Translate this page into:
Apoptosis gene reprograming of human peripheral blood mononuclear cells induced by radioiodine-131 (131I) irradiation
For correspondence: Dr Mu-Hua Cheng, Department of Nuclear Medicine, The Third Affiliated Hospital of Sun Yat-Sen University, No. 600, Tianhe Road, Guangzhou, Guangdong 510 630, PR China e-mail: chmarka@163.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The nature of adaptable change of B-cell lymphoma-2 (BCL-2) and/or Bcl2-associated X protein (BAX) gene expression in the human peripheral blood mononuclear cells (PBMCs) irradiated by radioiodine in thyroid diseases therapy is not fully understood. In this study, the alternation of apoptotic gene expression was evaluated while the PBMCs collected from healthy volunteers were irradiated by the radioiodine-131 (131I).
Methods:
Fasting blood samples were obtained from healthy volunteers. PBMCs from group 0 to 6 were incubated and exposed to different doses of 131I in cell suspension for 6, 12, 24 and 48 h. The apoptosis rates and expression of BCL-2 and BAX genes of PBMCs were examined.
Results:
The apoptosis rate in the human PBMCs was gradually enhanced after six hour irradiation. The values of BCL-2 and BAX gene expression in groups 1-6 were higher than in group 0 within 6 h of irradiation, and then, these were decreased gradually from 6 to 12 h. BCL-2 gene expression increased in groups 1-3 after 12 h irradiation, but there was no difference in groups 4-6. The ratio of BCL-2/BAX gene expression among groups 4-6 gradually decreased during the period from 6 to 12 h, and it was significantly lower than in the group 0 at 12, 24 and 48 h.
Interpretation & conclusions:
The expression of BCL-2 and BAX genes was initially upregulated following irradiation. Later, the balance of BCL-2/BAX genes expression was adjusted, and then, PBMCs underwent apoptosis at higher doses of radiation.
Keywords
Apoptosis
gene expression
131I
mononuclear cell
radiation
Radioiodine has been widely used in hyperthyroidism and post-operative ablation of remnant thyroid tissue or metastasis in thyroid carcinoma patients for several decades12. The irradiation emitted from radioiodine would harm the peripheral blood mononuclear cells (PBMCs) in vivo, which play an important role in the immune response and in host homoeostasis, inflammation and elimination of tumour cells3. The results of in vitro and in vivo study demonstrated that the γ-radiation would induce the programmed cell death (apoptosis) of PBMCs4. The apoptosis of PBMCs was induced by γ-irradiation in a dose-dependent manner5. However, Vokurková et al6 reported that apoptosis was not detected within the first six hours in PBMCs isolated from blood of healthy donors when irradiated by a dose of 7 Gy, but in 50 per cent of the cells after 16 h and almost all cells after 48 h.
The mechanism of PBMC apoptosis induced by radioiodine in thyroid disease therapy is not completely understood7. The mechanism of the radiation-induced apoptosis would be associated with the adjustment of apoptosis gene8. The key event in the initiation of apoptosis is activation of caspase gene910, which is initiated by two distinct mechanisms mediated by cell surface receptor11 and mitochondrial factors1213. The event of apoptosis is mainly controlled by several genes14, including B-cell lymphoma-2 (BCL-2) family members, p53, first apoptosis signal (FAS) and Fas ligand (FASL). The expression of the BCL-2 family members is a crucial factor in the sensitivity of cells to radiation-induced apoptosis5.
BCL-2 family members belong to two main categories15. One is restraining apoptosis genes, such as BCL-2, BCL-extra-large (BCL-XL1) and Bag family molecular chaperone regulator 1 (BAG1). The upregulated expression of BCL-2 enhances the ability of lymphocytes in resisting γ-radiation16. BCL-2 gene family plays an important role in radiation-induced apoptosis and its interaction determines the regulatory biological effect517. Another category is promoting apoptosis genes, for example, BCL-2-associated X protein (BAX). Bax proteins exist in the cytoplasm or attach loosely at membrane as monosomes1718. When stimulated by the death signal, these protein monosomes transpose to mitochondrial membrane, transform into intrinsic membrane protein and cross-link as homodimer and finally lead cell to death. Nakagawa et al19 demonstrated that expression of BAX was upregulated in NIH3T3 cells within eight hours after ultraviolet light irradiation, and more than 50 per cent of the NIH3T3 cells underwent apoptosis 48 h after irradiation. Bcl-2 and Bax proteins are always co-ordinately expressed in cells17, and the elevated Bcl-2/Bax ratio promotes cell survival and vice versa. Bcl-2/Bax change plays a vital role in determining cell fate2021. Increasing level of Bcl-2/Bax heterodimer may prevent Bax inserting into mitochondrial membrane and inhibit the release of apoptosis factors, e.g. cytochrome complex (Cyt c) from mitochondria into cytosol, thereby inhibiting apoptosis22.
It is contentious whether the radioiodine irradiation would damage human body's immune system during the radionuclide therapy in thyroid diseases17. The sequence of events and nature of adaptable change in B BCL-2 and BAX genes following irradiation of human PBMCs by radioiodine in thyroid diseases therapy are not fully understood. We here present the change in apoptotic genes expression in human PBMCs following irradiation of radioiodine (131I).
Material & Methods
The study was conducted in the departments of Nuclear Medicine and Breast and Thyroid Surgery, Third Affiliated Hospital of Sun Yat-Sen University, PR China. This study was approved by the clinical medical research Ethical Clearance Committee in the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, PR China. Informed written consents were obtained from healthy volunteers for the collection of blood specimens. The PBMC samples obtained from blood sample (5 ml) of 12 volunteers were divided into seven groups, and each group had four-hole plate sample for four test points. The study was repeated three times for apoptosis, BCL-2 and BAX gene analysis, respectively.
Human PBMCs were obtained from fresh whole blood after differential migration with Ficoll Paque1. Washed twice with cold phosphate buffered saline3, the cells were suspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, USA) supplemented with 10 per cent foetal bovine serum (Zhejiang Tianhang Biotechnology, PR China) at a density of 1.5×106 cells/ml unless otherwise noted. The viability of these cells was greater than 98 per cent as estimated by trypan blue exclusion test13. The fluorescent dye 4′, Annexin V/PI double-staining kit were purchased from Keygen, PR China. Total RNA Extraction Kit I was from Omega, USA. BCL-2 primer was procured from Takara, Japan. BAX primer was from Invitrogen, USA.
Exposure of human PBMC samples to 131I: PBMC samples with cell density of 1.5×106/ml were placed in each well of a 12-well culture plate. The radioactive concentration in the blood was approximately 74 kBq/ml in the hyperthyroidism patients administrated with 370-555 MBq radioiodine in the next morning and was approximately 1332 kBq/ml in differentiated thyroid carcinoma patients with 7400 MB 131I in our preparatory experiments. Hence, the PBMC samples were divided into six groups (0-6). The group 0 was set as control group without radioiodine, and groups 1-3 were set for lower irradiation dose and treated with 18.5, 37 and 74 kBq 131I/ml cell suspension, respectively. Groups 4 to 6 were set for higher irradiation and treated with 333, 666 and 1332 kBq 131I/ml cell suspension, respectively. Immediately, these were incubated at 37°C in five per cent CO2 humidified atmosphere for 6, 12, 24 and 48 h and collected for analysis. Each experiment was repeated thrice.
Cell apoptosis detection: Annexin V-FLUOS assay was used for apoptosis detection according to the manufacturer's instruction. Briefly, 5 μl FITC-Annexin V and 5 μl propidium iodide (PI) were added to 5×105 cells suspended in 500 μl binding buffer. The mixture was incubated for 15 min in the dark at room temperature and was analyzed using FACS Aria flow cytometry (BD Company, USA) within one hour.
Semi-quantitative RT-PCR detection of BCL-2 and BAX mRNA: Briefly, total RNA was isolated using Total RNA Extraction Kit I (Omega, USA) according to the manufacturer's protocol. Reverse transcription-generating cDNA was performed using first strand cDNA Synthesis Kit (Fermentas, USA). PCR of BCL-2 and BAX cDNA using Go-Taq Green Master Mix (Promega, USA) was performed, with β-actin as internal standard.
BCL-2 cDNA was amplified using forward (5´-ACAACATCGCCCTGT GGATGAC-3´) and reverse (5´-ATAGCTGATTCGACGTTTTGCC-3´) primers, which produced a 409 bp product. Amplification of BCL-2 cDNA was performed at 94°C for five minutes for preheating, followed by 40 cycles of 94°C for 30 sec, 63°C for 30 sec, 72°C for 10 sec and a final extension of 72°C for five minutes.
BAX cDNA was amplified using forward (5´-GGACGAACTGGACAGTAACA-3´) and reverse (5´-ACCACCCTGGTCTTGGAT-3´) primers, which produced a 271 bp product. Amplification of BAX cDNA was performed at 94°C for five minutes for preheating, followed by 31 cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec, extension at 72°C for 10 sec and a final extension of 72°C for five minutes.
β-actin was amplified using forward (5´-GCTCGTCGTCGACAACGGCTC-3´) and reverse (5´-CAAACATGATCTGGGTCATCTTCTC-3´) primers, which produced a 359 bp product. All primers used in this study were synthesized by Invitrogen.
All PCR products were electrophoresed on two per cent agarose gels containing ethidium bromide. The resulting bands were photographed under ultraviolet light and analyzed using a Gel Imaging System (Gel Doc2000, Bio-Rad, Hercules, CA, USA). The relative intensity of bands of interest was represented as the ratio to β-actin mRNA bands. The ratio of fluorescence intensity of target-specific product to the internal control product was represented as the relative levels of target mRNA expression.
Statistical analysis: Data were presented as percentages, mean and standard deviation. Significance was assessed by analysis of variance for repeated measures and least significant difference test to compare the groups. Statistical analysis was performed using SPSS for Windows Release 13.0 (SPSS Inc., USA).
Results
Apoptosis rates of PBMCs after radioiodine irradiation: The data of apoptosis rate from groups 0 to 6 are presented in the Table while the PBMCs were irradiated within 6 to 48 h. The apoptosis rate from group 1 to 6 was not significantly different from that in the group 0 at six hours but increased gradually during the time period of irradiation from 6 to 48 h. The apoptosis rates in groups 4-6 were higher at 48 h than at 24 h irradiation (P<0.001). The apoptosis rate among groups 4-6 was higher than for groups 0-3 at 48 h (P<0.001).
| Time (h) | Group 0 | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
|---|---|---|---|---|---|---|---|
| 6 | 6.26±1.30 | 6.87±0.51 | 5.80±1.57 | 6.37±0.85 | 5.47±0.84 | 6.23±1.17 | 6.63±0.41 |
| 12 | 9.55±0.78 | 11.79±1.59 | 10.9±0.77 | 11.49±1.24 | 11.24±1.15 | 12.35±0.79 | 14.73±1.32 |
| 24 | 13.69±4.24 | 12.09±3.27 | 12.52±3.23 | 13.42±2.91 | 16.84±2.25 | 20.79±3.26 | 26.47±3.45 |
| 48 | 22.23±4.67 | 22.96±5.79 | 22.45±4.61 | 23.63±1.86 | 34.80±1.01***††† | 41.32±2.64***††† | 51.79±8.51***††† |
Values are mean±SD (n=3)
***P<0.001 compared to respective values in the same group at 24 h
†††P<0.01 compared to values in groups 0-3 at 48 h
BCL-2 gene expression after radioiodine irradiation: The expression value of BCL-2 gene in human PBMCs from groups 1 to 6 increased compared to the control group at six hours (P<0.01). However, the expression value of BCL-2 in human PBMCs from groups 1 to 6 decreased gradually in the period from 6 to 12 h and increased in groups 1-3 from 12 to 48 h but had no significant change in groups 4-6 (Fig. 1). The gene expression value of BCL-2 in all groups had no significant difference at 12 and 24 h, but these were lower in groups 4-6 than in groups 0-2 at 48 h (P<0.001).

- Expression of B-cell lymphoma-2 (BCL-2) in the treatment groups decreased gradually from 6 to 12 h and increased gradually in groups 1-3 from 12 to 48 h but remained unchanged in groups 4-6. The expression of BCL-2 in treatment groups increased significantly than the control group at six hours (P<0.01). Values are mean±SD of three observations.
BAX gene expression after radioiodine irradiation: The value of BAX gene expression in the human PBMCs from groups 1 to 6 was significantly higher than in the control group 0 at 6 h (P<0.01). The value of BAX gene expression in groups 1-6 was decreased from 6 to 12 h. However, there was no significant difference in BAX gene expression among groups 1-6 compared with group 0 at 12, 24 and 48 h, though the value of BAX gene expression in all groups had an ascendant trend after 24 h (Fig. 2).
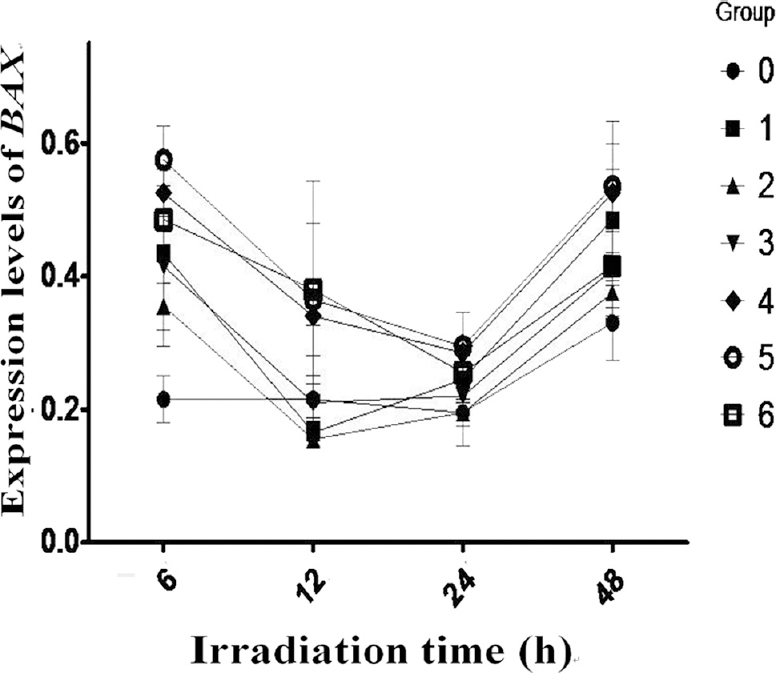
- Expression of Bcl-2 associated BAX protien in all treatment groups decreased gradually from 6 to 24 h and decreased more obviously in groups 4-6 than others. However it increased more obviously in groups 4-6 at 48 h. Values are mean±SD of three observations.
Ratio of BCL-2/BAX gene expression after radioiodine irradiation: The ratio of BCL-2/BAX gene expression in the human PBMCs in groups 4 to 6 was decreased from 6 to 12 h while that in groups 0-3 was relatively unchanged. However, it was significantly lower in groups 4-6 than in the group 0 at 12, 24 and 48 h (P<0.05). The ratio of BCL-2/BAX in groups 1 to 6 was similar to group 0 at 6 h.
Discussion
Programmed cell death, also known as apoptosis, plays a vital role in regulating the cell number of human body and function of organs31623. The major mechanism for cell injury and damage caused by ionizing radiation is regarded as being the radiation-induced apoptosis816. Apoptosis of PBMCs could be induced by γ-irradiation in a dose-dependent manner4. In the present study, the apoptosis rates among irradiated cells increased gradually during the time period of irradiation from 6 to 48 h. Though, the apoptosis rate of PBMCs in groups 1-3 had no increase compared with that in control group, but the apoptosis rate of PBMCs in relatively higher dose group (groups 4-6) was higher than in the lower dose groups and control group after the PBMCs were irradiated within 48 h. Those results indicated that the apoptosis event in PBMCs was dose dependent. Micronucleus formation in the lymphocytes was increased with the increasing radiation dose24. The apoptosis rate of PBMCs induced by irradiation was not only associated with radiation intensity but also with the exposure time25.
BCL-2 gene is considered as one of the strongest anti-apoptosis genes2627, and the BAX gene is one of main apoptosis promoting genes19. Earlier reports28 demonstrated that upregulating expression of BCL-2 might enhance the ability of lymphocytes in resisting to γ-radiation. In the present study, the PBMCs upregulated the BCL-2 and BAX gene expression within six hour of irradiation. It implied that the PBMCs might upregulate the expression of BCL-2 gene to prevent apoptosis induced by irradiation in early period. However, it was not clear as to why the level of both BCL-2 and BAX gene expression decreased during the time point from 6 to 12 h, while the PBMCs were irradiated by radioiodine in all group. This might be because all gene expressions in cells would be inhibited by the initial irradiation.
The BCL-2/BAX ratio plays an important role in mitochondria-mediated apoptosis18. The expression of BCL-2 and BAX genes in PBMCs can be changed after radiation1629. In our study the gene expression value of BCL-2 in PBMCs increased gradually in lower dose groups 1-3 during the time points from 12 to 48 h of irradiation. Gradual rise in BCL-2 expression in PBMCs receiving lower doses of irradiation (as in groups 1-3) may be due to an adaptive upregulation of BCL-2 to prevent apoptosis; however, at higher doses with greater extent of damage, the pro-apoptotic regulators prevail and cells undergo apoptosis.
BAX is one of main apoptosis promoting genes. In the present study, the BAX gene expression was up-regulated in the PBMCs irradiated within six hours also, but later the BAX gene expression was down- regulated close to the level in control group, and there were no significant difference in different radioactive dose samples compared with the control group after 12 h irradiation. These results indicated that the BAX gene expression might not be the key event while the apoptosis was induced by the radioiodine irradiation.
Bcl-2 and Bax proteins are co-ordinately expressed in cells; Bcl-2 and Bax ratio plays a vital role in determining cell fate20. In the present study, the ratio of BCL-2/BAX gene expression in higher dose group was lower than that in lower dose group after 12 h. These results implied that the mechanism of apoptosis induced by radioiodine radiation in PBMCs would be due to the decrease of BCL-2/BAX ratio. In the lower dose group, cells did not undergo apoptosis because the BCL-2 gene expression was upregulated and the BCL-2/BAX heterodimer was increased to prevent apoptosis.
The present study had certain limitations. The actual irradiation dose was not calculated because it was difficult and complicated to accurately calculate the irradiated dose of radioiodine in the PBMCs30. Further, the radioactive concentration in PBMCs was set according to the rough results of the radioactive concentration in the blood in several hyperthyroidism and differentiated thyroid carcinoma patients. The biological factor of radioiodine drainage was not considered while cumulative dose was evaluated in cytological experimental of PBMCs.
In conclusion, the expression of the BCL-2 family members is a crucial factor in the sensitivity of cells to radiation-induced apoptosis. The apoptosis rate in human PBMCs gradually increased after six hour of irradiation in the present study. The values of BCL-2 and BAX gene expression were enhanced at initial irradiation while the PBMCs were irradiated by radioiodine (131I), and then decreased gradually from 6 to 12 h. Later, the balance of BCL-2/BAX gene expression was adjusted, and PBMCs underwent apoptosis at higher cumulative doses.
Acknowledgment
Authors thank the technicians in the Nuclear Medical Department of the Third Hospital Affiliated Sun Yat-Sen University for technical support.
Financial support & sponsorship: This work was supported in part by the Foundation (No.2016A020215070) of Guangdong Provincial Science and Technology plan project (Department of Science and Technology of Guangdong Province, PR China).
Conflicts of Interest: None.
References
- DNA damage in peripheral blood lymphocytes of thyroid cancer patients after radioiodine therapy. J Nucl Med. 2016;57:173-9.
- [Google Scholar]
- Prognostic factor analysis in 325 patients with Graves’ disease treated with radioiodine therapy. Nucl Med Commun. 2018;39:16-21.
- [Google Scholar]
- MiR-126 in peripheral blood mononuclear cells negatively correlates with risk and severity and is associated with inflammatory cytokines as well as intercellular adhesion molecule-1 in patients with coronary artery disease. Cardiology. 2018;139:110-8.
- [Google Scholar]
- Changes in phosphorylation of histone H2A. X and p53 in response of peripheral blood lymphocytes to gamma irradiation. Acta Biochim Pol. 2008;55:381-90.
- [Google Scholar]
- Bcl-xL overexpression restricts gamma-radiation-induced apoptosis. Cell Biol Int. 2006;30:15-20.
- [Google Scholar]
- CD8+ natural killer cells have a potential of a sensitive and reliable biodosimetric marker in vitro . Physiol Res. 2006;55:689-98.
- [Google Scholar]
- Cytogenetic effects of radioiodine therapy: A 20-year follow-up study. Radiat Environ Biophys. 2016;55:203-13.
- [Google Scholar]
- New concepts in radiation-induced apoptosis: ‘premitotic apoptosis’ and ‘postmitotic apoptosis’. J Cell Mol Med. 2001;5:240-53.
- [Google Scholar]
- Caspase-mediated crosstalk between autophagy and apoptosis: Mutual adjustment or matter of dominance. J Cancer Res Ther. 2015;11:514-24.
- [Google Scholar]
- Silencing of the mRNA-binding protein huR increases the sensitivity of colorectal cancer cells to ionizing radiation through upregulation of caspase-2. Cancer Lett. 2017;393:103-12.
- [Google Scholar]
- Role of inhibitor of growth 4 in the suppression of human melanoma cells through the fas/fasl-mediated apoptosis pathway. Int J Mol Med. 2018;41:1055-61.
- [Google Scholar]
- Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion. Cell Death Dis. 2015;6:e1633.
- [Google Scholar]
- Combination of cinobufacini and doxorubicin increases apoptosis of hepatocellular carcinoma cells through the fas- and mitochondria-mediated pathways. Am J Chin Med. 2017;45:1537-56.
- [Google Scholar]
- Mesenchymal stem cells-conditioned medium protects PC12 cells against 2,5-hexanedione-induced apoptosis via inhibiting mitochondria-dependent caspase 3 pathway. Toxicol Ind Health. 2017;33:107-18.
- [Google Scholar]
- CBid, BAX and Bcl-xL exhibit opposite membrane remodeling activities. Cell Death Dis. 2016;7:e2121.
- [Google Scholar]
- Up-regulation of BCL-2 expression in cultured human lymphocytes after exposure to low doses of gamma radiation. J Med Phys. 2015;40:38-44.
- [Google Scholar]
- Live-cell imaging to measure BAX recruitment kinetics to mitochondria during apoptosis. PLoS One. 2017;12:e0184434.
- [Google Scholar]
- The relationship between the BCL-2/BAX proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS One. 2016;11:e0163327.
- [Google Scholar]
- Downregulation of BCL-xL is relevant to UV-induced apoptosis in fibroblasts. J Biochem Mol Biol. 2002;35:452-8.
- [Google Scholar]
- Radiation-induced gene expression profile of human cells deficient in 8-hydroxy-2'-deoxyguanine glycosylase. Int J Cancer. 2006;118:633-42.
- [Google Scholar]
- Rapamycin induces autophagy in the melanoma cell line M14 via regulation of the expression levels of BCL-2 and BAX. Oncology Letters. 2013;5:167-72.
- [Google Scholar]
- Selective peptide inhibitors of antiapoptotic cellular and viral BCL-2 proteins lead to cytochrome c release during latent kaposi's sarcoma-associated herpesvirus infection. Virus Res. 2016;211:86-8.
- [Google Scholar]
- Apoptosis and cell proliferation in short-term and long-term effects of radioiodine-131-induced kidney damage: An experimental and immunohistochemical study. Nucl Med Commun. 2018;39:131-9.
- [Google Scholar]
- Dose-dependent and gender-related radiation-induced transcription alterations of Gadd45a and Ier5 inhuman lymphocytes exposed to gamma ray emitted by (60)Co. Radiat Prot Dosimetry. 2013;154:37-44.
- [Google Scholar]
- An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat Cell Biol. 2015;17:1270-81.
- [Google Scholar]
- Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim Biophys Acta. 2015;1852:70-82.
- [Google Scholar]
- Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of fas or FADD/MORT1 signaling. Implications for cancer therapy. J Exp Med. 2000;191:195-200.
- [Google Scholar]
- Effects of hesperidin as a radio-protector on apoptosis in rat peripheral blood lymphocytes after gamma radiation. J Biomed Phys Eng. 2016;6:217-28.
- [Google Scholar]
- Dose calculations for ([131] I) meta-iodobenzylguanidine-induced bystander effects. Dose Response. 2014;12:1-23.
- [Google Scholar]






