Translate this page into:
Are Indian patients with juvenile-onset ankylosing spondylitis taller than reference population?
Reprint requests: Dr Debashish Danda, Department of Clinical Immunology and Rheumatology, Christian Medical College & Hospital, Vellore 632 004, Tamil Nadu, India e-mail: debashisdandacmc@hotmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Paucity of growth retardation has been observed by us in patients with juvenile-onset ankylosing spondylitis (JAS) in a tertiary care health centre in south India. We, therefore, undertook this pilot study to assess and compare anthropometry of patients with JAS who were 15 yr and older with that of adult onset ankylosing spondylitis (AAS) and matching Indian reference population.
Methods:
Consecutive male patients (December 2009- October 2012) with JAS and AAS fulfilling Modified New York Criteria were selected after applying inclusion and exclusion criteria. Demography and anthropometry were noted. Height of both patient groups as well as their parents and siblings were compared with that of the reference population. Mid-parental height and delta height were derived. Those with delta height of >8.5 cm were compared with the remaining. Multivariate logistic regression was done for variables that were found to be significant by chi-square in bivariate analysis. Similar analysis was done for BMI also.
Results:
There was no significant difference in anthropometric variables between JAS and AAS groups. Twenty eight of the 30 (93.33%) JAS patients were taller as compared to the reference population. Twenty six (86.67%) AAS patients were taller than the reference population. The mean heights of JAS (170.67 ± 6.94 cm) and AAS (168.2 ± 5.94 cm) patients were significantly higher than the reference value of 163.11 cm; both P<0.001. Logistic regression revealed that tallness in JAS was associated positively with hypermobility (OR=23.46,95%CI 1.2-447.2, P=0.036). No significant association was detected for height in AAS and for BMI in both JAS and AAS groups.
Interpretation & conclusions:
No growth retardation was seen in patients with JAS in our study. Majority of patients with JAS and AAS were taller than reference population. The difference between mean height of JAS and AAS was not significant. Larger studies involving different populations are required to confirm these findings.
Keywords
Anthropometry
height
hypermobility
juvenile
onset ankylosing spondylitis
Ankylosing spondylitis (AS) is a chronic inflammatory disease that primarily affects the sacroiliac joints and the spine. It has a strong genetic association with HLA B271. When this illness starts before the 16th birthday of an individual, it is called juvenile-onset ankylosing spondylitis (JAS). According to the International League of Associations for Rheumatology (ILAR) classification, most of the patients with JAS may fall within the entity of enthesitis related arthritis (ERA), one of the seven subtypes of juvenile idiopathic arthritis (JIA)2.
Growth retardation is a well known complication of juvenile onset chronic inflammatory diseases especially in systemic onset JIA and polyarticular disease, and is known to vary from 10 to 40 percent34567. Persistently elevated pro-inflammatory cytokines and altered status of growth hormone and related proteins in long-standing disease and use of corticosteroids are likely factors responsible for growth retardation in JIA7. Liem and Rosenber5 have reported normal growth patterns in RF negative polyarticular and oligoarticular subsets. An Indian study on outcome of juvenile rheumatoid arthritis (JRA, diagnosis based on American College of Rheumatology criteria) in adolescent boys reported that both height and weight remained suboptimal in all classes of JRA (pauciarticular, polyarticular and systemic onset) as compared to normal population8. Padeh et al9 reported growth retardation in 35.8 per cent of oligoarticular JIA (diagnosis based on ILAR classification2). The disparity between these two studies89 as compared to prior studies3456 could be due to the different classification criteria used as it is possible that pauciarticular and RF negative polyarticular arthritis described in earlier studies included some JAS or ERA patients as well.
In a tertiary care referral centre in south India, we have consistently noted a paucity of growth retardation in patients with JAS. This is in contrast to that observed in other forms of JIA and juvenile onset chronic inflammatory diseases. There are evidence for the latter from literature, however, there are no data on growth and anthropometry in JAS. This has led us to study anthropometry, particularly height in this patient population. Height is dependent on various factors including parental height, socio-economic factors, ethnicity, nutritional factors as well as disease activity and any study on height needs to take these factors into consideration. The present study was designed to assess anthropometry particularly in patients with JAS who were ≥15 yr of age and compare these parameters with those in patients with adult-onset ankylosing spondylitis (AAS) and reference Indian population.
Material & Methods
This cross-sectional study was conducted at the department of Clinical Immunology and Rheumatology, Christian Medical College and Hospital, Vellore, Tamil Nadu, India, between December 2009 and October 2012. Consecutive male patients with JAS and AAS fulfilling Modified New York Criteria for AS10 were recruited into the study from outpatient and inpatient services. Exclusion criteria were as follows: age less than 15 or greater than 45, patients on any hormone replacement or anticonvulsant therapy, use of corticosteroid with more than 15 mg prednisolone or equivalent/day for > 3 months, history of diabetes mellitus, thyroid or parathyroid disease, obesity, hip joint arthritis, spine pathology like kyphosis or vertebral fracture.
Written informed consent was obtained from all patients. The study protocol was approved by the Institutional Review Board. The following demographic characteristics were documented at baseline: current age, age at disease onset, gender and geographical origin/ethnicity. The revised Kuppuswamy scale was used for assessing socio-economic status with income modifications as described by Patro et al11 and patients were stratified as 1 (Upper), 2 (Upper middle), 3 (Lower middle), 4 (Upper lower), and 5 (Lower).
Detailed history (including history of drug therapy and family history), physical examination, scorings for disease activity, functional status and metrology by BASDAI (Bath Ankylosing Spondylitis Activity Index), BASFI (Bath Ankylosing Spondylitis Functional Index) and BASMI (Bath Ankylosing Spondylitis Metrology Index), respectively were noted. Weight, height, arm span, ratio of upper to lower segment were recorded as described earlier12. Inflammatory markers erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), and 25-hydroxy vitamin D levels were measured in all patients.
Height standard deviation scores (H-SDS) was calculated using the formula:
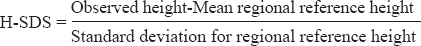
for both the patient groups using regional reference values described by Perkin et al13. The study is based on data derived from National Family Health Survey (NFHS) conducted in India from 2005-200614 on a nationally representative cross-sectional sample of women and men aged 15-49 and 15-54 yr, respectively from the 29 States of India. Data are available for different wealth quantile, education and childhood macroenvironment-birth cohort.
Body mass index (BMI) was calculated and categorized as: very severely underweight - < 15.0 kg/m2; severely underweight - 15.0 to 16.0 kg/m2; underweight-16.0 to 18.5 kg/m2; normal (healthy weight)-18.5 to 25 kg/m2; and overweight- 25 to 30 kg/m2. For purpose of further analysis, those with BMI <18.5 kg/m2 were defined as underweight and those with BMI ≥ 18.5 kg/m2 were defined as normal.
Age, height and weight of parents and siblings were also noted as far as possible at the clinic. Whenever this was not feasible, these were obtained by phone, email or post. Observed height of patients with AAS and JAS and their parents and siblings were compared with that of reference Indian population13.
The 3rd to 97th percentiles for anticipated adult height is represented by 8.5 cm on either side of the calculated mid-parental height15. Mid-parental height was calculated by

The difference between mid-parental height and height gave the delta height. For bivariate analysis, any patient with a delta height of >8.5 cm was considered as tall, since this definition would overcome the effect of parental factor on height.
For sample size calculation, the mean H-SDS was derived from five patients of JAS and AAS. The mean H-SDS of JAS and AAS were 0.88 ± 0.73 and 0.43 ± 0.71, respectively. Keeping α and β errors at 5 and 20%, respectively we needed to study 24 subjects in each arm.
Statistical analysis: Data are presented as mean ± SD or median (range) values. Unpaired t test was used for statistical analysis. One sample t test was used whenever comparison with population mean was done. Bivariate analyses were done between the ‘tall” subset of patients as defined above and the remaining “not so tall” patients to identify risk factors or associated features of ‘tallness’; similar analysis was done for BMI between underweight vs those with normal BMI. Chi-square test was done to find out any significant associations. The relevant variables with P≤0.30 by bivariate analysis were subjected to multivariable logistic regression analyses. Analyses were done using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Based on inclusion and exclusion criteria, 42 patients with JAS and 45 patients with AAS were included in the study. The first 30 patients in each group with all available data including anthropometry of their parents and siblings were considered for analysis (Table I). Heights of all 60 patients were recorded in the clinic. Sixty-nine out of 120 parental height measurements were also recorded during their visit to the Rheumatology out patient clinic and the remaining 51 were obtained by e-mail/postal mail. Of the 84 measurements of height of siblings, only 14 were recorded by us, the remaining 70 were obtained by email/post.
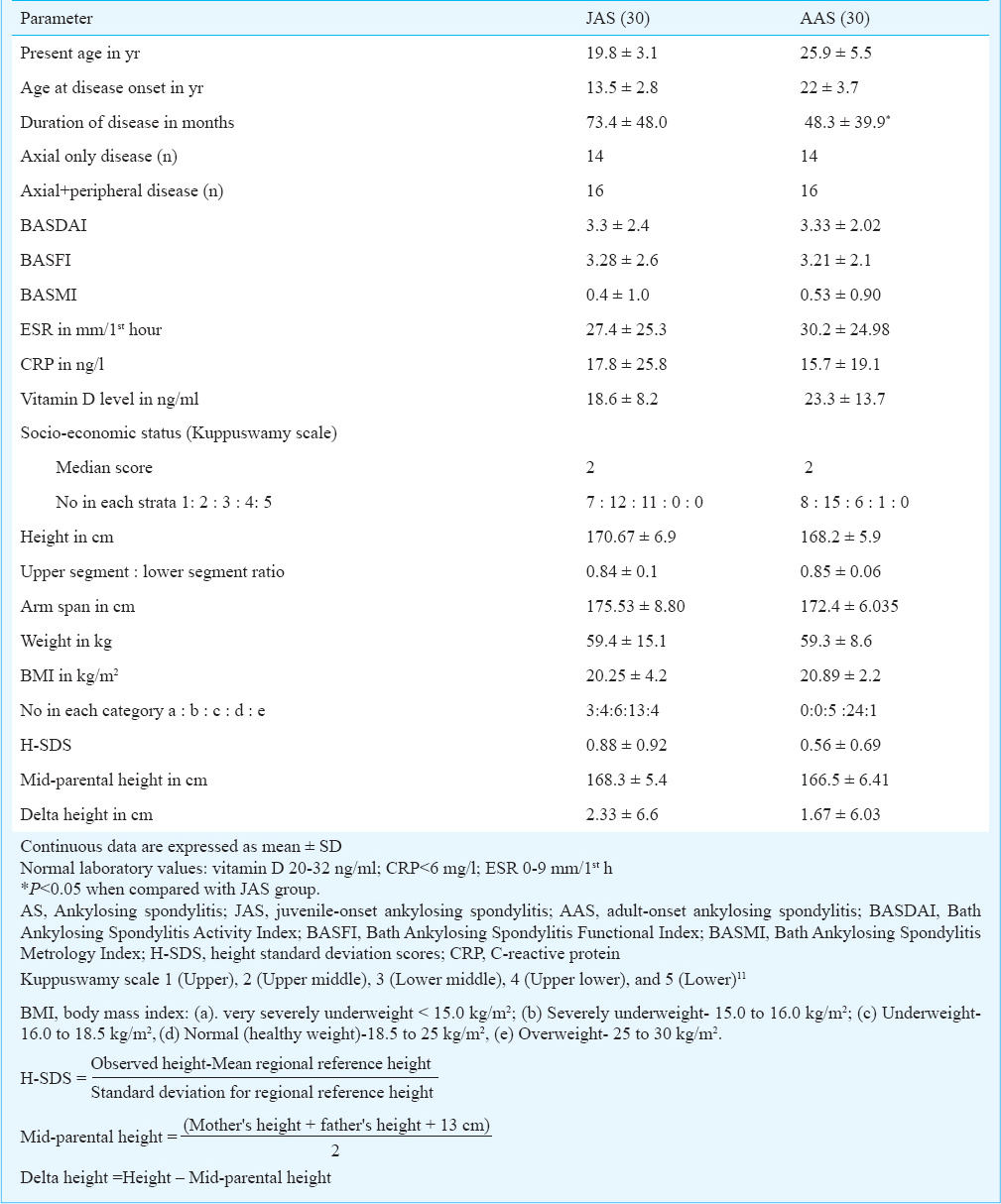
There was no significant difference in the baseline disease outcome measures, inflammatory markers (ESR and CRP) and 25 (OH) vitamin D levels between JAS and AAS groups. There was no observed or historically documented special dietary intervention including therapeutic doses of vitamin D for these patients either by the family, or by the previously treating physicians. Anthropometric variables between JAS and AAS were also comparable despite the former having a significantly higher disease duration (P<0.05). The regional distribution of JAS patients were as follows: West Bengal (16), Jharkand (6), Tamil Nadu (5), Bihar (2) and Uttar Pradesh (1). Among AAS patients, 23 were natives of West Bengal, three from Jharkhand, two from Tamil Nadu and one each from Bihar and Uttar Pradesh.
Reference population: The weighted mean height for different States and subgroups across the country are given in Table II. As majority of patients hailed from West Bengal, the mean height for the State (163.11 ± 9.94 cm for males and 150.87±6.81 for females)13 was used in analysis.

JAS: Socio-demographic parameters and anthropometry of JAS are given in Table I. JAS group had 12 patients (40%) with hypermobile joints with a mean Beighton's score of 6.
Of the 30 JAS patients, 28 (93.33%) had their observed height greater than that of the reference population (H-SDS >0). The mean ± SD of height of JAS patients (170.67±6.94 cm) was significantly higher than the reference value of 163.11±9.94 cm (P<0.001). Even the mean height of all male siblings and their fathers together as a group (n=47) and that of female siblings and their mothers together as a group (n=47) were significantly higher than that of regional sex matched reference values13 [Male relatives vs reference = 168.62 ± 8.12 cm vs 163.11 ± 9.94 cm, P<0.001); female relatives vs reference = 156.35 ± 6.89 cm vs 150.87±6.81 cm, (P<0.001)]. The mean ± SD of height of JAS patients was significantly higher even when compared against the reference value for the top wealth quantile (170.67± 6.94 cm vs 167.3±5.74 cm, P=0.013) and highest education (170.67± 6.94 cm vs 167.5±5.57 cm, P<0.05).
There was no difference between the mean height of patients with JAS and their corresponding mid-parental height (170.67 ± 6.94 vs 168.33 ± 5.43). However, seven out of 30 (23%) patients in JAS group had heights greater than 2SD of the mid-parental height. The mean delta height of these seven patients were 11±2 cm. Six out of these seven patients had hypermobile joints, an association more pronounced than that seen in the JAS group as a whole. These seven tall patients were compared with the remaining 23 patients within JAS. Bivariate analysis of seven tall patients vs remaining 23 patients followed by logistic regression analysis revealed that “tallness” was positively associated with hypermobility (OR=23.46, 95% CI 1.2-447.2, P=0.036) and negatively with vitamin D (OR=0.827, 95% CI 0.67-1.0, P=0.07). Tallness was not related to socio-economic status, duration of disease, type of disease (axial or peripheral disease), ESR or CRP values. Thirteen of the 30 (43.3%) patients in JAS group had a BMI of <18.5 kg/m2. Comparison of these 13 patients was done with the remaining 17 to determine the variables associated with BMI. Logistic regression analysis revealed no significant association.
AAS: Table I describes socio-demographic parameters and anthropometry of AAS group. There were eight patients (26.67%) with hypermobile joints with a mean Beighton's score of 5.75. Twenty six of the 30 patients (86.67%) had a height greater than that of the reference population (H-SDS>0). In case of AAS patients also, the mean±SD of height was significantly higher than the reference value13 (168.2±5.9 cm vs 163.11±9.94 cm; P<0.001). The mean height of immediate 1st degree male relatives (siblings and father; n=52) of AAS patients was significantly higher in comparison with the regional sex matched reference value13 (166.81±6.70 vs 163.11±9.94 cm; P<0.001). Similarly, the mean height of immediate 1st degree female relatives (siblings and mother; n=58) of AAS patients was higher in comparison with the regional sex matched reference mean height (154.57± 8.7 cm vs 150.87±6.8 cm; P=0.002). There was no significant difference when the mean height of AAS patients was compared with the subgroup with the highest education and top wealth quantile.
There was no difference in mean height between the AAS patients and their corresponding mid-parental height (168.2 ± 5.4 vs 166.53 ± 6.41). Four of the 30 (13.33%) patients in AAS group had heights greater than 2SD of the mid-parental height with a mean delta height of 12±4.76 cm. None of these four patients had hypermobility. When these four patients were compared with the remaining 26 patients, no significant association of height was detected with any of the variables. Of the 30 patients in the AAS group, only five (16.67%) had BMI<18.5 kg/m2. In regression analysis none of the variables studied were found to have significant correlation.
Discussion
In this study growth retardation was not seen in our patients with JAS. Majority of patients with JAS and AAS had height greater than that of reference population13. In JAS and AAS groups, 23 and 13.33 per cent patients, respectively were tall independent of any influence of parental height, as they had heights greater than 2SD of the mid-parental height.
Any study on height in a diseased state should take into account parental height as well as socio-economic factors, ethnic and regional factors and duration of disease. As already mentioned, our patients were not on any specific nutrient supplementation to the best of our knowledge; however, intake of subtherapeutic dose cannot be ruled out keeping recollection bias of patients in mind, especially because calcium with vitamin D are commonly prescribed in musculoskeletal disorders by referring primary physicians. The mean height of JAS patients was significantly higher when compared with the reference height of the best socio-economic class in the country and that of AAS was comparable. The mean heights of JAS and AAS groups as well as their parents and siblings were significantly higher, as compared with mean sex-matched regional reference population13. This could be due to a shared environmental factor such as socio-economic state or genetic factor, especially because of strong genetic predisposition and familial aggregation in AS. The other predictor variable influencing height is birth cohort. Perkins et al13 reported that for males, one year difference in year of birth was associated with a difference of 0.045 cm. Most of JAS were born between 1983-1995. Even after adjusting for birth cohort, the mean height of JAS would be higher than the reference population.
Twenty three per cent of JAS patients and 13.33 per cent in AAS group were tall independent of any influence of parental height, as they had heights greater than 2SD of the mid-parental height. Socio-economic status was not found to be a significant determinant of height in our study. Also, tallness of these patients could not be associated with duration of the disease, type of disease or inflammatory markers in logistic regression analysis; but association was seen with hypermobility of joints and low vitamin D state.
The link between benign hypermobility of joints and tallness is not an established one. Hypermobility was more common in JAS patients as compared with AAS; this could be because of decrease in hypermobility of joints with age. Increased incidence of hypermobility has been reported in Indian children with malnutrition16. However, in the logistic regression analysis, hypermobile joints were not found to have significant association with BMI in our patient groups. It is not clear if there is an increased incidence of hypermobility in AS, although there are a few studies describing coexistence of AS and hypermobility171819.
Negative association of vitamin D with height was seen in our study though not significant in logistic analysis. This does not imply that low vitamin D causes increase in height and this can be explained by higher requirement and utilization of vitamin D in tall people as described in literature20.
In earlier studies on JIA, short stature has been attributed to reduction in lower extremity measurements7. Also, reduced armspan measurements have been noted in 27 per cent of JIA patients in a study3. In our study, the armspan and the upper segment to lower segment ratio measurements were suggestive of absence of growth retardation of limbs.
The mean BMI values of both the population groups were in the normal range. However, it was notable that around 43.3 per cent of our JAS patients were underweight in spite of the fact that none of our patients belonged to the lowest strata by Kuppuswamy scale. Also, there was no significant association between low BMI and socio-economic status by logistic regression analysis.
In summary, no growth retardation was seen in our patients with JAS. Almost a quarter of JAS patients (23%) and 13.33 per cent in AAS group were tall independent of any influence of parental height, as they had heights greater than 2SD of the mid-parental height. This effect was also independent of socio-economic status, disease duration and inflammatory markers. There was significant positive association of height with hypermobility of joints and negative association with vitamin D level. Although 43.33 per cent of JAS patients were underweight, no significant association was detected for this observation. Lack of growth retardation in JAS patients observed in this study may seem contradictory to high prevalence of growth retardation described in other juvenile onset rheumatic diseases.
Our study was limited by small numbers. Height is influenced by many factors which makes comparison between patients difficult. We have tried to account for some of these factors such as socio-economic status using Kuppuswamy scale. Most of our patients belonged to upper middle class and this could be a cause for absence of growth retardation but it was interesting to note that the mean heights of both the patient groups were more than the mean height for the best economic class and the most educated strata of society. Being a cross-sectional study it was not possible to assess the contribution of disease activity and treatment on height. This could have been better studied using a prospective design.
Acknowledgment
The authors acknowledge CMC Vellore Fluid research grant and thank Ms Madhumathi and Ms Kavitha Devi for assistance in recruiting patients.
References
- International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-2.
- [Google Scholar]
- Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis. A long-term follow-up study. Horm Res. 1999;52:80-5.
- [Google Scholar]
- Adult outcomes of patients with juvenile idiopathic arthritis. Horm Res. 2009;72(Suppl 1):20-5.
- [Google Scholar]
- Growth patterns in juvenile rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:663-8.
- [Google Scholar]
- Growth retardation in non-steroid treated juvenile rheumatoid arthritis. Scand J Rheumatol. 1997;26:99-103.
- [Google Scholar]
- Growth retardation and delayed puberty in children and adolescents with juvenile idiopathic arthritis. Arch Med Sci. 2010;6:19-23.
- [Google Scholar]
- Longitudinal growth attainments of Indian boys with juvenile rheumatoid arthritis. Rheumatol Int. 2011;31:635-40.
- [Google Scholar]
- Children with oligoarticular juvenile idiopathic arthritis are at considerable risk for growth retardation. J Pediatr. 2011;159:832-7.
- [Google Scholar]
- Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-8.
- [Google Scholar]
- Kuppuswamy's socioeconomic status scale 2010-the need for periodic revision. Indian J Pediatr. 2012;79:395-6.
- [Google Scholar]
- Anthropometry and physical fitness in individuals with family history of type-2 diabetes mellitus: A comparative study. Indian J Endocrinol Metab. 2011;15:327-30.
- [Google Scholar]
- Patterns and trends of adult height in India in 2005-2006. Econ Hum Biol. 2011;9:184-93.
- [Google Scholar]
- International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-06: India; vol. I, Mumbai: IIPS; 2007
- [Google Scholar]
- Standards for children's height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970;45:755-62.
- [Google Scholar]
- Juvenile ankylosing spondylitis and the joint hypermobility syndrome. Srp Arh Celok Lek. 1992;120:58-60.
- [Google Scholar]
- Do pathological opposites cancel each other out. Do all patients with both hypermobility and spondylarthropathy fulfill a criterion of any disease? Scand J Rheumatol. 1999;28:120-2.
- [Google Scholar]
- Assessment of vitamin D status in healthy children and adolescents living in Tehran and its relation to iPTH, gender, weight and height. Ann Hum Biol. 2010;37:692-701.
- [Google Scholar]






