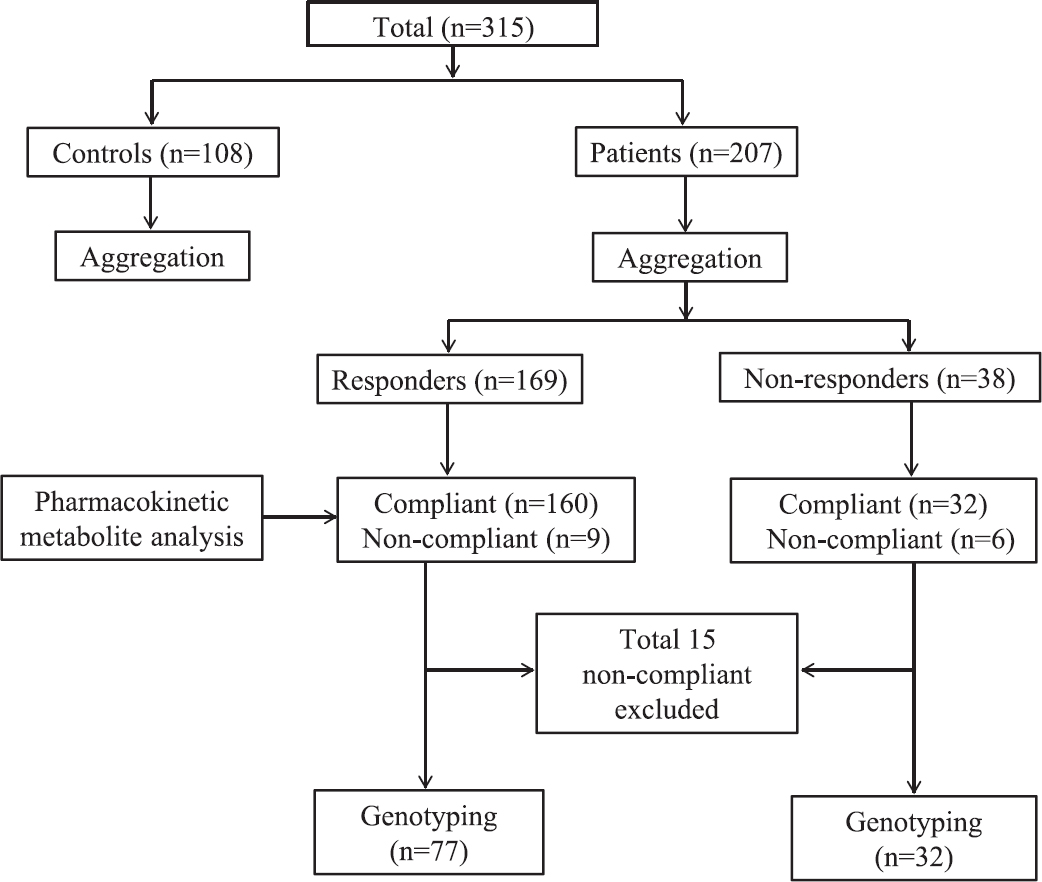
- Flowchart showing distribution of participants at different phases of study.
Inclusion/exclusion criteria: ST-elevation myocardial infarction (STEMI)/non-STEMI consecutive patients who were on a maintenance dose of DAPT, namely acetylsalicylic acid and clopidogrel ≥75 mg, daily for at least past 10 days prior to sample collection were included in the study. The patients with other chronic illnesses, bleeding disorders, allergic to aspirin and clopidogrel and concomitant use of other NSAIDs were excluded from the study. The study protocol was approved by the Institutional Ethics Committee of KGMU, Lucknow (XLIV ECM/A-P1). Written informed consent was obtained from each participant.
The sample size was primarily based on DAPT non-responsiveness in MI patients. To detect at least 7 per cent incidence (effect size) of DAPT non-responsiveness in MI patients, and considering 5 per cent margin of error (Type I error), 80 per cent power (Type II error) and 1:2 ratio, the study required minimum 300 individuals (100 controls and 200 patients)8. The sample size to deduce a significant association of SNPs with DAPT non-responsiveness in MI patients was based on allele frequency. To detect at least 5 per cent difference (effect size) in major allele frequency between responder and non-responder patients, and considering 5 per cent margin of error (Type I error), 80 per cent power (Type II error) and 1:2 ratio, the study required minimum 75 patients to be genotyped (25 non-responders and 50 responders)8.
Blood (9 ml) was drawn by venipuncture [3.8% sodium citrate (pH 6.5); 9:1 ratio (v/v)] 10-12 h after ingestion of antiplatelet drug. Samples were centrifuged at 200 ×g for 10 min to separate platelet-rich plasma (PRP). Further, centrifugation at 1500 ×g for 15 min at room temperature resulted in platelet poor plasma9. Plasma and buffy coat samples were stored at −80°C till further processed.
Platelet aggregation: Platelet aggregation was measured in PRP at 37°C by light transmittance aggregometry using an aggregometer (Model 700, Chrono-Log Corp., USA). The PRP was pre-warmed to 37°C for five minutes before addition of the agonists i.e., 5 μM adenosine diphosphate (ADP), 0.5 mM arachidonic acid (AA) and 2 μg/ml collagen (all from Chrono-Log Corp., USA)9. The aggregation response was monitored for at least five minutes, and the extent of aggregation was expressed as the percentage (%) aggregation calculated using Aggrolink software (Chrono-Log Corp., USA)
The aspirin non-responsiveness was defined as maximal platelet aggregation ≥20 per cent with AA as the agonist10 and clopidogrel non-responsiveness was defined as the mean inhibitory platelet aggregation ≤10 per cent as calculated from ADP-induced platelet aggregation11, which estimated a value of ≥59 per cent. Collagen was used to measure high platelet reactivity in patients, and was defined as maximal aggregation ≥90th percentile of the distribution with collagen (2 μg/ml)12, which estimated a value of ≥28 per cent. Collagen is a strong platelet agonist that acts on GPVI receptor and directly activates the function of GPIIb/IIIa receptor to induce a maximal platelet aggregation response12. Signalling by these receptors causes the secretion of ADP and TxB2 which further amplifies GPIIb/IIIa activation and subsequent platelet aggregation response. Collagen-induced platelet aggregation is highly sensitive to aspirin and clopidogrel treatment and is routinely used to assess high platelet reactivity and anti-platelet drug responsiveness in patients1013. Based on platelet aggregation, 38 patients were non-responders and 169 were responders (Fig. 1).
Measurement of platelet activation markers: The levels of TxB2 were estimated in platelet poor plasma of patients using TxB2 ELISA kit (Amersham Biosciences USA), whereas sCD40L release was measured using human sCD40 Ligand assay kit (R&D Systems, Inc., USA) as per the manufacturer's instructions.
Measurement of acetyl salicylic acid, clopidogrel and its carboxy metabolite in plasma: Drug compliance for DAPT was assessed by (i) direct and detailed questioning, and (ii) measuring salicylate and clopidogrel carboxy metabolite levels in patient's plasma by liquid chromatography-mass spectrometry/mass spectrometry. All patients confirmed drug intake as per the prescribed regimen before their enrolment in the study. Acetylsalicylic acid, clopidogrel and its carboxy metabolite were estimated in plasma as previously described14. Acetyl benzoic acid for aspirin (acetyl salicylic acid) and ticlopidine for clopidogrel were used as internal standard (IS)14. Clopidogrel and its carboxy metabolite from plasma were extracted by mixing 100 μl plasma with 10 μl of IS solution (IS 0.5 μg/ml) and extraction solvent (3 ml ether and ethyl acetate, 70:30 v/v) on a cyclomixer (Spinix, Tarsons). The mixture was centrifuged for 10 min at 2000 ×g at 20°C. The organic layer (2.6 ml) was separated and evaporated to dryness under vacuum. The residue was reconstituted in 100 μl of acetonitrile and 20 μl was injected onto analytical column (C18, Merck, USA). A simple protein precipitation method for the extraction of aspirin and salicylic acid from human plasma was used. To 100 μl of plasma, 10 μl of IS solution (3 μl/ml) was added and vortexed on a cyclomixer for 15 sec. Acetonitrile (300 μl) was added to precipitate proteins. The mixture was centrifuged for 20 min at 12000 ×g. The supernatant was injected onto analytical column. Mass spectrometric detection of acetyl salicylic acid, clopidogrel and its carboxy metabolite was performed on an API 4000 mass spectrometer (Applied Biosystems, MDS Sciex Toronto, Canada).
Based on the above assessment, 15 (7.2%) patients were non-compliant and thus excluded from the study (Fig. 1). Of these 15 patients, nine were responders and six were non-responder. Thus, of the total 192 compliant patients, 32 non-responders and 160 responders were subjected to SNP analysis. Of the total 32 non-responders, 20 (62.5%) showed failure of inhibition of platelet aggregation when AA was the agonist and 12 (37.5%) when ADP was the agonist. Amongst these 32 non-responders, 17 (53.1%) showed high platelet reactivity to collagen induced aggregation.
Genotyping: Genomic DNA was extracted from the buffy coat using a genomic DNA purification kit (Fermentas, USA) as per the manufacturer's protocol. The genomic DNA was subjected to PCR amplification followed by agarose gel electrophoresis for separation. The samples were outsourced for genotyping (Cromdx Solutions Pvt. Ltd., India) to determine polymorphisms in genes coding for (i) enzymes involved in hepatic metabolism of clopidogrel [CYP2C19*2 (681G>A) (rs4244285), CYP2C19*3 (636 G>A) (rs4986893) and CYP3A4 (IVS10+12 G>A) (rs2242480)], (ii) target proteins of clopidogrel and aspirin [P2Y12 (IVS1+744 T>C) (rs2046934) and COX1 (842A>G) (rs10306114)], and (iii) platelet collagen receptor [GPVI (T>C) (rs1613662)] using primers described in Table I15161718. The quality value (QV) for base calling was reported to be ≥20. Genotyping was done on 109 patients, all 32 non-responders and ≥2 fold randomly selected responders (77 out of 160) (Fig. 1).
| Gene (SNP) | Primer sequence | PCR conditions |
|---|---|---|
| CYP2C19*2 (681 G>A) | 5’- CAACCAGAGCTTGGCATATT-3’ (F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- TACGCAAGCAGTCACATAAC-3’ (R) | 51°C for 45 sec, 72ºC for 30 sec | |
| CYP2C19*3 (636 G>A) | 5’- CCCTGTGATCCCACTTTCAT-3’(F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- ATGGCTGTCTAGGCAAGACT-3’ (R) | 51°C for 45 sec, 72°C for 30 sec | |
| CYP3A4 (IVS10+12G>A) | 5’- TGTATTAACTGGCCACTCAC-3’(F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- GAGCCTTCCTACATAGAGTC-3’(R) | 51°C for 45 sec, 72°C for 30 sec | |
| P2Y12 (IVS1+744T>C) | 5’- TGATAGGTCAGAGGCTAACG-3’(F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- CCTGCTACTCCTTCAGATCA-3’ (R) | 51°C for 45 sec, 72°C for 30 sec | |
| COX1 (A842G) | 5’ -TTCCGATAACTGACCACCTACTACATGCTG-3’ (F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- CCAAACTCCAAGAGAGCCTAGTTCAAATCC-3’(R) | 51°C for 45 sec, 72°C for 30 sec | |
| GPVI (T>C) | 5’- ACATCCACAACAGTCCAGTG-3’ (F) | 94°C for 5 min, 94°C for 45 sec, |
| 5’- ATCGAGAAGTCTAGGCAGAG-3’ (R) | 47°C for 45 sec, 72°C for 30 sec |
Statistical analysis: Continuous data were summarized as mean ± standard error of the mean whereas discrete (categorical) in number and percentage (%). Continuous groups were compared by independent Student's t test whereas categorical groups were compared by Chi-square test. Continuous groups were also compared by one-way analysis of variance (ANOVA) and significance of mean difference between the groups was done by Newman-Keuls post hoc test. Pearson's correlation analysis was done to assess the association between the variables. Univariate and multivariate binary logistic regression analysis was done to assess independent predictor(s) of non-responsiveness. The power of all statistical tests was 80 per cent. Analyses were performed on STATISTICA software v 7.1 (StatSoft, Inc., USA).
Results
The basic characteristics (demographic: age, sex, smoking, hypertension, diabetes mellitus and concomitant drugs and clinical: platelet aggregation and activation) of 108 controls and 207 patients are summarized in Table II. Among patients, 23 (11.1%) had smoking habits, 186 (89.9%) had STEMI and 39 (18.8%) had family history of heart disease; 187 (90.3%) patients were on statins, 184 (88.9%) on β-blockers, 76 (36.7%) on angiotensin-converting enzyme inhibitors, eight (3.9%) on calcium channel blockers, 18 (8.7%) on nitrates and eight (3.9%) on diuretics. The frequency of smokers, hypertension and diabetes mellitus differed significantly and was higher in patients as compared to controls. In contrast, the mean platelet aggregation (AA, ADP and collagen) and platelet activation (TxB2 and sCD40L) were lowered significantly (P<0.001) in patients as compared to controls.
| Variables | Controls (n=108) (%) | Patients (n=207) (%) |
|---|---|---|
| Age (yr) | 52.33±1.17 | 54.67±0.68 |
| Gender | ||
| Female | 19 (17.6) | 23 (11.1) |
| Male | 89 (82.4) | 184 (88.9) |
| Smoking | ||
| No | 106 (98.1) | 184 (88.9)** |
| Yes | 2 (1.9) | 23 (11.1) |
| Hypertension | ||
| No | 98 (90.7) | 157 (75.8)*** |
| Yes | 10 (9.3) | 50 (24.2) |
| Diabetes mellitus | ||
| No | 107 (99.1) | 166 (80.2)*** |
| Yes | 1 (0.9) | 41 (19.8) |
| Concomitant drugs | ||
| Statins | - | 187 (90.3) |
| β-blockers | - | 184 (88.9) |
| Platelet aggregation | ||
| ADP (5 μM) | 65.93±0.93 | 24.35±1.13*** |
| AA (0.5 mM) | 65.55±1.08 | 9.22±0.86*** |
| Collagen (2 μg/ml) | 67.58±0.95 | 10.99±0.97*** |
| Platelet activation | ||
| TxB2 (pg/ml) | 169.88±18.23 | 75.05±9.55*** |
| sCD40L (pg/ml) | 63.68±7.72 | 35.58±3.05*** |
P**<0.01, ***<0.001 compared to controls. The continuous parameters age, platelet activation and platelet aggregation between two groups were compared by Student’s t test while categorical parameters gender, smoking, hypertension and diabetes mellitus were compared by Chi-square test. AA, arachidonic acid; ADP, adenosine diphosphate; TxB2, thromboxane B2; sCD40L, soluble CD40 ligand
The quantitative measurements (platelet aggregation and platelet activation) of the three groups (108 controls and 169 responder and 38 non-responder patients) are summarized in Table III. The mean platelet aggregation and activation levels of the three groups were significantly (P<0.001) different. The mean platelet aggregation in both responder and non-responder patients was significantly (P<0.001) lower in patients as compared to controls. The mean platelet aggregation was significantly (P<0.001) lower in responders as compared to non-responders. The mean TxB2 was significantly (P<0.01 or P<0.001) lower in responders as compared to both controls and non-responders, but not differed between controls and non-responders.
| Variables | Controls (n=108) | MI patients (n=207) | |
|---|---|---|---|
| Responders (n=169) | Non-responders (n=38) | ||
| Platelet aggregation | |||
| ADP (5 μM) | 65.93±0.93 | 21.63±0.99***† | 36.47±3.72*** |
| AA (0.5 mM) | 65.55±1.08 | 5.62±0.35***† | 25.26±3.38*** |
| Collagen (2 µg/ml) | 67.58±0.95 | 7.02±0.48***† | 28.61±3.71*** |
| Platelet activation | |||
| TxB2 (pg/ml) | 169.99±18.23 | 62.54±6.23***†† | 130.67±43.35 |
| sCD40L (pg/ml) | 63.68±7.72 | 33.64±2.87**† | 44.24±10.66 |
P**<0.01, ***<0.001 compared to controls. P †<0.05, ††<0.01 compared to non-responders. Values are mean±SE. SE, standard error; AA, arachidonic acid; ADP, adenosine diphosphate; TxB2, thromboxane B2; sCD40L, soluble CD40 ligand; MI, myocardial infarction
The correlation between TxB2 and sCD40L in controls (n=108), total patients (n=207) and responder (n=169) and non-responder (n=38) patients is shown in Fig. 2. The Pearson's correlation analysis showed insignificant correlation between TxB2 and sCD40L in controls (r=−0.06, P>0.05), total patients (r=0.01, P>0.05) and responder (r=0.02, P>0.05) and non-responder (r=−0.03, P>0.05) patients. Of the 207 patients, 32 (15.5%) were found non-responsive (non-responder) to aspirin and clopidogrel (after excluding 15 non-compliant patients).
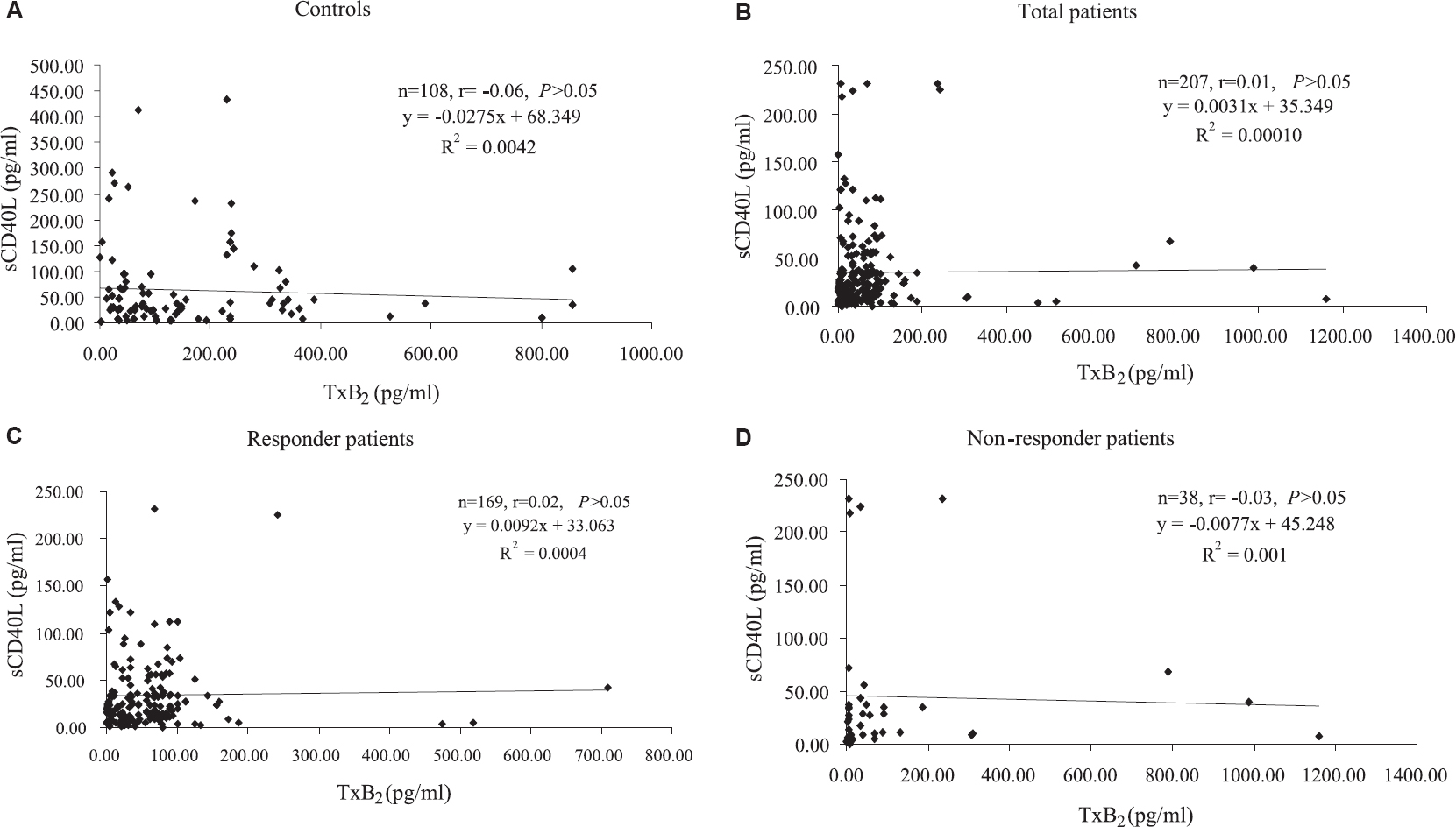
- Scatter plot showing correlation between (A) thromboxane B2 and sCD40L in controls, (B) total patients, (C) responder patients and (D) non-responder patients.
Responder and non-responder patients: Genotyping was done on 109 patients, 32 non-responders and 77 responders. The demographic, clinical and genetic (SNPs) factors of two groups are summarized in Table IV. The mean age and frequency of smokers, diabetes mellitus, use of concomitant drugs (statins and β-blockers) and SNPs (CYP2C19*3 G>A, CYP3A4 G>A, P2Y12 T>C and COX1 A>G) were found similar in the two groups. However, gender, hypertension and SNPs (CYP2C19*2 G>A and GPVI T>C) and platelet activation (TxB2 and sCD40L) differed significantly between the two groups. The frequency of female gender, hypertension and homozygous AA genotype of CYP2C19*2 G>A SNP was significantly (P<0.05) higher in non-responders as compared to responders. In contrast, frequency of homozygous CC genotype of GPVI T>C SNP was significantly (P<0.01) lower in non-responders as compared to responders. Further, the mean TxB2 and sCD40L both were lowered significantly (P<0.001) in responders as compared to non-responders.
| Variables | Responders (n=77) (%) | Non-responders (n=32) (%) | P value |
|---|---|---|---|
| Age (yr) | 53.56±1.17 | 56.69±1.77 | 0.147 |
| Gender | |||
| Female | 6 (7.8) | 7 (21.9) | 0.039 |
| Male | 71 (92.2) | 25 (78.1) | |
| Smoking | |||
| No | 66 (85.7) | 29 (90.6) | 0.485 |
| Yes | 11 (14.3) | 3 (9.4) | |
| Hypertension | |||
| No | 60 (77.9) | 19 (59.4) | 0.048 |
| Yes | 17 (22.1) | 13 (40.6) | |
| Diabetes mellitus | |||
| No | 60 (77.9) | 26 (81.3) | 0.698 |
| Yes | 17 (22.1) | 6 (18.8) | |
| Statins | |||
| No | 8 (10.4) | 2 (6.3) | 0.495 |
| Yes | 69 (89.6) | 30 (93.8) | |
| β-blockers | |||
| No | 9 (11.7) | 3 (9.4) | 0.725 |
| Yes | 68 (88.3) | 29 (90.6) | |
| Platelet activation | |||
| TxB2 (pg/ml) | 62.25±9.70 | 149.37±50.84 | 0.017 |
| sCD40L (pg/ml) | 27.05±3.97 | 48.52±12.50 | 0.036 |
| CYP2C19*2 G>A | |||
| GG | 60 (77.9) | 20 (62.5) | 0.011 |
| GA | 13 (16.9) | 4 (12.5) | |
| AA | 4 (5.2) | 8 (25.0) | |
| CYP2C19*3 G>A | |||
| GG | 75 (97.4) | 30 (93.8) | 0.292 |
| GA | 2 (2.6) | 1 (3.1) | |
| AA | 0 (0.0) | 1 (3.1) | |
| CYP3A4 G>A | |||
| GG | 31 (40.3) | 16 (50.0) | 0.079 |
| GA | 40 (51.9) | 10 (31.3) | |
| AA | 6 (7.8) | 6 (18.8) | |
| P2Y12 T>C | |||
| TT | 44 (57.1) | 22 (68.8) | 0.525 |
| TC | 27 (35.1) | 8 (25.0) | |
| CC | 6 (7.8) | 2 (6.3) | |
| COX1 A>G | |||
| AA | 65 (84.4) | 29 (90.6) | 0.674 |
| AG | 5 (6.5) | 1 (3.1) | |
| GG | 7 (9.1) | 2 (6.3) | |
| GPVI T>C | |||
| TT | 18 (23.4) | 17 (53.1) | 0.008 |
| TC | 25 (32.5) | 8 (25.0) | |
| CC | 34 (44.2) | 7 (21.9) |
The allele frequency of two groups is summarized in Table V. The frequency of major allele A of CYP2C19*2 G>A SNP was found significantly (P<0.01) different and 17.7 per cent higher in non-responders as compared to responders. In contrast, frequency of major allele C of GPVI T>C SNP was found significantly (P<0.001) different and 30.0 per cent lower in non-responders as compared to responders. However, the allele frequency of other SNPs (CYP2C19*3 G>A, CYP3A4 G>A, P2Y12 T>C and COX1 A>G) did not differ between the two groups.
| SNP/allele | Responders (n=154) (%) | Non-responders (n=64) (%) | OR (95% CI) |
|---|---|---|---|
| CYP2C19*2 G>A | |||
| G | 133 (86.4) | 44 (68.8) | 2.88 (1.43-5.80) |
| A | 21 (13.6) | 20 (31.3)** | |
| CYP2C19*3 G>A | |||
| G | 152 (98.7) | 61 (95.3) | 3.74 (0.61-22.93) |
| A | 2 (1.3) | 3 (4.7) | |
| CYP3A4 G>A | |||
| G | 102 (66.2) | 42 (65.6) | 1.03 (0.56-1.90) |
| A | 52 (33.8) | 22 (34.4) | |
| P2Y12 T>C | |||
| T | 115 (74.7) | 52 (81.3) | 0.68 (0.33-1.41) |
| C | 39 (25.3) | 12 (18.8) | |
| COX1 A>G | |||
| A | 135 (87.7) | 59 (92.2) | 0.60 (0.21-1.69) |
| G | 19 (12.3) | 5 (7.8) | |
| GPVI T>C | |||
| T | 61 (39.6) | 42 (65.6) | 0.34 (0.19-0.63) |
| C | 93 (60.4) | 22 (34.4)*** |
P**<0.01 ***<0.001 compared to responders. The allele frequency between two groups was compared by Chi-square test. OR, odds ratio; CI, confidence interval. Abbreviations are as given in Table I.
To find out independent predictor of non-responsiveness in MI patients, the demographic, clinical and genetic factors of the two groups were subjected to univariate (crude or unadjusted) and multivariate (adjusted) binary logistic regression analysis and findings are summarized in Table VI. In univariate analysis, gender, TxB2 and GPVI T>C showed a significant (P<0.05 or <0.01) association with non-responsiveness. In multivariate analysis, gender, TxB2, CYP2C19*2 G>A and GPVI T>C showed a significant (P<0.05 or P<0.01) association with the non-responsiveness after adjusting the demographic, clinical and genetic confounding factors, suggesting these to be significant and independent predictors of non-responsiveness in MI patients.
| Predictor | Univariate (unadjusted) | Multivariate (adjusted) | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (yr) | 1.03 (0.99-1.07) | 0.148 | 1.03 (0.98-1.09) | 0.274 |
| Gender | ||||
| Female | Ref | Ref | 0.023 | |
| Male | 0.30 (0.09-0.98) | 0.047 | 0.18 (0.04-0.78) | |
| Smoking | ||||
| No | Ref | Ref | 0.830 | |
| Yes | 0.62 (0.16-2.39) | 0.488 | 0.84 (0.17-4.11) | |
| Hypertension | ||||
| No | Ref | Ref | 0.346 | |
| Yes | 2.41 (0.99-5.87) | 0.052 | 1.78 (0.54-5.94) | |
| Diabetes mellitus | ||||
| No | Ref | Ref | 0.922 | |
| Yes | 0.81 (0.29-2.30) | 0.698 | 0.94 (0.25-3.56) | |
| Statins | ||||
| No | Ref | Ref | 0.386 | |
| Yes | 1.74 (0.35-8.68) | 0.500 | 3.72 (0.19-72.73) | |
| β-blockers | ||||
| No | Ref | Ref | 0.999 | |
| Yes | 1.28 (0.32-5.07) | 0.726 | 1.00 (0.09-11.12) | |
| TxB2 (pg/ml) | 1.00 (1.00-1.01) | 0.047 | 1.00 (1.00-1.01) | 0.013 |
| sCD40L (pg/ml) | 1.01 (1.00-1.02) | 0.051 | 1.01 (1.00-1.02) | 0.214 |
| CYP2C19*2 G>A | ||||
| GG | Ref | Ref | 0.044 | |
| GA/AA | 2.12 (0.86-5.19) | 0.101 | 3.33 (1.04-10.69) | |
| CYP2C19*3 G>A | ||||
| GG | Ref | Ref | 0.951 | |
| GA/AA | 2.50 (0.34-18.57) | 0.370 | 1.09 (0.07-16.81) | |
| CYP3A4 G>A | ||||
| GG | Ref | Ref | 0.653 | |
| GA/AA | 0.67 (0.29-1.54) | 0.351 | 0.79 (0.28-2.24) | |
| P2Y12 T>C | ||||
| TT | Ref | Ref | 0.435 | |
| TC/CC | 0.61 (0.25-1.45) | 0.261 | 0.64 (0.20-1.99) | |
| COX1 A>G | ||||
| AA | Ref | Ref | 0.432 | |
| AG/GG | 0.56 (0.15-2.14) | 0.396 | 0.49 (0.08-2.89) | |
| GPVI T>C | ||||
| TT | Ref | Ref | 0.007 | |
| TC/CC | 0.27 (0.11-0.64) | 0.003 | 0.23 (0.08-0.67) | |
Discussion
Amongst 207 MI patients, 32 drug compliant patients were identified as DAPT non-responders, so the dual non-responsiveness to aspirin and clopidogrel was 15.5 per cent as reported in other studies1013. More than one-half of non-responders (17/32) showed high residual platelet reactivity by collagen that activates platelets via GPVI receptor and involves mechanisms different from COX1- and P2Y12-mediated platelet functions. These associations can be explained, at least in part, by the fact that the different pathways involved in platelet reactivity can influence each other at different degrees. An abnormal platelet response to various physiological platelet agonists may help understand the underlying mechanisms of DAPT non-responsiveness in patients.
In the present study, the high percentage of collagen-induced platelet hyperactivity in aspirin and clopidogrel DAPT non-responders could be due to the cross-talk of different platelet activation mechanisms regulating platelet functions. AA activates platelets via thromboxane A2 that is synthesized by COX while ADP targets platelet G-protein-coupled receptor P2Y1 and P2Y12 to initiate aggregation1. Signalling by collagen receptors causes the secretion of both ADP and thromboxane A2, which subsequently binds to their target receptors and amplifies the aggregation response. Collagen-induced platelet activation and aggregation is significantly hampered if either or both of the two mechanisms are disrupted. It has been previously shown that ADP and collagen-induced platelet aggregation are affected to some extent by aspirin191. Thus, an abnormal response to 2 or 3 different platelet aggregation stimuli after aspirin and clopidogrel maintenance dose could identify abnormal platelet functions in MI patients.
In the present study, non-responders to aspirin and clopidogrel showed significantly enhanced plasma TxB2 levels in addition to sustained ADP, AA and collagen-induced aggregation, reflecting increased systemic levels of TxA2 in agreement with previous studies20. The plasma TxB2 levels in patients were also found to be associated with DAPT non-responsiveness. It has been proposed that measurement of TxB2 in both serum and plasma of patients may provide better assessment of aspirin response in patients taking dual antiplatelet drugs (aspirin and clopidogrel)21. There was a significant increase in the levels of plasma sCD40L in drug non-responders in the present study, which was in agreement with a previous report22. Soluble CD40 ligand is a well-known biomarker of activated platelets that is released into the systemic circulation22. An insignificant correlation was observed between TxB2 and sCD40L when analyzed in controls, total patients, responders and non-responder patients. The lack of correlation between TxB2 and sCD40L indicated that the increase or decrease in one might not be associated with increase or decrease in the other.
Poor adherence to anti platelet therapy can also be one of the underlying factors for DAPT non-responsiveness and may reflect variability in the prescribed therapy and patient's response to treatment benefits. It is not only the acute treatment of MI but also long-term medication adherence, follow up and lifestyle modification which are equally responsible for the increase in the chances of survival2324. Previous reports indicated variable frequencies of poor drug compliance ranging from 9 to 40 per cent in patients with MI who were on antiplatelet therapy2324. In the present study, 15 (7.2%) non-complaint patients were found. The patients who did not show any trace amount (<1 ng/ml) of salicylate or carboxy metabolites in their plasma or no effect on inhibition of platelet aggregation were considered as non-complaint. These observations collectively suggested poor compliance. On the basis of platelet aggregation and compliance, 32 patients were found to be DAPT non-responsive. Compliance, therefore, is one of the critical factors that should be taken into account while assessing drug non-responsiveness in patients.
Patients’ response to aspirin and clopidogrel is critically regulated by their specific receptor functions as well as by their hepatic metabolism and intestinal absorption. All these mechanisms are strongly regulated by genetic polymorphisms. Hence, elucidation of individual patient pharmacogenomics is critical for maximizing effective drug therapy. In our study, COX1 SNP-PTGS1 was not found to be associated with DAPT non-responsiveness and was in agreement with a previous report regarding aspirin resistance in Chinese young healthy volunteers26.
Considerable variation in the distribution of CYP2C19*2 allele frequency has been found in different populations worldwide. The allelic frequency of CYP2C19*2 has been reported to be significantly higher in Asians (∼30%) in comparison to Caucasian (∼13%) and the African-American (∼18%) populations5. The CYP2C19*3 variant expression shows more frequency in the Asians (∼10%) than in other population groups (∼< 1%)1819. Healthy volunteers with CYP2C19 polymorphism have shown a weak antiplatelet response to clopidogrel28. CYP2C19*2 has been reported as a loss-of-function allele, and patients with CYP2C19*2 polymorphism showed enhanced platelet activation compared to patients with normal genotype29. In the present study, DAPT non-responsiveness was associated with the polymorphism of CYP2C19*2, but not with CYP2C19*3 or CYP3A4 SNPs. Moreover, P2Y12 receptor polymorphism was also not associated with patient variability towards DAPT. It was observed that 25 per cent of total DAPT non-responding patients were homozygous to this variant. CYP2C19*2 allele was present in significantly higher frequency in aspirin and clopidogrel DAPT non-responders (31.3%) than in responders (13.6%).
Genetic polymorphism in platelet collagen receptor GPVI has been shown to associate with myocardial infarction7. An association was found between the GPVI receptor (C13254T) polymorphism and DAPT non-responsiveness in our study. Genetic variation in GPVI is assumed to alter platelet collagen interaction and hence might contribute towards the risk of cardiovascular disease. In the present study, a significant association of CYP2C19*2 and GPVI was found with DAPT non-responsiveness. The association of GPVI with DAPT non-responsiveness in the present study highlights the importance of collagen-induced platelet activation pathway in thrombotic disorders and therapy.
Our study had certain limitations. Lack of recording of several clinical factors such as actual dosage of aspirin and clopidogrel, duration of disease, lack of clinical follow up, controls and patients matched with confounding risk factors, not genotyping healthy controls and not genotyping all responder patients etc., were limitations of the study.
In conclusion, the DAPT non-compliance in MI patients was found to be 7.2 per cent (15 of 207 patients) and non-responsiveness was 15.5 per cent (32 of 207 patients). The mean platelet aggregation and activation both were lowered significantly in patients as compared to controls. The non-responsiveness was found significantly associated with gender, TxB2 and genetic polymorphisms (CYP2C19*2 G>A and GPVI T>C). The findings of this study need further validation on a large cohort of patients with clinical follow up.
Financial support & sponsorship: The last author (MD) acknowledges the financial support received from the Council of Scientific & Industrial Research (THUNDER, BSC0102) and the Department of Science & Technology-SERB [JC Bose National Fellowship (SB/SE/JCB-017/2015)], New Delhi.
Conflicts of Interest: None.
References
- Anti-platelet therapy for acute coronary syndrome: A Review of currently available agents and what the future holds. Cardiovasc Hematol Disord Drug Targets. 2011;11:79-86.
- [Google Scholar]
- Antiplatelet drug resistance: Molecular insights and clinical implications. Prostaglandins Other Lipid Mediat. 2015;120:21-7.
- [Google Scholar]
- Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541-7.
- [Google Scholar]
- Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363-75.
- [Google Scholar]
- Pharmacogenetics and cardiovascular disease – Implications for personalized medicine. Pharmacol Rev. 2013;65:987-1009.
- [Google Scholar]
- Genetic polymorphisms of platelet receptors in patients with acute myocardial infarction and resistance to antiplatelet therapy. Genet Test Mol Biomarkers. 2014;18:599-604.
- [Google Scholar]
- Novel platelet membrane glycoprotein VI dimorphism is a risk factor for myocardial infarction. Circulation. 2001;104:1459-63.
- [Google Scholar]
- Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Erlbaum, L; 1988.
- Synthesis and evaluation of dual antiplatelet activity of bispidine derivatives of N-substituted pyroglutamic acids. Eur J Med Chem. 2016;110:1-2.
- [Google Scholar]
- A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961-5.
- [Google Scholar]
- The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005;45:1392-6.
- [Google Scholar]
- Thrombotic events in high risk patients are predicted by evaluating different pathways of platelet function. Thromb Haemost. 2008;100:1136-45.
- [Google Scholar]
- Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312-7.
- [Google Scholar]
- Simultaneous quantitation of acetylsalicylic acid and clopidogrel along with their metabolites in human plasma using liquid chromatography tandem mass spectrometry. Biomed Chromatogr. 2016;30:466-73.
- [Google Scholar]
- Clopidogrel resistance in Japanese patients scheduled for percutaneous coronary intervention. Circ J. 2009;73:336-42.
- [Google Scholar]
- Impact of CYP2C19 polymorphisms on the antiplatelet effect of clopidogrel in an actual clinical setting in Japan. Circ J. 2009;73:1498-503.
- [Google Scholar]
- Polymorphisms of COX-1 and GPVI associate with the antiplatelet effect of aspirin in coronary artery disease patients. Thromb Haemost. 2006;95:253-9.
- [Google Scholar]
- Distribution of genetic polymorphisms of genes encoding drug metabolizing enzymes & drug transporters – A review with Indian perspective. Indian J Med Res. 2014;139:27-65.
- [Google Scholar]
- Association of CYP2C19, CYP3A5 and GPIIb/IIIa gene polymorphisms with aspirin and clopidogrel resistance in a cohort of Indian patients with coronary artery disease. Int J Lab Hematol. 2015;37:809-18.
- [Google Scholar]
- Increased platelet aggregation and serum thromboxane levels in aspirin-treated patients with prior myocardial infarction. Thromb Haemost. 2012;108:140-7.
- [Google Scholar]
- Variation in thromboxane B2 concentrations in serum and plasma in patients taking regular aspirin before and after clopidogrel therapy. Platelets. 2015;26:17-24.
- [Google Scholar]
- Impact of CYP2C19 variants on clinical efficacy of clopidogrel and 1-year clinical outcomes in coronary heart patients undergoing percutaneous coronary intervention. Front Pharmacol. 2016;7:453.
- [Google Scholar]
- Compliance as a critical consideration in patients who appear to be resistant to aspirin after healing of myocardial infarction. Am J Cardiol. 2005;95:973-5.
- [Google Scholar]
- Determinants of reduced antiplatelet effect of aspirin in patients with stable coronary artery disease. PLoS One. 2015;10:e0126767.
- [Google Scholar]
- Medication adherence and its determinants in myocardial infarction patients: An Indian scenario. J Clin Prev Cardiol. 2016;5:2-8.
- [Google Scholar]
- Frequency of genetic polymorphisms of COX1, GPIIIa and P2Y1 in a Chinese population and association with attenuated response to aspirin. Pharmacogenomics. 2007;8:577-86.
- [Google Scholar]
- The impact of genetic polymorphisms of P2Y12, CYP3A5 and CYP2C19 on clopidogrel response variability in Iranian patients. Biochem Pharmacol. 2012;83:903-8.
- [Google Scholar]
- Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244-7.
- [Google Scholar]
- The influence of CYP2C19*2 polymorphism on platelet function testing during single antiplatelet treatment with clopidogrel. Thromb J. 2011;9:4.
- [Google Scholar]






